Abstract
The development of oxygen (O2) carrying blood substitutes has evolved from the goal of replicating blood O2 transports properties to that of preserving microvascular and organ function, reducing the inherent or potential toxicity of the material used to carry O2, and treating pathologies initiated by anemia and hypoxia. Furthermore, the emphasis has shifted from blood replacement fluid to “O2 therapeutics” that restore tissue oxygenation to specific tissues regions. This review covers the different alternatives, potential and limitations of hemoglobin based O2 carriers (HBOCs) and perfluorocarbon based O2 carriers (PFCOCs), with emphasis on the physiological conditions disturbed in the situation that they will be used. It describes how concepts learned from plasma expanders without O2 carrying capacity can be applied to maintain O2 delivery and summarizes the microvascular responses due to HBOCs and PFCOCs. This review also presents alternative applications of HBOCs and PFCOCs namely: 1) How HBOC O2 affinity can be engineered to target O2 delivery to hypoxic tissues; and 2) How the high gas solubility of PFCOCs provides new opportunities for carrying, dissolving and delivering gases with biological activity. It is concluded that current blood substitutes development has amplified their applications horizon by devising therapeutic functions for oxygen carriers requiring limited O2 delivery capacity restoration. Conversely, full, blood-like O2 carrying capacity re-establishment awaits control of O2 carrier toxicity.
Keywords: Plasma expander, oxygen, artificial blood, hemoglobin, perfluorocarbon, gas carriers, artificial organs
In the United States allogeneic RBC transfusion has long been considered an important treatment option for patients suffering from blood loss.1 However, the recent emergence of infectious agents such as the H1N1 influenza virus and others have the potential of placing the blood supply at risk.2 Currently, the American Red Cross tests donated blood for hepatitis B and C viruses, human immunodeficiency virus (HIV), human T-cell lymphotropic virus, syphilis, West Nile virus and the agent of Chagas disease.3-6 As a result, the safety of the blood supply in terms of transfusion transmitted diseases is quite good. However, as new infectious agents emerge, the unit of blood cost increases, since additional screening tests may have to be conducted before blood can be distributed to health care providers. Of greater concern is that donated blood may contain infectious agents yet to be identified.4 Moreover, short- and long-term transfusion-related adverse events are among the costliest contributors to health care expenditures.7 In addition, there are new concerns regarding the safety of blood transfusions following extended durations of storage (i.e., the storage lesion).8-9 To further compound the problem, the availability of human blood is even more limited in emergency situations such as wars or natural disasters.10 Therefore, it has been a long-term goal to develop an efficacious and safe universal blood substitute (i.e., O2 bridge) for use in transfusion medicine.
Hemoglobin (Hb) is the protein responsible for storage and transport of O2 and other gaseous ligands in RBCs.11 Hb is also the precursor for synthesis/formulation of Hb based O2 carriers (HBOCs) that can be used as RBC substitutes.12-14 It is not surprising that the first HBOC to be developed as a RBC substitute consisted of stroma-free Hb.15-16 However, transfusion of acellular Hb leads to several major side-effects.17-21 In the circulation extracellular tetrameric Hb (α2β2) easily dissociates into two pairs of αβ dimers18-19, which are extremely prone to oxidation21 and enhanced renal excretion.18, 22-23 The process of Hb oxidation to methemoglobin (metHb) promotes unfolding of the globin chains and releases cytotoxic heme into the circulation, leading to kidney tubule damage and eventual renal failure.18-19 MetHb can also generate harmful reactive O2 species (ROS)17 that can damage cell membranes and oxidize nucleic acids and proteins.20 Therefore, the presence of extracellular Hb in the circulation can elicit tissue toxicity due to heme release and ROS generation, causing vasoconstriction and systemic hypertension by various mechanisms.24-27 These include scavenging nitric oxide (NO) generated by endothelial cells, which acts as a vasodilator to the surrounding smooth muscle cells24-25 and an “autoregulatory” response in which extracellular Hb facilitates O2 transport in the lumen of the blood vessel over oxygenating the surrounding tissues, thereby eliciting vasoconstriction in order to reduce blood flow.26-27 Regardless of the exact mechanism for the development of vasoconstriction and systemic hypertension, stroma-free Hb can be modified in order to eliminate or reduce these adverse effects.
Physiological deficits due to blood losses
Historically, blood substitutes have been formulated to address the deficit of blood’s intrinsic oxygen carrying capacity (O2CC) resulting from the loss of red blood cells (RBCs), however the decrease of circulating RBCs and the associated blood volume are not the only factors affected by blood loss. Studies of the biomechanics of the systemic circulation and the microcirculation have shown that blood fluid mechanical properties are optimized to maintain blood flow and to uniformly perfuse the capillary network.28 Currently, this can only be accomplished by the transfusion of fresh blood, a commodity whose increasing scarcity drives the development of blood substitutes. In order to design a blood replacement fluid, it is essential to understand the changes in physiological conditions underlying the need for blood transfusion, since this fluid will have to address these changes and restore O2CC.
Blood loss lowers blood pressure, and most vascular beds initiate reflexes aimed at maintaining pressure via vasoconstriction. The combination of low blood pressure and vasoconstriction reduces the hydrostatic pressure at the arteriolar end of the capillaries. Low capillary pressure affects fluid movement across the capillary membrane (filtration). Therefore, blood losses reduce capillary blood pressure where fluid filters from plasma into the tissues and increases capillary pressure in the region of the capillary where fluid is reabsorbed, causing a net gain of fluid to the plasma. This fluid “autotransfusion” increases the severity of the anemia induced by blood losses. The most apparent deficits associated with blood loss are:
Intrinsic oxygen carrying capacity
The intrinsic O2CC is the amount of O2 contained in a volume of blood. It comprises O2 carried by Hb and that dissolved in plasma. For saturated blood this amounts to 20 ml O2/dl blood carried by RBCs and 0.2 ml by plasma (at Hct 45%). Blood losses change these proportions, however O2 carried by plasma never becomes a prominent factor, even in conditions of 100% O2 inhalation since the partial pressur of O2 (pO2) of hemodiluted blood reaching the tissue is much lower due to the O2 transit losses.
Blood viscosity
The viscosity imparted to blood by blood substitutes is the most overlooked parameters in the design of blood substitutes. This arises in part from the perception embodied in medicine since antiquity29 that lowering blood viscosity is beneficial, an axiom formerly applied throughout the practice of medicine. Deliberate or un-intended adherence to this concept cause blood substitutes viscosity to have plasma-like viscosity of the order 1.5 cP even when formulated at 14 g Hb/dl. Infusion of a fluid with low viscosity decreases blood viscosity, beyond the effect of reducing the concentration of RBCs. A moderate reduction of blood viscosity can have positive effects due to the increase in flow and perfusion which maintain WSS constant, as the increased flow compensates for the decreased viscosity. However, further lowering Hct is not compensated by a corresponding increase in blood flow because the heart is unable to sustain the increased workload, as the diluted blood delivers less O2 to the heart muscle.30 As a consequence WSS decreases, leading to vasoconstriction and reduced blood flow. Therefore, the positive effects of lowering viscosity are limited to Hct levels that maintain heart function, increased blood flow and maintenance of WSS sufficient to prevent the increase of vascular hindrance, a process that can be delayed by increasing plasma viscosity during hemodilution.
Vessel wall shear stress (WSS)
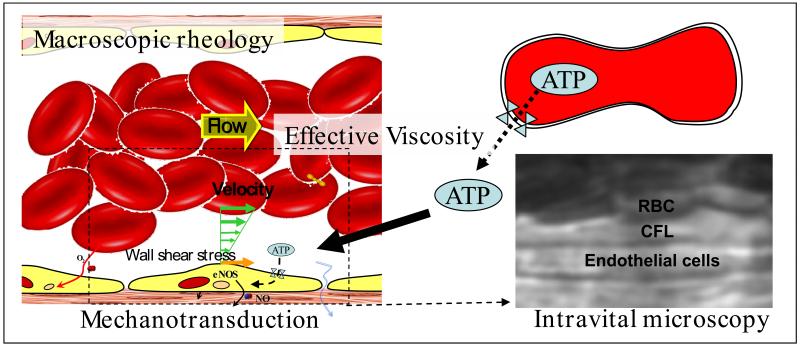
Shear stress is the product of blood viscosity and shear rate determined by the flow velocity profile. It has a principal cardiovascular role since it causes the endothelium to produce NO via mechanotransduction, modulating peripheral vascular resistance and blood flow. Hemorrhage depletes circulating blood volume, lowers blood pressure throughout the circulation including the capillaries, promoting fluid absorption from interstitial spaces, which continues until plasma colloidal osmotic pressure lowers to the new average capillary pressure. This process lowers blood viscosity and reduces the viscous component of vascular resistance.29 Upon fluid resuscitation from hemorrhagic shock, blood flow increases, although it is not able to restore WSS, due to limitations in the increase in cardiac output and therefore in blood flow velocity. The decrease in WSS lowers mechanotransduction, hinders the production of NO by the endothelium, and prevents normal vascular signaling and regulation31-33 and the production of vasorelaxants that modulate vessel diameter, which determines the geometric component of vascular resistance and blood flow.
Cell free layer (CFL)
The CFL separates the RBC blood column from the vessel wall, i.e., the endothelial cell membrane. This hydrodynamic layer is generated by axial migration due to resulting from the flexibility of RBCs that cause their displacement toward the center of the flow stream.34-37 The presence of the CFL contributes to the reduction of flow resistance by decreasing blood viscosity near the vessel wall. Hemodilution decreases blood viscosity and increases the CFL width, further decreasing flow hindrance.38 In this scenario using low viscosity (1.0 - 1.3 cP) plasma expanders decreases WSS because of the increased CFL width, while plasma viscosity which in normal conditions is in the range of 1.0 to 1.3 cP is mostly unchanged.38
NO distribution
The physiological effects due to hemodilution tend to lower the NO concentration in the arteriolar wall, causing vasoconstriction and a pro-inflammatory environment.39 Vasoconstriction lowers blood flow and WSS, decreasing vascular mechanotransduction, a sequence of events that is aggravated if hemodilution is associated with the presence of acellular Hb or HBOCs in the circulation.39-40 Hb undergoes several reactions reducing smooth muscle NO levels.41-42 The extent of the physiological consequences of Hb-associated NO scavenging depends on whether Hb is localized within RBCs or circulates as acellular Hb due to hemolysis or infusion of HBOCs.41-47 Hb scavenges NO, and effectively block NO bioactivity, resulting in vasoconstriction and transient hypertension.48-50 In the presence of acellular Hb, NO is quenched through an NO dioxygenation reaction and by the formation of the “dead end” (highly stable and relatively non-reactive) ferrous heme-nitrosyl form.49, 51-53 The NO dioxygenation reaction involves the efficient reaction of NO with oxygenated hemes to form met (ferric) heme and nitrate.42, 54-55 Therefore, the NO reactions with Hb will depend on the redox and ligation states of the heme iron modulated by O2 levels.49, 53, 56 Scavenging of NO by acellular Hb has been viewed as a major source of HBOC induced toxicity, which has in part limited the development of these materials for clinical use.49-50, 57 The relevance of the NO dioxygenation reaction as a contributor to HBOC-induced toxicity was compellingly demonstrated through the use of recombinant Hbs engineered to slow down NO dioxygenation reaction rates.54-55, 58 Implications of HBOCs induced NO scavenging and vasoconstriction will be analyzed for various HBOCs later in this review.
ATP release from erythrocytes
RBCs have been shown to enable the controlled, nonlytic release of adenosine-5′-triphosphate (ATP), not involving cell membrane rupture in response to mechanical stimuli.59 They release ATP following exposure to mechanical deformation, reduced O2 tension, or acidosis.60 These are conditions to which erythrocytes are exposed in the vasculature, e.g., when passing through constricted vessels or vessels in contracting striated muscle.61 Once in the extracellular medium, extracellular ATP triggers different cellular responses by interacting with receptors on the cell surface, while at the same time its concentration is controlled by the activities of one or more ectonucleotidases.62 Although intracellular ATP signaling is well known, there is comparatively little information on the mechanism of ATP release. Since the RBC spectrin-actin cytoskeleton appears to control ATP mechanosensitive release,59 it is likely that blood viscosity is a factor affecting vascular and RBC ATP signaling to maintain perfusion and oxygenation. Significant decreases in Hct as well as WSS could therefore be a factor in lowering ATP bioavailability.
The combination of these deficits causes the decrease in O2 delivery capacity of the circulation and the initiation of microvascular dysfunction, a condition characterized by the decrease of functional capillary density (FCD). This microvascular parameter measures the circulation’s capacity for maintaining uniform tissue oxygenation and the adequate extraction of metabolic by-products from the tissue. Notably microvascular dysfunction is mostly independent of tissue oxygenation, and is present when Hct is reduced by 50%, which is the nominal reduction of RBC concentration that signals the need to restore O2 carrying capacity.
Sensing and regulation of the O2 supply at the local level in the microcirculation has undergone a profound transformation since the finding that RBCs are not only O2 carriers but also O2 sensors.63 One of the mechanisms to sense O2 concentration by the RBCs is based on the release ATP in conditions of increased shear stress and decreased O2 tension. Once released from RBCs ATP forms adenosine stimulating the endothelium to produce the vasodilator NO. ATP is a signaling molecule that can stimulate the ATP-sensitive P2Y-purinergic receptor in the plasma membrane of vascular smooth muscle and endothelial cells. It contributes to blood pressure regulation since the PY2 purinergic receptor modulates the production of NO and therefore mediates vasoconstriction and dilatation.64-65 Studies in vitro, in experimental models and in humans have shown that RBC-derived NO is a factor in regulating the match between O2 demand and O2 supply through the control of vessel diameter. Furthermore, this regulation is present at various Hct levels66 and extends to other flow regulation processes such as thermoregulation.67
Novel HBOC formulations include ATP-adenosine and glutathione (GSH) cross-linked to Hb (ATP-ADO-GSH-Hb), which can delivery O2 and stimulate erythropoiesis.68-69 In children with sickle-cell anemia, a single injection of ATP-ADO-GSH-Hb stimulated erythropoiesis.68 ATP-ADO-GSH-Hb’s erythropoietic activity and O2 carrying capacity can reduce the need for supplementary transfusions. The chemical modification with ATP, adenosine, and GSH does not interfere with Hb O2 transport capacity, and provides the Hb molecule with properties that counteract the intrinsic toxic effects of Hb, namely vasoconstriction, oxidative stress, and inflammation.70 In this HBOC, the ATP stabilizes the Hb tetramer and prevents its dimerization, and adenosine allows the creation of Hb oligomers,70 in addition ATP and adenosine counteract the vasoconstrictive and proinflammatory properties of acellular Hb, and prevents platelet aggregation.70 Lastly, GSH shields heme from reactive O2 species (ROS), and introduces a electronegative charge onto the surface of Hb reducing renal excretion, extravasation, and phagocytociss.70 Studies by Simoni et al. investigated the potential of ATP-ADO-GSH-Hb as an erythropoiesis stimulating agent.68-70
Blood rheology and plasma expansion
The viscosity of blood is primarily determined by its exponential dependence on Hct. As blood flows in a vascular network of branching arterial blood vessels, the presence of the CFL becomes an increasingly significant volume compartment. Therefore, the tube Hct in blood vessels progressively decreases from the systemic value, about 45% in men and 42% in women, to about 10% in the capillary circulation.71 This phenomenon is known as Fahraeus effect, which is due to the motion of RBCs away from the vessel wall in microvessels (and small tubes),72 which decreases blood effective viscosity near the vessel wall (the Fahraeus-Lindqvist effect).73 This decreases the Hct near the vessel wall and compacts cells in the vessel core, producing a non-uniform Hct in the microvascular cross-section.74 Since blood close to the vessel wall supplies side branches, the feeding Hct of these branches has a lower Hct, an effect known as “plasma skimming” that becomes more significant as blood vessel diameter decreases.74 As a consequence, capillary Hct is low (0 - 20%) and capillary blood viscosity is only slightly higher than plasma viscosity while systemic blood viscosity averages about 4.5 cP. Given the exponential relationship between blood viscosity and Hct, and the dependence of blood effective viscosity on vessel diameter, hemodilution with a fluid of a plasma-like viscosity primarily lowers the effective blood viscosity in large blood vessels. Hemodilution has a lesser effect on blood viscosity in micro-vessels and has practically no effect in capillaries.
Blood viscosity is a determinant of peripheral vascular resistance and therefore cardiac output and blood pressure. The two-phase nature of blood (RBCs and plasma) and RBC deformability and aggregability cause blood to exhibit a varying non-Newtonian viscosity as it passes through the different vascular compartments. These factors also cause blood to be shear-thinning, whereby its viscosity decreases as the shear rate increases.75 Blood shear thinning properties redistribute shear rate dependent energy expenditure from the bulk of the flow (RBC core) to the periphery (CFL), increasing WSS. This effect is in part counteracted by the increase in CFL width.38
Blood rheology and hemodilution
Blood losses entail hemodilution, since the organism shifts fluids into the circulation from the tissue compartment. Correcting blood loss with a solution further lowers Hct, i.e., hemodilutes the circulation. To date, plasma expander that are used to treat hypovolemia and to reinstate blood volume, have plasma-like viscosity and are Newtonian fluids. An exception is polyethylene glycol (PEG) conjugated albumin (PEG-Alb), presently under development, which when mixed with diluted blood increases its natural shear thinning behavior, i.e., the slope of the dependence of shear stress vs. shear rate increases as the shear rate decreases, according to measurements in a conventional cone and plate viscometer. This leads to blunter velocity profiles, increased WSS and production of NO76 and lower vascular resistance due to vasodilatation. However, the exact mechanism by which PEG-Alb affects blood viscosity is not presently known.
The PEG-Alb molecule has a large exclusion volume, which significantly increases the colloidal osmotic pressures (COP) of the albumin molecule per se, limiting its plasma concentration and therefore the blood viscosity obtained when introduced in the circulation.76 There is presently little information on the aggregability induced by PEG-Alb or its intrinsic pseudoplasticity.
Hemodilution with PEG-polymers
PEG-Alb is a remarkable plasma expander that yields close to normal microvascular function even on conditions of extreme hemodilution.77 The same effect has been obtained with significantly higher viscosity fluids such as dextran 500 kDa and alginates.78 Both types of plasma expansion provide near optimal resuscitation from hemorrhagic shock induced by the experimental exsanguination of 50% blood volume.79-80 The principal difference between plasma expanders is that dextran 6% 500 kDa (6 cP) and 1% alginate (7 cP) hemodiluted blood have comparatively high viscosity compared to 4% PEG-Alb (1.8 cP). The high viscosity causes an additional load on heart function that is absent with PEG-Alb.30 PEG-Alb is a low viscosity plasma expander, that when mixed with blood increases blood flow driven by a constant pressure gradient or decreased the pressure drop under constant flow conditions.76 Blood diluted with PEG-Alb restores blood’s shear thinning behavior at low Hct leading to different hemodynamic effects at various locations within the circulation. In particular the inherent hemodilution associated with its introduction and it is shear thinning endowing properties reduces blood viscosity in the heart, therefore reducing its workload.30, 81
The effect of polymerization
Solution viscosity is a direct function of the solute dimension (molecular structure and mass) and concentration, therefore polymerization of globular proteins provides the means for increasing solution viscosity without increasing COP. Polymerizing Hb increases O2CC of the solution.82 Hemoglobin polymers of up to about 30 MDa have been synthesized and occur in nature. Natural acellular Hb of terrestrial and marine worms is configured in this manner.83 Hemoglobin from the marine worm Arenicola marina is being developed as an HBOC84 and has properties similar to the synthesized zero linked polymeric Hb OxiVita85 developed by Prof. Bucci’s group.86
The rheology of hemoglobin vesicles
A different means for introducing Hb in the circulation is to encapsulate it in vesicles (HbV). This technology was developed by the group formerly directed by Prof. Tsuchida87 and consists in encapsulating Hb in 250 nm diameter phospholipid vesicles coated with PEG. This approach allows high Hb concentrations, making the O2CC of the PEG-HbV suspension similar to blood. PEG-HbV requires suspension in a solution whose osmolarity, COP and solution viscosity can be adjusted. PEG-HbVs were tested using different plasma expanders including recombinant human serum albumin (rHSA), dextran 70 kDa, modified gelatin 45k Da, and hydroxyethyl starch 140 kDa. The HbV suspension in rHSA solution was nearly Newtonian, while PEG-HbV suspension in hydroxyethyl starch, dextran and gelatin made non-Newtonian suspensions due to PEG-HbV aggregation at a HbV concentration of 16% w/v (Hb = 10 g/dL and lipids 6 g/dL, which corresponds to a Hb volume fraction of about 60% v/v).88
The rheological component of hemorrhagic shock resuscitation
Blood is generally transfused to treat hemorrhage when Hct or Hb are reduced by 50%. This approach is undeniably successful in restoring O2CC; however, it is questionable if this is the sole origin of the resulting improvements. Resuscitation from hemorrhagic shock with non-O2 carrying fresh blood saturated with carbon monoxide or whose Hb was completely converted to methemoglobin was not different by comparison to fresh blood.89-90 In this scenario, blood provides resuscitation by restoring blood properties independent of O2CC, where the most conspicuous effect is the significant increase of blood viscosity. Since the decision to transfuse blood is frequently driven by medical experience, and there is evidence that the intervention provides an acute health benefit, it is possible that increasing blood viscosity of a hemodiluted individual is beneficial per se. This suggests that the transfusion trigger in many instances is a “blood viscosity trigger”. Furthermore, if the principal purpose of a blood transfusion is to restore blood viscosity, this would not be the optimal use of this resource.
Can plasma expansion increase oxygen delivery?
Plasma expanders do not carry O2 per se, therefore increased tissue O2 in hemodilution, if it occurs, must depend on effects equivalent to increasing O2CC. Through the combination of vasodilatation and hemodilution, plasma expanders like PEG-Alb, dextran 500 kDa and alginates induce a condition of supra-perfusion, whereby microvascular flow is significantly increased, in some cases up to 50% above baseline.77 Oxygen delivery is the resultant of blood flow or convection through the vasculature and diffusion from the vasculature. Increasing blood flow increases convection while diffusion remains comparatively constant, with the net effect that more O2 arrives to the capillaries.91 Experimental studies show that supra-perfusion is associated with a significant increase in vessel wall NO concentration.39, 76
Interplay between NO levels and O2 utilization
Endothelial NO synthase can affect O2 metabolism at the mitochondrial level.92 Studies in conscious dogs at rest and during exercise show that NO inhibition increases O2 consumption independently of changes of skeletal muscle blood flow.93 Positron emission tomography measurements of human skeletal muscle blood flow and uptake shows that NO formation inhibition enhances resting muscle O2 uptake by 20%. Conversely, cell respiration is inhibited where inducible NO synthase increases NO generation.94 Angiotensin-converting enzyme inhibitors (enalapril) increases intrarenal O2 tension by decreasing O2 consumption through stimulation of NO synthesis.95 Dietary nitrate supplements NO production independently of NO synthase.96 Thus, increasing circulating nitrite increases NO bioavailability.97 Nitrite and nitrate are reduced in physiological conditions to NO. Dietary nitrates have been reported to reduce O2 consumption during maximal arm and leg exercise.98 Therefore, increasing NO concentration should lower O2 metabolism and reduce O2 demand, which can be equivalent to increasing O2CC.
Oxygen carrying plasma expanders
Increasing O2CC of a volume replacement fluid is in principle as simple as substituting albumin with Hb in a human serum albumin-based plasma expander, since albumin and Hb are globular proteins of virtually identical solution properties. This can be accomplished across species for Hb due to the lack of Hb antigens, which is not the case for albumin precluding the use of the bovine albumin source in humans. However, albumin and Hb are structurally very different molecules, since albumin is a folded protein, while Hb is a tetramer with 4 identical subunits that can relatively easily dis-aggregate in solution, creating problems for kidney function. These limitations were recognized early on, leading to various approaches to stabilize the tetramer by crosslinking its subunits, or polymerizing the Hb molecule.99-101
The pressor effect of hemoglobin solutions
Regardless of the source and method of stabilization of molecular Hb, its introduction into the circulation causes hypertension, assumed to be one of the causes for the negative outcome of clinical trials with HBOCs. However, it is questionable whether this is directly and uniquely due to the pressor effect. It should be noted that hemorrhage and other forms of shock are treated with vasopressors102, and there are reports proposing that the Hb pressor effect could be beneficial103-104 in the management of hemorrhage, providing immediate treatment independently of other pathologies.105 Systemic pressure is routinely increased during resuscitation by giving fluid and using vasopressor agents when fluid alone is not sufficient. However, the effect of vasopressors does not necessarily transmit central pressure to the periphery, and can lead to necrosis and multi organ failure as seen clinically when vasopressors dosage is exceeded using noradrenaline or arginine-vasopressin.102, 106
The principal finding in studies of hemorrhage resuscitation with a viscogenic plasma expander (Hextend, Hospira, IL) PEG-Alb and the vasoactive polymerized bovine Hb HBOC-301 (OPK Biotech, Cambridge, MA) was that HBOC-301 transfusion in a hamster window chamber model of hemodilution prior to a major bleed yields 100% survival after one hour.104 This result is identical to that obtained using PEG-Alb previously reported107 to also yield the same final blood pressure and pH. The same procedure carried out with Hextend reduces survival rate. FCD was the principal microhemodynamic difference, while O2 distribution evidenced anoxia in all groups. Thus, as in previous studies108, outcome appears to be determined by FCD rather than tissue O2109-110, a direct consequence of maintaining normal capillary hydraulic pressure, requiring the transmission of central blood pressure, which is the rationale for restoring central blood pressure in resuscitation.110-111
Hypertension due to using cross linked Hb solutions has been associated with focal necrosis112 as a result of vasoconstriction due to NO-scavenging.113 Notably, there is no evidence in the literature that the use of vasopressors in the treatment of hemorrhagic shock leads to micronecrosis. Studies show that vasoconstriction is effective in transmitting central pressure to the capillary circulation, suggesting that pressure elevation by vasoconstriction “leaks through” to the capillary circulation, providing a beneficial effect.
Microhemodynamic effects of vasoactive hemoglobin solutions
The effect of HBOCs vasoactivity in hemorrhage resuscitation was also studied as a function of Hb concentration administering HBOC-301, at 4 and 13 g/dL concentration by weight, and comparing this with an albumin solution of the same COP. It was found that even though this HBOC is vasoactive, it improved survival when used in small dosage in comparison to a non-O2 carrying colloidal fluid such as albumin used at higher dosages.114-115 The balance between vasoconstriction and increased O2CC was also found to have an optimal value when HBOC-301 was used to remedy extreme hemodilution, providing improved FCD at a specific plasma Hb concentration, FCD being lower at higher and lower dosages.116 The extent of blood exchange was also found to be a factor in determining tissue conditions. During hemodilution, studies where 80% of blood was substituted with vasoactive Hb, tissue oxygenation was significantly impaired due to an apparent increase of vessel wall O2 metabolism.117
These results show that the effects of vasoactivity are strongly related to free Hb concentration in the circulation. The range of free Hb concentration where vasoactive HBOCs are beneficial is low; therefore, the gain in O2CC is probably not physiologically relevant. These findings contribute to explain the lack of side effects noted with the use of PEG-Hb based fluid replacement fluids.118-119 They scavenge NO being potential vasoconstrictors; however, their high COP precludes their achieving high blood concentrations. They cause modest increases of O2 transport while fully exerting their beneficial effects as plasma expanders, a property that they share with PEG-Alb.120
Hemoglobin polymerization
Glutaraldehyde has been widely employed to non-specifically cross-link/polymerize Hb.121-124 Two polymerized Hbs (PolyHb) have undergone phase III clinical trials. Hemopure® (OPK Biotech, Cambridge, MA) consists of polymerized bovine Hb (bHb) with a P50 (i.e. O2 affinity) of 38 mm Hg and average MW of 250 kDa with < 2% α2β2. 14, 125-127 In contrast, PolyHeme®(Northfield Laboratories Inc., Evanston, IL) consists of a pyridoxylated polymerized human Hb with a P50 of 28 - 30 mm Hg, and average MW of 150 kDa with < 1% α2β2. 12, 128-129 These PolyHbs were commercially developed, however hypertension and safety and toxicity issues attributed to vasoactivity and oxidative events130 were critical impediments for their further clinical use in the U.S.131-132
Unmodified Hb interactions with the vascular endothelium or sub-endothelium may include NO scavenging, increased facilitated diffusion of O2 to surrounding tissues, and oxidative reactions.133 The vasoconstriction and hypertension elicited by these small-sized PolyHbs indicates that these materials are capable of extravasating into the interstitial space scavenging NO before it can reach the smooth muscle cells, or facilitating O2 diffusion to the blood vessel wall due to their small size.125, 129 This has led to the development of larger sized PolyHbs that limit these effects. Polymerized bovine Hb (bHb) in the low O2 affinity state (low P50 PolybHb) with the highest cross-link density (50:1, i.e., molar ratio of glutaraldehyde to bHb) yielded no vasoconstriction and was least hypertensive when compared to other PolybHb with lower molar ratios of glutaraldehyde to bHb.101 Vasoconstriction and blood pressure were inversely proportional to the absolute MW of low P50 PolybHb (L-PolybHb) solutions. Additionally, the ability to engineer the O2 affinity of PolybHbs allowed for targeted and regulated O2 delivery to tissues in the microcirculation under extreme hemodilution (i.e., anemic conditions).134 Specifically, high MW L-PolybHb improved tissue oxygenation versus high MW high O2 affinity PolybHb (H-PolybHb).134 In another study, high MW L-PolybHb exhibited longer circulatory half-life and reduced oxidation in vivo versus high MW H-PolybHb.100
In summary, engineering PolyHbs with tunable O2 affinity can regulate the extent of vasoconstriction, hypertension, tissue oxygenation and circulatory half-life. Yu et al. showed that lowering the α2β2 content in PolybHb solutions reduced the hypertensive effect observed in vivo.135 This result is supported by a recent study showing that reducing the α2β2 content of PolybHb solutions from 31% (Oxyglobin®, OPK Biotech) to 2% (Hemopure®, OPK Biotech) significantly reduced the hypertensive effect observed in vivo.136 It is important to note that Oxyglobin® is chemically identical to Hemopure®, except that the latter has undergone extensive purification to remove the majority of α2β2 from solution. Therefore, in light of this wide body of literature, synthesis/formulation of PolybHbs with large MWs and no α2β2 should enhance Hb compartmentalization within the vascular space, extend retention times and limit vasoconstriction/hypertension as well as tissue oxidative injury. This novel but simple design addresses two potential mechanisms for the development of vasoconstriction and hypertension upon administration of PolyHbs and may optimize circulation times for therapeutic applications of extended duration.
Current studies lead to a new strategy for developing RBC substitutes based on molecular engineering the appropriate plasma expander properties into the HBOC.110, 137-141 An optimal O2 carrier plasma expander should possess: high molecular volume (i.e., high MW) compared to α2β2 (i.e., > 64 kDa) and high viscosity compared to plasma (i.e., >1.2 cP, however it should not increase blood viscosity above baseline after infusion).142-143 As previously mentioned, the first property will prevent extravasation of the PolyHb through the blood vessel wall and will therefore limit/prevent vasoconstriction, systemic hypertension and oxidative tissue toxicity. Interestingly, the second property of PolyHb solutions elicits mechanotransduction in the endothelium, generating NO and consequently dilating blood vessels.
ATP releasing HBOCs
Linking ATP with Hb provides a means for simultaneously carrying and delivering O2 while counteracting the vasoconstrictive effects of Hb.70 Exploitation of this regulatory mechanism is the basis the HBOC develop by HemoBioTech, Inc. (Dallas, TX) using the method of “pharmacologic cross-linking” developed by Simoni and collaborators at Texas Tech University.70 This material uses bovine Hb cross-linked intramolecularly with ATP and inter-molecularly with adenosine, and is conjugated with reduced glutathione (GSH). This configuration is proposed to simultaneously regulate blood vessel tone and counteract vasoconstriction and Hb pro-inflammatory effects, while GSH prevents Hb extravasation shielding heme from NO and ROS.70 This material is being commercially developed and has entered the regulatory process in the US. This HBOC presents a combination of biochemical features that address the toxicity issues found in many Hb based materials. It remains to be established what is its maximal and most effective dose and plasma expansion properties.
Large Hb olygomers
Circulatory retention time and the hypertensive effect of acellular Hb appear to be a direct function of the molecular dimension of the modified Hb molecule.48 Very large O2 transporting HBOC molecules are presented in organisms such crustaceans and worms whose Hb is not contained within a cell.144 This configuration can be obtained by oligomerization of Hb, an approach that was used by Bucci and colleagues.86 Their intra-molecularly cross-linked bovine Hb is obtained by means of a chemistry referred to as “zero length cross linking” that yields a low O2 affinity Hb polymer with 36 nm hydrodynamic radius and average molecular weight 17 MDa.86
This large “super-polymeric” Hb called “zero link Hb”, is commercialized by OXYVITA, Inc. (New Windsor, NY), with the name of OxyVita. Its large molecular dimensions are accompanied by increased solution viscosity and the attenuation of Hb’s vasoconstrictive activity and extravasation.86 This formulation has also shown to be highly resistant to dissociation by comparison to natural acellular polymeric Hbs found in the terrestrial and marine organisms.85 Furthermore, OxyVita has a comparatively high heme stability.145 Preclinical studies of the regulation of cerebral blood flow by Koheler et al. show that there are no adverse effects due to the substitution of blood with OxyVita.146
Naturally occurring large Hb polymers
The circulatory system of the marine invertebrate Arenicola marina transports and stores O2 by means of a large Hb polymer consisting of globin and non-globin linker chain complexes with total molecular weight 3.6 MDa and large O2 binding capacity.147 This Hb is harvested and used to produce the O2 carrying therapeutic HEMOXYCarrier® by Hemarina S.A., Morlaix, France.148 Its NO binding rate is somewhat lower than human Hb while CO binding rate is somewhat greater.84 Evaluation of HEMOXYCarrier® in rodent preparations showed that administration of 40 mg/mL Hb solution in saline caused a transient increase of blood pressure not statistically different from that due to administering saline.84 This HBOC is reported to be stable over a wide range conditions (ionic compositions, osmolarities, and oxidative environments) and to have natural antioxidant properties in the presence of natural superoxide dismutase like enzymes.149
Tempol conjugated hemoglobin
Nitroxides limit the formation of toxic hydroxyl radicals and are effective in neutralizing the effects of ROS in mammalian tissues.150 An extensively studied nitroxide is Tempol (4-Hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) which can be administered intravenously reducing blood pressure in hypertensive rodent models and correcting for the effects of endothelial dysfunction.150-151 Tempol is a free radical scavenger that can treat the toxicity due to excessive superoxide production and NO depletion arising from the presence of acellular Hb or the infusion of HBOCs.152 SynZyme Technologies LLC (Irvine, CA) has introduced polynitroxylation of αα-fumaryl Hb as an approach to attenuate the vasoconstrictive activity of acellular Hb, exploiting its antioxidant properties.153 Their multifunctional product polynitroxylated pegylated Hb (PNPH) transports O2, has antioxidant properties and plasma expansion (hyper-colloid) properties due to pegylation.154 Preclinical data shows that PNPH also has neurovascular protective properties.155 PNPH should have a low or non-existent toxicity profile, however it combines three materials that at present have substantial costs, namely PEG, bovine Hb and tempol, therefore its use as blood substitute for transfusion medicine could be limited. Conversely it should be successful as an O2 therapeutic.
Pegylation of hemoglobin
Conjugation of Hb with PEG (PEGylation) pioneered by Enzon Inc. (Piscataway Township, NJ) introduced a fundamentally new approach for dealing with some of the shortcomings of free Hb in plasma.156 In the original formulation, bovine Hb was conjugated to 10 copies of 5 kDa PEG by means of a urethane linkage (PEG-isocyanate), yielding a molecule with a very large hydrodynamic volume. As a consequence, COP and viscosity were significantly increased relative to the unPEGylated molecule, with a significantly reduced pressor effect. Ajinomoto Inc. (Tokyo, Japan) developed a human PEG Hb which is pyridoxylated, oligomerized and surface decorated with 3 kDa PEG using an isopeptide linkage. Its P50 was 20 mmHg and it showed a transient hypertension upon transfusion.157
Sangart Inc. (San Diego, Ca) developed the product Hemospan™ using 2-iminothiolation based extension arm facilitated (EAF) PEGylation formulated as a 4 gm% solution using a propyl maleimide linkage.158 This PEGylated Hb, currently called MP4OX, was extensively studied in rodents118 and large animals159, and is now in clinical trials in Europe.160 Its plasma expansion characteristics are similar to those obtained with high viscosity plasma expanders, even though its viscosity, similar to that of PEG-Alb, is considerably lower.161 Extensive microcirculatory studies have shown that PEG-Hb maintains and at times improves microvascular function when used to restore blood volume after blood losses and to preserve volume during hemodilution, a result attributed to PEG-Hb optimal plasma expansion properties and high O2 affinity.
The viscosity, molecular size and COP of PEG-Hb are similar to those of PEG-Alb, since Hb and albumin have virtually the same molecular weight and are both globular proteins. When PEG-Alb or PEG-Hb are mixed with blood they yield similar rheological properties. However, PEG-Hb lowers perivascular NO due to NO scavenging.40 The high COP of PEG-Hb prevents achieving blood concentrations that are sufficiently high to sustain the O2 metabolic demand of mammalian organisms. However, its high O2 affinity, low viscogenicity, and supra-plasma expansion due to the low plasma concentration results in targeting O2 delivery to ischemic tissues.162 PEG-Hb scavenges NO, but does not cause vasoconstriction or hypertension.40, 163 This result is probably due the low concentration that PEG-Hb achieves in the circulation, since its high COP pulls fluid from the interstitial space decreasing the concentration in plasma. In addition, PEG-Hb compensates for the NO scavenged by increasing the generation of NO due to increased WSS and its enhanced nitrite to NO reductase.164
NO generation from nitrite by HBOCs
Nitrite may be an important reagent in the production of bioactive NO via Hb in RBCs or from acellular Hb in the circulation. A central role for nitrite in NO production from Hb emerged when it was shown that deoxy Hb can catalyze the conversion of nitrite to NO though a nitrite reductase reaction.47 In this reaction, penta-coordinate ferrous heme reacts with nitrite to produce NO and ferric heme.165 Furthermore, the rate of the reaction is allosterically controlled.166 Studies on chemically modified Hbs167-168, sol-gel encapsulated Hbs169, and Hb dimers bound to haptoglobin170 all indicate that R state deoxy hemes manifest at a faster initial rate by a factor of approximately 10 over the corresponding T state deoxy hemes.170-171 Nitrite reduction by Hb reaction is determined by a balance between the two quaternary structures of the Hb tetramer, Hb in the oxygenated and deoxygenated conformation. The redox potential of oxygenated Hb favors nitrite reductase reaction, while deoxygenated Hb provided the unligated heme sites necessary for nitrite binding, supporting the role for nitrite reductase by Hb as a sensor and effector of hypoxic vasodilation.164 This allosterically responsive behavior links this reaction with hypoxic vasodilation. As part of the mechanism, a half-oxygenated R state Hb would have maximum NO generating efficacy since it represents a compromise between accessible reactive deoxy heme sites and the higher reactivity associated with the R state: Too much deoxygenation results in T state formation and too much oxygenation results in too few sites available for the reaction with nitrite.166 The importance of this reaction is also apparent from HBOC studies. Hbs with the highest rates of nitrite reductase typically show the lowest levels of induced vasoconstriction due to significantly reversed NO inhibition.166 Although NO is a potent vasodilator that produces significant effects at low concentrations, acellular Hb with high nitrite reductase may still causes vasoconstriction since it is difficult for NO to escape from Hb.
NO and NO precursors have been used to compensate for the NO consumed by HBOCs and to reduce vasoconstriction, including nitroglycerin (NTG). NTG was administered during fluid resuscitation from hemorrhagic shock using polymerized Hb, to attenuate the hypertensive, vasoconstrictive and toxic effects of acellular Hb.172 NTG produces vasodilatation being a weak NO donor (an organic nitrates that require a three-electron reduction to be converted into NO).173 NTG did not eliminate vasoconstriction; however, it elevated acellular MetHb levels, reducing O2 delivery to tissues. Similarly, when sodium nitrite was used in fluid resuscitation from hemorrhagic shock with polymerized Hb vasoconstriction was attenuated only with high doses of nitrite.174 The nitrite dose effective in preventing hypertension, increased MetHb levels, reduced platelet function and increased pulmonary complications (edema and congestion).174-175
Pure NO inhalation was used to treat NO depletion.176-177 Inhaled NO prevented pulmonary and systemic hypertension induced by polymerized Hb.176 However, inhaled NO reacts quickly with Hb (NO dioxygenation reaction) in the lung and increases MetHb levels without restoring the NO bioavailability to peripheral tissues. The largest family of NO donors currently in use is NONOates, also known as diazeniumdiolates. NONOates decomposition rate depends on pH, and temperature.178-179 NONOates have been used to mitigate the vascular inflammation induced by plasma Hb during malaria infection.180 NONOates have limited application to control HBOCs induced NO depletion, due to short half-life and increased formation of MetHb.
Alternatives to prevent vasoconstriction induced by HBOCs include adjunct therapy with Sildenafil, a phosphodiesterase type 5 inhibitor; however, it partially prevented some of the hemodynamic changes induced by acellular Hb.181 Lastly, NO releasing nanoparticles (NOnps) have been used to restore NO bioavailability after infusion of polymerized Hb.182 The slow release of NO from circulating NOnps, allows small amounts of NO to diffuse abluminally, thus reducing the effects of NO scavenging and limiting MetHb formation.33, 182-183
Conclusions from the use of these various approaches to supplement NO in the presence of acellular Hb in the circulation are: 1) NO and NO precursors react with Hb reducing O2 capacity and producing MetHb; 2) NO supplementation has to be systemic; 3) A combination of NO supplementation and enhanced sensitivity to NO with phosphodiesterase inhibitors may reduce the amount of NO and MetHb formation while counteracting acellular Hb vasoconstriction.
Transport of NO in the form of S-nitrosoHb of by HBOCs
The potential role of Hb as a source of bioactive NO started with the proposal that Hb could transport and deliver NO in an allosterically controlled manner through the formation of S-nitrosoHb (SNO-Hb).184 SNO-Hb is a derivative of Hb in which the reactive thiols associated with the two β93 Cys undergo S-nitrosation.185-187 Both the formation of the β93 Cys SNOs and the efficacy for the transfer or release of these SNO associated NOs were proposed to be sensitive to the quaternary structure of the Hb tetramer.188 Although SNO-Hb has been observed in vivo, its role remains controversial. The ability of PEGylated Hbs without the reactive thiols to function as vasodilators indicates that SNO-Hb formation is not a requirement for this activity.164, 189 Similarly, there are studies showing that transport of NO bioactivity by RBCs, including hypoxic vasodilation, does not require SNO-Hb participation.190-192 Thus SNO-Hb may function to facilitate RBC-generated NO bioactivity, but it is not essential.96, 193 Since SNO-containing molecules are likely to be the basis for the long lived transportable NO bioactivity, the more intriguing and as yet unanswered question is through what reactions can Hb create reactive species capable of S-nitrosation of reactive thiols on either Hb or other thiol-containing peptides. HBOCs can stabilize the effects of NO. The release of NO, or its metabolites, from the SNO-Hb was facilitated by addition of GSH, which aids in the decomposition of S-nitrosothiols.194 SNO-Hb significantly reduced, but did not eliminate microvascular damage. Acellular Hb derivates based on S-nitrosylated PEG-modified Hb (SNO-PEG-Hb), deliver O2 and NO. SNO-PEG-Hb modification increase Hb molecular mass, and when administrated to anesthetized rats caused a hypertensive reaction, while SNO-Hb and SNO-PEG-Hb did not raise blood pressure.195 The mayor limitation of SNO-Hb formulation is the limited stability of the compounds. The SNOHb mechanism is intriguing and thought provoking. Several reports have already generated useful discussions and will stimulate further experimentation and possibly clinical trials, benefiting transfusion medicine and leading to better care for patients.196
Pefluorocarbon based oxygen carriers (PFCOCs)
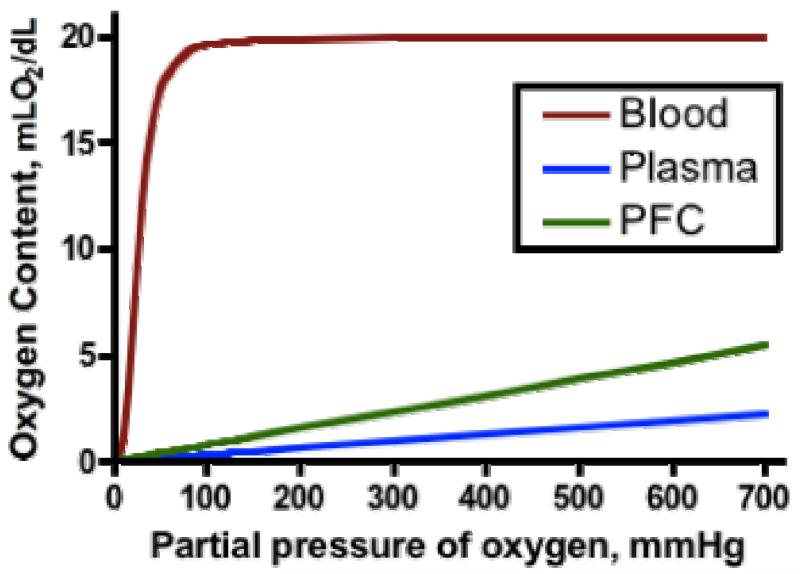
Perfluorocarbons (PFCs) are chemically and biologically inert, and can dissolve large amounts of O2 and other gases. However, PFCs have low water and lipid solubility. Their gas solubility reflects the very low intermolecular interactions (fluorine’s low polarizability translates into low van der Waals forces) within the PFC. The basic difference between O2 transport by PFCs and Hb is that PFCs dissolve, whereas the Hb bind O2. In the case of Hb, a strong bond is established between O2 and the heme. In the case of PFCs, there is only a physical weak equilibrium between concentration and solubility. The difference between Hb and PFC O2 transport is clearly shown by the differences in profiles of the O2 content curves as a function of pO2, i.e., sigmoidal for Hb vs. linear for PFCOC (Figure 2). With PFCOCs, there is no saturation and no possibility for chemical binding, and as O2 is released, carbon dioxide (CO2) is absorbed. Oxygen dissolved in PFCOC is immediately available to tissues; furthermore, dissolution and release to tissues can increase when temperature decreases.
Figure 2.
Oxygen content for whole blood, Perfluorocarbon emulsion (PFCOC, Oxycyte™, Synthetic Blood International, Inc., Costa Mesa, CA) and plasma as a function of O2 partial pressure.
PFCs are hydrophobic and must be emulsified for intravenous use. It is currently feasible to produce stable PFCs emulsions with particles of median diameter < 0.2 μm.197 PFCs, as opposed to Hb, are among the most inert organic materials. PFCs were initially developed for handling extremely corrosive uranium fluorides. PFCOC are not subject to oxidation, and there is no indication that any sort of chemical modification occurs under conditions of processing, storage, and use. PFCOC can typically be heated to 300 °C and higher for several days without detectable changes198. Therefore, appropriately formulated PFCOCs can be terminally heat-sterilized.197, 199
PFCs are a synthetic material available in bulk at modest costs. Therefore, PFCOC could in principle lead to a convenient, largely available, economic, pathogen-free and storable O2 carrier. However, emulsification of PFCs for parenteral administration requires phospholipids. PFCs droplets size is less than 0.5 micrometers and is coated with a surfactant that serves as emulsifier and stabilizes the suspension. PFCs emulsions are produced at concentrations ranging from 20% to 120% weight/volume (PFCs specific gravity is ~2). Osmolarity of the suspending media is independent of PFCOC concentration and is adjusted by the addition of tonicity agents. Principal challenges in the development of infusible PFCOC include: i) selecting the appropriate PFC (easily eliminable, highly pure and easy to emulsify); ii) preparing stable emulsions (small-sized, heat-sterilizable and well tolerated surfactant); and iii) counteracting molecular diffusion, responsible for particle size growth over time.200-201
HBOCs carry O2 via a reversible chemical reaction between Hb and O2, while PFCOs carry O2 by dissolving the O2, thus PFOCs O2CC is limited by the O2 concentration.202 PFCOC emulsion’s O2CC is about twice that of plasma at room air, and about 10 times less than blood Hb.203 The limited O2CC of PFCOCs emulsion can be overcome by increasing the fraction of inspired O2 (FiO2).202 Effects due to superposition of hyperoxia on the changes of circulating fluid composition are additional variables in the analysis of the effectiveness of PFCOCs.202-203
Few PFCs are acceptable for parenteral use because of their slow excretion and emulsion stability. It has been determined that in vivo PFCs excretion is mostly determined by their MW, rapid excretion corresponding to the lower MWs, while emulsion stability requires PFCs with higher MWs, conditions that cannot be satisfied simultaneously. Additionally, vapor pressure, which also depends on MW, is an important parameter, which can favor retention of air in the alveoli, resulting in increased pulmonary residual volume (also known as pulmonary gas trapping). To avoid this phenomenon, the vapor pressure of the final PFCOC phase at body temperature should not exceed about 10 mmHg.204-206 The interaction of PFCOC with some colloids (dextrans and gelatins) clinically used as plasma expanders triggers the activation of leukocytes during hemodilution.207 This study also reported that PFCOC has a minor interaction with hydroxyl ethyl starch (HES), but it did not impair microvascular perfusion.207
Oxygen transport with PFCOCs
Loading and releasing O2 and others gases from PFCOCs to tissues is not subordinated to any change in conformation and does not require the assistance of an allosteric effector. In the case of O2, the van der Waals interactions between O2 and PFCOC molecules are an order of magnitude lower than Hb and O2 reactions, resulting in higher extraction rates and ratios.208 In normal conditions with central arterial pO2 of 100 mmHg and venous pO2 of 35 mmHg, PFCOC emulsions can release 65% of O2, compared to about 30% for Hb in the RBCs. Oxygen release from PFCOCs is effective at any physiologically relevant partial pressure, rendering a cooperativity-like effect unnecessary. Likewise, O2 release by PFCOCs is not dependent on pH and is not adversely affected by temperature.209 Since PFCOCs undergo no oxidation or other modification over time, their O2 uptake and release characteristics are not affected by storage or during circulation. Introducing a PFCOC emulsion into the circulation is akin to increasing the O2 solubility of blood’s plasma compartment. When Hb and a PFCOC are present in the circulation simultaneously, the PFC will always release its O2 first, thus conserving the Hb bound O2 until it is released to the hypoxic tissues.210 A valuable consequence of PFCOCs, following Henry’s law, is that the O2 content of a PFCOC emulsion can be increased several fold by increasing FiO2, which is simple to do in a rescue vehicle and critical care or surgical settings.
Oxygen diffusion from the RBCs into tissues is driven by the blood/tissue pO2 gradient.211 Thus, the rates of both oxygenation and deoxygenation of Hb solutions are limited by diffusion and governed by the O2 gradient between internal and external spaces.212 Diffusion should be facilitated when the RBC membrane is absent and in the presence of numerous small size (as compared to RBCs) highly mobile O2 reservoirs. The CFL between the RBC column and the endothelium is particularly large during anemic or in hemodiluted states. Cell free Hb molecules, Hb-loaded liposomes and PFCOC droplet fill the CFL in large numbers, increase O2 content and potentially facilitate O2 diffusion by providing numerous “stepping stones” or dynamic chains of particles over which O2 can travel. Much more numerous than RBCs, such particles also offer an area several orders of magnitude larger for gas exchange. A near-wall excess of the smaller particles is likely to develop in smaller vessels, as the shear rate gradient creates a force which counteracts dispersion forces and tends to move elastic RBCs away from the vessel wall.
The large O2 gradients caused at high FiO2 associated with the use of PFCOCs provide a strong driving force for O2 diffusion from the PFCOC droplets to the tissues. The movement of emulsion microdroplets in the blood stream was proposed to create dynamic chains of particles; hence, channels which would help transfer O2 from the RBCs to tissues.206 PFCs deliver O2 due to their physical characteristics and the convective delivery to tissues. By reason of their small particle size, PFC emulsions penetrate collateral capillaries of an ischemic microcirculation, both supplying oxygenation and possibly restoring flexibility of acidotically stiffened erythrocytes by reinstituting aerobic metabolism. This theory represents a new concept in O2 delivery and may define a new class of therapeutic agents that can improve tissue O2 supply in pathological states involving low O2 delivery and ischemia.213 The rationalization that small PFCOC droplets (< 0.2 μm diameter) augmented O2 delivery because they are able to pass through narrow microvascular channels by comparison with large RBCs (7 - 8 μm diameter) has been discarded.214 Infusion of PFCOC emulsion during partial ischemia did not have an effect on oxygenation, and resulted in trapping of the droplets in ischemic tissue, which increased tissue damage.214-215 The study of systemic and microvascular O2 exchange proposed that PFCOCs and increased FiO2 increases O2 delivery to the tissue; however, the remnant Hb delivers most of the O2 since PFCOCs allow RBCs to remain partially oxygenated when they arrive to the tissue.210
PFCOC O2 transport capacity was investigated in the microcirculation hamster window chamber model during extreme hemodilution.210 Pentaspan (10%, B. Brown, Medical, Irvine, CA, HES, 200 kDa MW) was used as a plasma expander to reduce Hct to 18% by two isovolemic hemodilution steps. A third step reduced the Hct to 11% and was completed with either HES or Oxycyte™ (Synthetic Blood International, Inc., Costa Mesa, CA). Comparisons of HES only hemodiluted animals vs. animals that received 4.2 g/kg of emulsion were made at normoxia (FiO2 = 0.21) and hyperoxia (FiO2 = 1.0). Systemic and microvascular O2 delivery for PFCOC was 25% (normoxia) and 400% (hyperoxia) higher than for HES. The combination of PFCOCs and hyperoxic ventilation delivered O2 to the tissue without causing vasoconstriction or impairing microvascular perfusion. Positive acid-base balance, restoration of mean arterial pressure and cardiac output suggested correspondence between microvascular and systemic events. PFCOC and increased FiO2 increased systemic O2 delivery and extraction when compared to a plasma expander due to the higher plasma O2 content when PFCOC is present. In the microcirculation, O2 delivery was also increased by PFCOC, although O2 was mostly released from RBC Hb.210
Transport of other gases with PFCOC
The capacity for PFCOCs to dissolve nitrogen (and air) may find applications in the treatment of decompression sickness and for protection from neurologic damage caused by air microemboli during cardiopulmonary bypass surgery.216-217 The solubility of xenon in PFCOCs can be exploited for magnetic resonance imaging (MRI). Both Hb and PFCOCs transport NO but by different mechanisms, which change the availability of the gas.218-219 During cardiopulmonary bypass (CPB) volatile anesthetics can be added to the oxygenator to provide anesthesia regulated systemic vascular resistance and reduce hormonal responses to CPB. The rate of wash-in and wash-out of volatile anesthetics via oxygenators depends on their solubility in blood. Two important factors affect the solubility of volatile anesthetics: hypothermia increases solubility and crystalloid hemodilution decreases it. PFCOC unique physicochemical characteristics, chemically inert with high solubility, provide the ideal media to dissolve larger quantities of gases. Volatile anesthetics also have a higher solubility in perfluorodecalin, the main component of Fluosol™ (Alpha Therapeutic Corp., Los Angeles, CA), a first-generation emulsion.220 Fluosol is the only PFCOC approved to date by the U.S. Food and Drug Administration (FDA) and regulatory agencies in 8 other countries.197 Fluosol was not intended to reduce or replace allogenic blood transfusions. Fluosol which was approved for use during cardiac angioplasty increased mycoardial oxygenation, prevented ST segment elevation, and preserved the ejection fraction.221 Fluosol was used for 3 years (1989 to 1992) in more than 40,000 patients and was discontinued due to problems with emulsion stability arising from rewarming the frozen suspension prior to infusion.
Transport of carbon dioxide
Intravascular CO2 transport relies on several mechanisms, including physical dissolution in plasma, carbonic anhydrase induced transformation into bicarbonate and chemical binding consequent to reaction with the Hb. About 25% of total CO2 is transported by RBCs, which affects the Bohr Effect. As CO2 affects pH and respiratory acid-base regulation the high solubility of CO2 PFCOCs (typically 3 to 5 times higher than for O2), infusion of PFCOCs may affect the amounts of CO2 transported by red cells and plasma, reduce carbonic acid levels and affect blood O2 transport. Clinical and experimental result have shown a tendency to faster recovery of acid balance when PFCOCs are in circulation.210, 222-224
Volatile anesthetics transport by PFCOCs
The question therefore arises whether clinically relevant volumes of PFCOC might significantly increase the blood solubility of volatile anesthetics. Studies on the solubility of desflurane, sevoflurane, and isoflurane were performed using human blood in vitro mixed with clinical concentrations of perflubron based PFCOC emulsion, Oxygent™ (Alliance Pharmaceutical Corp., San Diego, CA) which has 3 times greater concentration than Fluosol (30% vs. 10% by volume; 60% vs. 20% by weight). At PFCOC concentrations equivalent to in vivo doses of 1.8 to 5.4 g/kg, the amount of sevoflurane carried by blood increased by a factor of 2.6, whereas desflurane did not increase (0.9 times).225 However, their study concluded that this finding lacked apparent clinical implications.225
Nitric oxide transport by PFCOCs
Nitric oxide (NO) is enzymatically produced by various types of cells and is a central mediator in the cardiovascular system. As a free radical, NO is highly unstable in vivo. Its primary targets include heme proteins such as guanylyl cyclase and free radical species. NO is readily oxidized by O2 in an aqueous media and mainly converted into nitrite anion. The partition coefficient of NO for PFCOC emulsion is defined as the ratio of the equilibrium concentrations between the aqueous and PFCOC phases. Partition coefficient of NO for Perftoran was found to be ~200.226 The sequestration of NO by PFCOC emulsion, without the presence of O2 to account for oxidation, was significant. Thus, the partition coefficient indicates that PFCOC micelle can increase micellar concentrations of NO to a higher level than an aqueous solution, and readily collect NO from regions with overproduction.
The reaction of NO and O2 with thiols results in thiol nitrosalation through the intermediary dinitrogen trioxide (N2O3) formed from the combination of NO with NO2.226 In the presence of PFCOC (hydrophobic compartments) NO should be quickly sequestrated by PFCOC micelles. The high local concentration of NO within the hydrophobic compartment leads to the acceleration of NO oxidation and the formation of nitrosative N2O3 from the combination of NO with NO2.226 According to the theory of micellar catalysis227, the rate of NO oxidation and S-nitrosothiols formation depends on the volume of the hydrophobic phase. Larger or smaller amounts of PFCOC could be progressively less effective as a tool for modulating plasma NO bioavailability.226 Consistently, thiols are in excess relative to NO, and a large fraction of NO2 should react with thiols rather than NO itself. However, it remains unclear whether intrinsic plasma NO or S-nitrosothiols play a significant role in cardiovascular processes. Notably, the administration of a large dose of PFCOC caused vasoconstriction, reduction of erythrocyte velocity in postcapillary venules and increased venular leukocyte sticking, which is probably due to rapid sequestration of NO.226-227
Oxygen transport with perfluorocarbon microbubbles
PFCOC microbubbles have been developed and reported to dissolve clinically relevant amounts of O2 when administered in dosages that are about 1/500 of usual quantities in which PFCOCs are administered to provide O2CC.228-229 The presence of gas bubbles in the circulation is generally considered medical anathema. However, it has been proposed that the diameter of these intravascular bubbles will remain subcapillary in size and they will transport significant amounts of O2. Intravascular microbubbles volume-stabilized by dodecafluoropentane gas are formed when a dodecafluoropentane-emulsion is injected into the circulation at normothermic conditions. The dodecafluoropentane has a boiling point of 29 °C at atmospheric pressure and the particles in this emulsion undergo a phase shift and form bubbles when warmed in the bloodstream. Initial bubble size depends on the size of the emulsion particles but, theoretically, the bubbles undergo size variations as they exchange O2, nitrogen and CO2 by diffusion in the lungs and the tissues.228-231
Future research objectives of PFCOC emulsions aim to prolong the intravascular persistence, reduce phagocytosis by macrophages, and increase stability and surface-modified PFCOCs. Substantial efforts are currently being devoted to investigating targeted PFCOCs for the purpose of molecular imaging - i.e., detection of molecular markers, such as proteins and other cell-surface receptors, characteristic of given pathologies.232 Such emulsions also have potential for site-directed drug delivery and monitoring of therapy. Important potential target pathologies include inflammation, atherosclerosis, tumor-related angiogenesis, and thrombi.
Engineering an oxygen carrying plasma expander
The products that went through clinical trials reflect the physiological know-how of the 1970s, when their effects could not be analyzed at the level of microscopic blood vessels, and the blood flow regulation by NO was unknown.233 Commercial developments disregarded the microvascular autoregulation theory and assumed that the endothelium is a passive cellular lining responsible of preventing extravasation of plasma proteins.234 High resolution methods for measuring microvascular perfusion and tissue pO2 became available at the beginning of the 1990s,235-236 and when these were applied to the analysis of the effects of HBOCs and PFCOCs, they elucidated several misperceptions and principles used to design the first generation of artificial O2 carriers.237-238 Studies showed that O2 is delivered by the arterioles and the capillaries, and that this rate of delivery was modulated by the consumption of O2 in the arteriolar wall. Vasoconstrictor effects were found to increase O2 consumption of the arterioles limiting tissue oxygenation.239 This was particularly true for Hb solutions formulated with small molecules, such as alpha-alpha-crosslinked Hb, which also presented another of the presumed required properties, namely low viscosity.240
The vasoconstrictor effect of HBOCs has been attributed to NO scavenging by acellular Hb, since NO is quenched through an NO dioxygenation reaction as described before in this review.48-50 However, other mechanisms may be involved or superimposed to the NO scavenging by Hb.27, 113
The distribution of oxygen near the endothelium is in part determined by the size/molecular mass of the HBOC molecule and the presence of the endothelial glycocalyx which creates an unstirred layer next to the vascular wall. Acellular HBOCs increase the concentration of O2 near the endothelium since they bring O2 bound to Hb into the CFL101 an effect that may trigger autoregulatory vasoconstriction aimed at preventing the oversupply of O2 to the tissue.241
The endothelial glycocalyx permeability to macromolecules decreases as a function of the distance from the endothelial surface due to the decreasing density and motion of its molecular branches, being impermeable to molecules > 70 kDa 242. This decreases scavenging of NO and O2 delivery by large molecular Hb configurations, relative to the smaller Hb based HBOCs. Large HBOCs also have a higher solution viscosity, increasing WSS dependent NO production and ATP release from the remaining erythrocytes, which can counteract NO consumption by the HBOC.243
Oxygenation of hypoxic tissues is increased by the use of high O2 affinity HBOCs which prevent O2 release in arterioles and maintain O2 bound to Hb until the HBOC arrives to regions of the microcirculation with low pO2.162, 244 Lastly, it is critical how much HBOC or PFCOC is necessary to obtain the desired effects. According to present experimental evidence none of the current formulation of HBOC or PFCOC can be administered in sufficient amounts to preserve aerobic metabolism of all tissues without toxic effects.245 On the other hand, HBOCs appear to be able to restore oxygenation to O2-deficient tissues, which otherwise may be damaged by ischemia-reperfusion-reoxygenation.
Controlling the problems associated with ischemia-reperfusion injury has proven difficult with HBOCs246-248 since the associated pathogenesis is strongly linked to oxidative stress and the formation of ROS, contributing to vascular inflammation and endothelial dysfunction.249 Restoration of vascular levels of NO has been shown to sustain mitochondrial and cellular function, thus reducing oxidative damage.247, 250 In the case of HBOCs, the maintenance of the ferrous form of Hb is necessary for O2 transport and preventing oxidation to metHb (non-O2 carrying), which leaves Hb void of efficacy and prone to degradation.251
Conclusions
Our knowledge of the fluids necessary for the treatment of blood loss is still under development. This is in part due to the quest for a blood substitute having begun much before the discovery of the role of NO, our understanding of how the microcirculation manages O2 distribution, and the role of shear stress in cardiovascular hemostasis. The substitution of blood with an equivalent fluid could be defined as a process of reducing the transfusion trigger, with the goal of postponing the introduction of blood to treat blood loss. This criterion suggests exploiting additional mechanism for achieving this goal, such as reducing O2 demand and improving the efficiency of O2 delivery. These effects can be obtained in the pre-transfusion setting, when the volume component of blood loss is treated with plasma expanders. Discarding this possibility places the entire burden of restoring O2CC on the O2 carrier, increasing its concentration and the potential for the development of significant negative side effects.
An optimal O2 carrier has not yet been devised, and banked blood is probably not adequate in this context.252 The properties of native blood may be unattainable, particularly because of the difficulty of reproducing the physical and chemical interplay between native blood and the vascular endothelium. Nevertheless, attaining this goal with blood substitutes may be aided by recognizing the importance of maintaining the functionality of the microcirculation, which is significantly dependent on adequate levels of O2, NO and hemodynamic interactions with the vascular endothelium.
The Center for Biologics Evaluation and Research (CBER) is the review body for the FDA in the arena of biology has implemented a comprehensive safety evaluation process for O2 carrying plasma expanders. These points encompass characterization of the product, animal safety testing, and human studies and address the theoretic concerns of Hb solutions raised previously (including pulmonary and systemic hypertension, organ dysfunction, oxidative tissue injury, synergy with bacterial pathogens, and immunomodulation). Documenting a direct clinical endpoint for O2 carrying plasma expanders is challenging because these endpoint were never established for RBCs.
In conclusion it is proposed that the initial step in the design of blood substitutes is the optimization of the plasma expander properties of proposed O2 carrying fluids in terms of their fluid mechanical, solution and biochemical properties, for the eventual integration of plasma expansion and gas transport properties. Fulfillment of these conditions allows for subsequently tailoring the properties of the O2 carrier and select between Hb molecular solutions, encapsulated Hb and fluorocarbons, including their potential as generalized gas carriers.
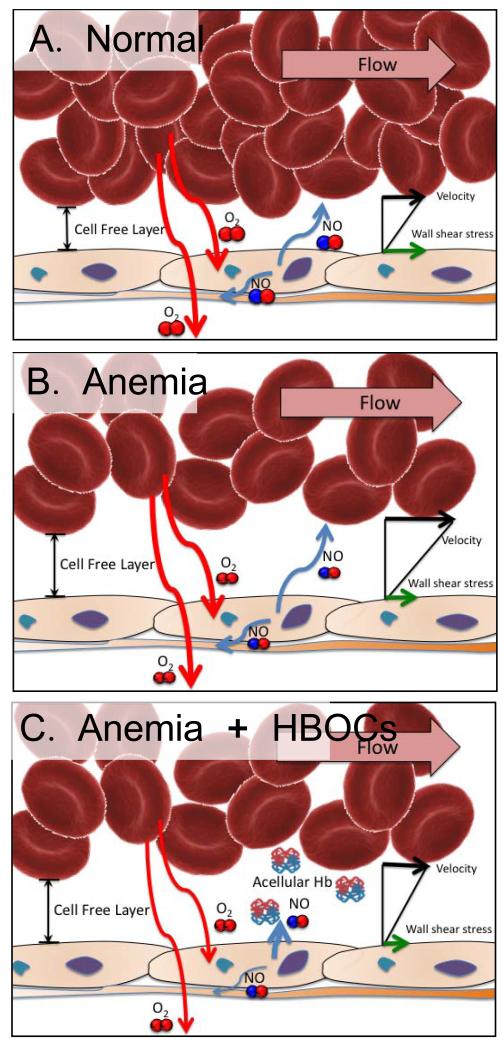
Figure 1.
In physiological conditions (A. normal), blood flow provides O2 and mechanical signals to the vasculature, which serve to self-regulate local blood flow. After severe blood losses (B. anemic), anemia or any condition that originates a need for blood transfusion, oxygenation and vascular shear stress are compromised, thus the local vascular regulatory process becomes impaired. Consequently, when HBOCs are transfused (C. Acellular Hb in circulation), they do not achieve their intended goal since they only increase O2CC without reversing the hypoperfusion generated pre-transfusion. Even once perfusion is recovered, vascular regulatory processes are not fully functional, such as eNOS, which becomes uncoupled and instead of generating NO produces superoxide, contributing to oxidative stress and triggering an inflammatory cascade. The additive role of oxidative and nitrosative stress results from increased NO release from inducible NOS (iNOS) sources, including tissues and macrophages/monocytes. The NO generated by iNOS is especially metabolic to peroxynitrite, which is cytotoxic to tissues.
Figure 3.
Universal O2 carrying solutions may replace the O2 carrying capacity and O2 transport functions of allogeneic blood. These O2 carriers will also prevent the many complications associated with blood transfusions. The two platforms to develop O2 carrying solutions include perfluorocarbon (PFC) based O2 carriers (PFCOCs) and hemoglobin based O2 carriers (HBOCs). PFCOCs are emulsions of PFC with high O2 solubility. HBOCs are chemically modified natural or recombinant Hb or encapsulated Hb, they include large HBOC like PEG decorated Hb vesicles (PEG-HbVs), and small HBOC such as, polymerized Hb (PolyHbs) and PEG surface conjugated Hb (PEG-Hb). Various design strategies for PFCOCs and HBOCs have resulted in vasoconstriction and the development of systemic hypertension. The root cause of this effect stems from the ability of acellular Hb to extravasate, scavenge NO and hyper-oxygenation of the vessel wall. Increasing the molecular size of HBOCs prevents extravasation, increases plasma viscosity and O2 carrying capacity without increasing colloidal oncotic pressure.
ACKNOWLEDGMENTS
This work was partially supported by Bioengineering Research Partnership grant R24-HL64395, Program Project P01-HL071064 and grants R01-HL62354 and R01-HL62318; and ARMY: W81XWH-11-2-0012.
List of abbreviations
- CFL
Cell free layer
- COP
Colloidal osmotic pressure
- CPB
Cardiopulmonary bypass
- FCD
Functional capillary density
- FiO2
Fraction of inspired oxygen
- HSA
Human serum albumin
- HBOC:
Hemoglobin based oxygen carrier
- HES
Hydroxyl ethyl starch
- Hb
Hemoglobin
- HbV
Hemoglobin vesicle
- MetHb
Methemoglobin
- MW
Molecular weight
- NO
Nitric oxide
- O2CC
Oxygen carrying capacity
- PEG
Polyethylene glycol
- PEG-HbV
Polyethylene glycol covered hemoglobin vesicle
- PFC
Perfluorocarbon
- PFCOC
Perfluorocarbon based oxygen carrier
- pO2
Partial pressure of oxygen
- RBC
Red blood cell
- rHSA
Recombinant human serum albumin
- ROS
Reactive oxygen species
- WSS
Wall shear stress
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenburg AG. Benefits and risks of blood transfusion in surgical patients. World J Surg. 1996;20:1189–93. doi: 10.1007/s002689900181. [DOI] [PubMed] [Google Scholar]
- 2.Hourfar MK, Themann A, Eickmann M, Puthavathana P, Laue T, Seifried E, et al. Blood screening for influenza. Emerg Infect Dis. 2007;13:1081–3. doi: 10.3201/eid1307.060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–62. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 4.Finucane ML, Slovic P, Mertz CK. Public perception of the risk of blood transfusion. Transfusion. 2000;40:1017–22. doi: 10.1046/j.1537-2995.2000.40081017.x. [DOI] [PubMed] [Google Scholar]
- 5.Stramer SL. Current risks of transfusion-transmitted agents - A review. Arch Pathol Lab Med. 2007;131:702–7. doi: 10.5858/2007-131-702-CROTAA. [DOI] [PubMed] [Google Scholar]
- 6.Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49:1S–235S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol. 1997;34:34–40. [PubMed] [Google Scholar]
- 8.Koch C, Li L, Figueroa P, Mihaljevic T, Svensson L, Blackstone EH. Transfusion and pulmonary morbidity after cardiac surgery. Ann Thorac Surg. 2009;88:1410–8. doi: 10.1016/j.athoracsur.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 10.Stowell CP, Levin J, Spiess BD, Winslow RM. Progress in the development of RBC substitutes. Transfusion. 2001;41:287–99. doi: 10.1046/j.1537-2995.2001.41020287.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodwell V,W, Kennelly P. Proteins: Myoglobin & Hemoglobin. In: Murray RK, Granner DK, Mayes PA, Rodwell V,W, editors. Harper’s Illustrated Biochemistry. 26th Edition ed McGraw-Hill; New York: 2003. pp. 40–8. [Google Scholar]
- 12.Gould SA, Moore EE, Hoyt DB, Burch JM, Haenel JB, Garcia J, et al. The first randomized trial of human polymerized hemoglobin as a blood substitute in acute trauma and emergent surgery. J Am Coll Surg. 1998;187:113–20. doi: 10.1016/s1072-7515(98)00095-7. discussion 20-2. [DOI] [PubMed] [Google Scholar]
- 13.Greenburg AG, Kim HW. Use of an oxygen therapeutic as an adjunct to intraoperative autologous donation to reduce transfusion requirements in patients undergoing coronary artery bypass graft surgery. J Am Coll Surg. 2004;198:373–83. doi: 10.1016/j.jamcollsurg.2003.11.020. discussion 84-5. [DOI] [PubMed] [Google Scholar]
- 14.Jahr JS, Moallempour M, Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (R) (Biopure Corporation) Expert Opin Biol Ther. 2008;8:1425–33. doi: 10.1517/14712598.8.9.1425. [DOI] [PubMed] [Google Scholar]
- 15.Amberson WR, Jennings JJ, Rhode CM. Clinical experience with hemoglobin-saline solutions. J Appl Physiol. 1949;1:469–89. doi: 10.1152/jappl.1949.1.7.469. [DOI] [PubMed] [Google Scholar]
- 16.Gilligan DR, Altschule MD, Katersky EM. Studies of Hemoglobinemia and Hemoglobinuria Produced in Man by Intravenous Injection of Hemoglobin Solutions. J Clin Invest. 1941;20:177–87. doi: 10.1172/JCI101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: Biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–27. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 18.Bunn HF, Esham WT, Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129:909–23. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan WL, Tang NLS, Yim CCW, Lai FM, Tam MSC. New features of renal lesion induced by stroma free hemoglobin. Toxicol Pathol. 2000;28:635–42. doi: 10.1177/019262330002800501. [DOI] [PubMed] [Google Scholar]
- 20.Dunne J, Caron A, Menu P, Alayash AI, Buehler PW, Wilson MT, et al. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J. 2006;399:513–24. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Levy A, Rifkind JM. Autoxidation of Hemoglobin Enhanced by Dissociation into Dimers. J Biol Chem. 1991;266:24698–701. [PubMed] [Google Scholar]
- 22.Bunn HF, Jandl JH. The renal handling of hemoglobin. Trans Assoc Am Physicians. 1968;81:147–52. [PubMed] [Google Scholar]
- 23.Bunn HF, Jandl JH. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969;129:925–34. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–53. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 25.Gibson QH, Roughton FJW. The kinetics and equilibria of the reactions of nitric oxide with sheep haemoglobin. J Physiol. 1957;136 doi: 10.1113/jphysiol.1957.sp005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92:103–17. doi: 10.1016/s0301-4622(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 27.Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003;253:508–17. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 28.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287:H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kuriyama S. Interpreting the history of bloodletting. Journal of the History of Medicine and Allied Sciences. 1995;50:11–46. doi: 10.1093/jhmas/50.1.11. [DOI] [PubMed] [Google Scholar]
- 30.Chatpun S, Cabrales P. Effects on cardiac function of a novel low viscosity plasma expander based on polyethylene glycol conjugated albumin. Minerva Anestesiol. 2011;77:704–14. [PubMed] [Google Scholar]
- 31.Khazaei M, Barmaki B. Role of exogenous nitric oxide donor in treatment of decompensated hemorrhagic shock in normotensive and hypertensive rats. J Biomed Biotechnol. 2012;2012:365195. doi: 10.1155/2012/365195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–12. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachuraju P, Friedman AJ, Friedman JM, Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82:607–13. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SK, Hwang NH. On transport of suspended particulates in tube flow. Biorheology. 1992;29(2-3):353–77. doi: 10.3233/bir-1992-292-313. [DOI] [PubMed] [Google Scholar]
- 35.Segre G, Silberberg A. Behaviour of macroscopic rigid spheres in Poiseuille flow. J Fluid Mech. 1962;14:115–35. [Google Scholar]
- 36.Secomb TW, Hsu R, Pries AR. A model for red blood cell motion in glycocalyx-lined capillaries. Am J Physiol. 1998;274:H1016–22. doi: 10.1152/ajpheart.1998.274.3.H1016. [DOI] [PubMed] [Google Scholar]
- 37.Munn LL, Dupin MM. Blood cell interactions and segregation in flow. Ann Biomed Eng. 2008;36(4):534–44. doi: 10.1007/s10439-007-9429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yalcin O, Wang Q, Johnson PC, Palmer AF, Cabrales P. Plasma expander viscosity effects on red cell-free layer thickness after moderate hemodilution. Biorheology. 2011;48:277–91. doi: 10.3233/BIR-2012-0598. [DOI] [PubMed] [Google Scholar]
- 39.Tsai AG, Acero C, Nance PR, Cabrales P, Frangos JA, Buerk DG, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H1730–9. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 40.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–10. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyle MP, LePoire DM, Pickering RA. Oxidation of hemoglobin and myoglobin by alkyl nitrites inhibition by oxygen. J Biol Chem. 1981;256:12399–404. [PubMed] [Google Scholar]
- 42.Sharma VS, Traylor TG, Gardiner R, Mizukami H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry. 1987;26:3837–43. doi: 10.1021/bi00387a015. [DOI] [PubMed] [Google Scholar]
- 43.Bouwer ST, Hoofd L, Kreuzer F. Diffusion coefficients of oxygen and hemoglobin measured by facilitated oxygen diffusion through hemoglobin solutions. Biochim Biophys Acta. 1997;1338:127–36. doi: 10.1016/s0167-4838(96)00197-5. [DOI] [PubMed] [Google Scholar]
- 44.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–7. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hrinczenko BW, Schechter AN, Wojtkowski TL, Pannell LK, Cashon RE, Alayash AI. Nitric oxide-mediated heme oxidation and selective beta-globin nitrosation of hemoglobin from normal and sickle erythrocytes. Biochem Biophys Res Commun. 2000;275:962–7. doi: 10.1006/bbrc.2000.3413. [DOI] [PubMed] [Google Scholar]
- 46.Joshi MS, Ferguson TB, Jr., Han TH, Hyduke DR, Liao JC, Rassaf T, et al. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A. 2002;99:10341–6. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 48.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, et al. Molecular dimensions of Hb-based O2 carriers determine constriction of resistance arteries and hypertension. Am J Physiol. 2000;279:H908–H15. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 49.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–97. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Buehler PW, Alayash AI. All hemoglobin-based oxygen carriers are not created equally. Biochim Biophys Acta. 2008;1784:1378–81. doi: 10.1016/j.bbapap.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Cho M, Spencer NY, Patel N, Huang Z, Shields H, et al. Measurements of nitric oxide on the heme iron and beta-93 thiol of human hemoglobin during cycles of oxygenation and deoxygenation. Proc Natl Acad Sci U S A. 2003;100:11303–8. doi: 10.1073/pnas.2033883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar MR, Fukuto JM, Miranda KM, Farmer PJ. Reactions of HNO with heme proteins: new routes to HNO-heme complexes and insight into physiological effects. Inorg Chem. 2010;49:6283–92. doi: 10.1021/ic902319d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinakoulaki E, Ohta T, Soulimane T, Kitagawa T, Varotsis C. Detection of the His-heme Fe2+-NO species in the reduction of NO to N2O by ba3-oxidase from thermus thermophilus. J Am Chem Soc. 2005;127:15161–7. doi: 10.1021/ja0539490. [DOI] [PubMed] [Google Scholar]
- 54.Olson JS, Mathews AJ, Rohlfs RJ, Springer BA, Egeberg KD, Sligar SG, et al. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988;336:265–6. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- 55.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Gossman W, Oldfield E. A density functional theory investigation of Fe-N-O bonding in heme proteins and model systems. J Am Chem Soc. 2003;125:16387–96. doi: 10.1021/ja030340v. [DOI] [PubMed] [Google Scholar]
- 57.Awasthi V, Yee SH, Jerabek P, Goins B, Phillips WT. Cerebral oxygen delivery by liposome-encapsulated hemoglobin: a positron-emission tomographic evaluation in a rat model of hemorrhagic shock. J Appl Physiol. 2007;103:28–38. doi: 10.1152/japplphysiol.00136.2006. [DOI] [PubMed] [Google Scholar]
- 58.Mawjood AH, Miyazaki G, Kaneko R, Wada Y, Imai K. Site-directed mutagenesis in hemoglobin: test of functional homology of the F9 amino acid residues of hemoglobin alpha and beta chains. Protein Eng. 2000;13:113–20. doi: 10.1093/protein/13.2.113. [DOI] [PubMed] [Google Scholar]
- 59.Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci U S A. 2008;105:16432–7. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–5. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Sprague RS, Goldman D, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, et al. Divergent effects of low-O(2) tension and iloprost on ATP release from erythrocytes of humans with type 2 diabetes: implications for O(2) supply to skeletal muscle. Am J Physiol Heart Circ Physiol. 2010;299:H566–73. doi: 10.1152/ajpheart.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ. Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem. 2011;286:38397–407. doi: 10.1074/jbc.M111.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: Oxygen Sensors and Modulators of Vascular Tone. Physiology. 2009;24:107–16. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 65.Relevic V, Rubino A, Burnstock G. Augmented sensory-motor vasodilatation of the rat mesenteric arterial bed after chronic infusion of the P1-purinoceptor antagonist, DPSPX. Br J Pharmacol. 1996;118:1675–80. doi: 10.1111/j.1476-5381.1996.tb15591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. The Journal of Physiology. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalsi KK, González-Alonso J. Temperature-dependent release of ATP from human erythrocytes: mechanism for the control of local tissue perfusion. Experimental Physiology. 2012;97:419–32. doi: 10.1113/expphysiol.2011.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feola M, Simoni J, Angelillo R, Luhruma Z, Kabakele M, Manzombi M, et al. Clinical trial of a hemoglobin based blood substitute in patients with sickle cell anemia. Surg Gynecol Obstet. 1992;174:379–86. [PubMed] [Google Scholar]
- 69.Simoni J, Simoni G, Newman G, Feola M. An improved blood substitute. In vivo evaluation of its hemodynamic effects. ASAIO J. 1996;42:M773–82. [PubMed] [Google Scholar]
- 70.Simoni J, Simoni G, Wesson DE, Feola M. ATP-adenosine-glutathione cross-linked hemoglobin as clinically useful oxygen carrier. Curr Drug Discov Technol. 2012;9:173–87. doi: 10.2174/157016312802650797. [DOI] [PubMed] [Google Scholar]
- 71.Lipowsky HH, Firrell JC. Microvascular hemodynamics during systemic hemodilution and hemoconcentration. Am J Physiol. 1986;250:H908–H22. doi: 10.1152/ajpheart.1986.250.6.H908. [DOI] [PubMed] [Google Scholar]
- 72.Fahraeus R. The suspension stability of the blood. Physiol Rev. 1929;9:241–74. [Google Scholar]
- 73.Fahraeus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. Am J Physiol. 1931;96:562–8. [Google Scholar]
- 74.Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks. Experiments and simulation. Circ Res. 1990;67:826–34. doi: 10.1161/01.res.67.4.826. [DOI] [PubMed] [Google Scholar]
- 75.Sakai H, Sato A, Takeoka S, Tsuchida E. Rheological properties of hemoglobin vesicles (artificial oxygen carriers) suspended in a series of plasma-substitute solutions. Langmuir. 2007;23:8121–8. doi: 10.1021/la7004503. [DOI] [PubMed] [Google Scholar]
- 76.Sriram K, Tsai AG, Cabrales P, Meng F, Acharya SA, Tartakovsky DM, et al. PEG-albumin supraplasma expansion is due to increased vessel wall shear stress induced by blood viscosity shear thinning. Am J Physiol Heart Circ Physiol. 2012;302:H2489–97. doi: 10.1152/ajpheart.01090.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabrales P, Tsai AG, Winslow RM, Intaglietta M. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol Heart Circ Physiol. 2005;289:H2392–400. doi: 10.1152/ajpheart.00225.2005. [DOI] [PubMed] [Google Scholar]
- 78.Cabrales P, Tsai AG, Intaglietta M. Alginate plasma expander maintains perfusion and plasma viscosity during extreme hemodilution. Am J Physiol Heart Circ Physiol. 2005;288:H1708–16. doi: 10.1152/ajpheart.00911.2004. [DOI] [PubMed] [Google Scholar]
- 79.Cabrales P, Intaglietta M, Tsai AG. Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock. 2005;23:549–55. [PubMed] [Google Scholar]
- 80.Cabrales P, Tsai AG, Ananda K, Acharya SA, Intaglietta M. Volume resuscitation from hemorrhagic shock with albumin and hexaPEGylated human serum albumin. Resuscitation. 2008;79:139–46. doi: 10.1016/j.resuscitation.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chatpun S, Nacharaju P, Cabrales P. Improving cardiac function with new-generation plasma volume expanders. Am J Emerg Med. 2013;31:54–63. doi: 10.1016/j.ajem.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Messmer C, Yalcin O, Palmer AF, Cabrales P. Small-volume resuscitation from hemorrhagic shock with polymerized human serum albumin. Am J Emerg Med. 2011 doi: 10.1016/j.ajem.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elmer J, Zorc K, Rameez S, Zhou Y, Cabrales P, Palmer AF. Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai AG, Intaglietta M, Sakai H, Delpy E, Drieu LARC, Rousselot M, et al. Microcirculation and NO-CO studies of a natural extracellular hemoglobin developed for an oxygen therapeutic carrier. Curr Drug Discov Technol. 2012 doi: 10.2174/157016312802650814. [DOI] [PubMed] [Google Scholar]
- 85.Harrington JP, Orlik K, Zito SL, Wollocko J, Wollocko H. Structural and redox behavior of OxyVita, a zero-linked polymeric hemoglobin: comparison with natural acellular polymeric hemoglobins. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:64–8. doi: 10.3109/10731191003634562. [DOI] [PubMed] [Google Scholar]
- 86.Bucci E, Kwansa H, Koehler RC, Matheson B. Development of zero-link polymers of hemoglobin, which do not extravasate and do not induce pressure increases upon infusion. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:11–8. doi: 10.1080/10731190600974277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsuchida E, Sou K, Nakagawa A, Sakai H, Komatsu T, Kobayashi K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjug Chem. 2009;20:1419–40. doi: 10.1021/bc800431d. [DOI] [PubMed] [Google Scholar]
- 88.Sakai H, Sato A, Takeoka S, Tsuchida E. Mechanism of Flocculate Formation of Highly Concentrated Phospholipid Vesicles Suspended in a Series of Water-Soluble Biopolymers. Biomacromolecules. 2009;10:2344–50. doi: 10.1021/bm900455e. [DOI] [PubMed] [Google Scholar]
- 89.Cabrales P, Intaglietta M, Tsai AG. Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation. 2007;75:124–34. doi: 10.1016/j.resuscitation.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabrales P, Tsai AG, Intaglietta M. Hemorrhagic shock resuscitation with carbon monoxide saturated blood. Resuscitation. 2007;72:306–18. doi: 10.1016/j.resuscitation.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 91.Mirhashemi S, Ertefai S, Messmer K, Intaglietta M. Model analysis of the enhancement of tissue oxygenation by hemodilution due to increased microvascular flow velocity. Microvasc Res. 1987;34:290–301. doi: 10.1016/0026-2862(87)90062-8. [DOI] [PubMed] [Google Scholar]
- 92.Gao YT, Roman LJ, Martasek P, Panda SP, Ishimura Y, Masters BS. Oxygen metabolism by endothelial nitric-oxide synthase. J Biol Chem. 2007;282:28557–65. doi: 10.1074/jbc.M704890200. [DOI] [PubMed] [Google Scholar]
- 93.Shen W, Xu X, Ochoa M, Zhao G, Bernstein RD, Forfia P, et al. Endogenous nitric oxide in the control of skeletal muscle oxygen extraction during exercise. Acta Physiol Scand. 2000;168:675–86. doi: 10.1046/j.1365-201x.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 94.Bolaños JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, et al. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem. 1997;68:2227–40. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 95.Adler S, Huang H. Impaired regulation of renal oxygen consumption in spontaneously hypertensive rats. J Am Soc Nephrol. 2002;13:1788–94. doi: 10.1097/01.asn.0000019781.90630.0f. [DOI] [PubMed] [Google Scholar]
- 96.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–14. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 97.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 98.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–7. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Elmer J, Cabrales P, Wang Q, Zhang N, Palmer AF. Synthesis and biophysical properties of polymerized human serum albumin. Biotechnol Prog. 2011;27:290–6. doi: 10.1002/btpr.531. [DOI] [PubMed] [Google Scholar]
- 100.Buehler PW, Zhou Y, Cabrales P, Jia Y, Sun G, Harris DR, et al. Synthesis, biophysical properties and pharmacokinetics of ultrahigh molecular weight tense and relaxed state polymerized bovine hemoglobins. Biomaterials. 2010;31:3723–35. doi: 10.1016/j.biomaterials.2010.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cabrales P, Sun G, Zhou Y, Harris DR, Tsai AG, Intaglietta M, et al. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107:1548–58. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Friesenecker BE, Tsai AG, Martini J, Ulmer H, Wenzel V, Hasibeder WR, et al. Arteriolar vasoconstrictive response: comparing the effects of arginine vasopressin and norepinephrine. Crit Care. 2006;10:R75. doi: 10.1186/cc4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reah G, Bodenham AR, Mallick A, Daily EK, Przybelski RJ. Initial evaluation of diaspirin cross-linked hemoglobin (DCLHb) as a vasopressor in critically ill patients. Crit Care Med. 1997;25(9):1480–8. doi: 10.1097/00003246-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Salazar Vázquez BY, Hightower CM, Martini J, Messmer C, Frienesenecker B, Cabrales P, et al. Crit Care Med. 2011;39(6):1451–66. doi: 10.1097/CCM.0b013e3182120cdb. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alayash AI. Oxygen therapeutics: Can we tame haemoglobin? Nature Reviews Drug Discovery. 2004;3:152–9. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 106.Friesenecker B, Tsai AG, Dunser MW, Mayr AJ, Martini J, Knotzer H, et al. Oxygen distribution in microcirculation after arginine vasopressin-induced arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2004;287:H1792–800. doi: 10.1152/ajpheart.00283.2004. [DOI] [PubMed] [Google Scholar]
- 107.Martini J, Cabrales P, K A, Acharya SA, Intaglietta M, Tsai AG. Survival time in severe hemorrhagic shock after perioperative hemodilution is longer with PEG-conjugated human serum albumin than with HES 130/0.4: a microvascular perspective. Crit Care. 2008;12:R54. doi: 10.1186/cc6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerger H, Saltzman DJ, Menger MD, Messmer K, Intaglietta M. Systemic and subcutaneous microvascular Po2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–36. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- 109.Kerger H, Saltzman DJ, Menger MD, Messmer K, Intaglietta M. Systemic and subcutaneous microvascular pO2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–H36. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- 110.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol. 2004;287:H363–H73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 111.Lindbom L, Tuma R, Arfors KE. Influence of oxygen on perfused capillary density and capillary red cell velocity in rabbit skeletal muscle. Microvasc Res. 1980;19:197–208. doi: 10.1016/0026-2862(80)90040-0. [DOI] [PubMed] [Google Scholar]
- 112.Burhop K, Gordon D, Estep T. Review of hemoglobin-induced myocardial lesions. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:353–74. doi: 10.1081/bio-200027429. [DOI] [PubMed] [Google Scholar]
- 113.Winslow RM. Alternative oxygen therapeutics: products, status of clinical trials, and future prospects. Curr Hemat Reports. 2003;2:503–10. [PubMed] [Google Scholar]
- 114.Cabrales P, Tsai AG, Intaglietta M. Polymerized bovine hemoglobin can improve small-volume resuscitation from hemorrhagic shock in hamsters. Shock. 2009;31:300–7. doi: 10.1097/SHK.0b013e318180ff63. [DOI] [PubMed] [Google Scholar]
- 115.Cabrales P, Tsai AG, Intaglietta M. Isovolemic exchange transfusion with increasing concentrations of low oxygen affinity hemoglobin solution limits oxygen delivery due to vasoconstriction. Am J Physiol Heart Circ Physiol. 2008;295:H2212–8. doi: 10.1152/ajpheart.00751.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cabrales P, Tsai AG, Intaglietta M. Balance between vasoconstriction and enhanced oxygen delivery. Transfusion. 2008;48:2087–95. doi: 10.1111/j.1537-2995.2008.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cabrales P, Tsai AG, Intaglietta M. Deferoxamine Lowers Tissue Damage After 80% Exchange Transfusion with Polymerized Hemoglobin. Antioxid Redox Signal. 2007 doi: 10.1089/ars.2006.1379. [DOI] [PubMed] [Google Scholar]
- 118.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285:H1411–H9. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 119.Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 2004;28:800–6. doi: 10.1111/j.1525-1594.2004.07392.x. [DOI] [PubMed] [Google Scholar]
- 120.Rohlfs RJ, Brunner E, Chiu A, Gonzales A, Gonzales ML, Magde D, et al. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–34. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 121.Alayash AI. Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic Res. 2000;33:341–8. doi: 10.1080/10715760000300881. [DOI] [PubMed] [Google Scholar]
- 122.Alayash AI, Summers AG, Wood F, Jia Y. Effects of glutaraldehyde polymerization on oxygen transport and redox properties of bovine hemoglobin. Arch Biochem Biophys. 2001;391:225–34. doi: 10.1006/abbi.2001.2426. [DOI] [PubMed] [Google Scholar]
- 123.Guillochon D, Esclade L, Remy MH, Thomas D. Studies on haemoglobin immobilized by cross-linking with glutaraldehyde. Cross-linked soluble polymers and artificial membranes. Biochim Biophys Acta. 1981;670:332–40. doi: 10.1016/0005-2795(81)90105-7. [DOI] [PubMed] [Google Scholar]
- 124.Guillochon D, Esclade L, Thomas D. Effect of glutaraldehyde on haemoglobin: oxidation-reduction potentials and stability. Biochem Pharmacol. 1986;35:317–23. doi: 10.1016/0006-2952(86)90532-0. [DOI] [PubMed] [Google Scholar]
- 125.Freilich D, Pearce LB, Pitman A, Greenburg G, Berzins M, Bebris L, et al. HBOC-201 Vasoactivity in a Phase III Clinical Trial in Orthopedic Surgery Subjects-Extrapolation of Potential Risk for Acute Trauma Trials. J Trauma. 2009;66:365–76. doi: 10.1097/TA.0b013e3181820d5c. [DOI] [PubMed] [Google Scholar]
- 126.Lowe KC. Blood substitutes: from chemistry to clinic. J Mater Chem. 2006;16:4189–96. [Google Scholar]
- 127.Marinaro J, Smith J, Tawil I, Billstrand M, Crookston KP. HBOC-201 use in traumatic brain injury: case report and review of literature. Transfusion. 2009;49:2054–9. doi: 10.1111/j.1537-2995.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- 128.Jahr JS, Varma N. PolyHeme. Northfield Laboratories. IDrugs. 2004;7:478–82. [PubMed] [Google Scholar]
- 129.Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 130.Cooper CE, Silaghi-Dumitrescu R, Rukengwa M, Alayash AI, Buehler PW. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC) Biochim Biophys Acta. 2008;1784:1415–20. doi: 10.1016/j.bbapap.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 131.Hayward R, Lefer AM. Administration of polymerized bovine hemoglobin improves survival in a rat model of traumatic shock. Methods Find Exp Clin Pharmacol. 1999;21:427–33. doi: 10.1358/mf.1999.21.6.541924. [DOI] [PubMed] [Google Scholar]
- 132.Standl T, Wilhelm S, Horn EP, Burmeister M, Gundlach M, Schulte am Esch J. [Preoperative hemodilution with bovine hemoglobin. Acute hemodynamic effects in liver surgery patients] Anaesthesist. 1997;46:763–70. doi: 10.1007/s001010050466. [DOI] [PubMed] [Google Scholar]
- 133.Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 134.Cabrales P, Zhou Y, Harris DR, Palmer AF. Tissue oxygenation after exchange transfusion with ultrahigh-molecular-weight tense- and relaxed-state polymerized bovine hemoglobins. Am J Physiol Heart Circ Physiol. 2010;298:H1062–71. doi: 10.1152/ajpheart.01022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu B, Liu Z, Chang TM. Polyhemoglobin with different percentage of tetrameric hemoglobin and effects on vasoactivity and electrocardiogram. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:159–73. doi: 10.1080/10731190600580223. [DOI] [PubMed] [Google Scholar]
- 136.Rice J, Philbin N, Light R, Arnaud F, Steinbach T, McGwin G, et al. The effects of decreasing low-molecular weight hemoglobin components of hemoglobin-based oxygen carriers in swine with hemorrhagic shock. J Trauma. 2008;64:1240–57. doi: 10.1097/TA.0b013e318058245e. [DOI] [PubMed] [Google Scholar]
- 137.Cabrales P, Tsai AG, Intaglietta M. Increased tissue PO2 and decreased O2 delivery and consumption after 80% exchange transfusion with polymerized hemoglobin. Am J Physiol. 2004;287:H2825–33. doi: 10.1152/ajpheart.00654.2004. [DOI] [PubMed] [Google Scholar]
- 138.Intaglietta M, Cabrales P, Tsai AG. Microvascular perspective of oxygen-carrying and -noncarrying blood substitutes. Annu Rev Biomed Eng. 2006;8:289–321. doi: 10.1146/annurev.bioeng.8.061505.095713. [DOI] [PubMed] [Google Scholar]
- 139.Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–10. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsai AG, Sakai H, Wettstein R, Kerger H, Intaglietta M. An effective blood replacement fluid that targets oxygen delivery, increases plasma viscosityand has high oxygen affinity. TATM. 2004;5:507–14. [Google Scholar]
- 141.Tsai AG, Intaglietta M. The unusual properties of effective blood substitutes. Keio J Med. 2002;51:17–20. doi: 10.2302/kjm.51.17. [DOI] [PubMed] [Google Scholar]
- 142.Cabrales P, Nacharaju P, Manjula BN, Tsai AG, Acharya SA, Intaglietta M. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 143.Webb AR, Barclay SA, Bennett ED. In vitro colloid osmotic pressure of commonly used plasma expanders and substitutes: a study of the diffusibility of colloid molecules. Int Care Med. 1989;15:116–20. doi: 10.1007/BF00295988. [DOI] [PubMed] [Google Scholar]
- 144.Harrington JP, Kobayashi S, Dorman SC, Zito SL, Hirsch RE. Acellular invertebrate hemoglobins as model therapeutic oxygen carriers: unique redox potentials. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:53–67. doi: 10.1080/10731190600974491. [DOI] [PubMed] [Google Scholar]
- 145.Jia Y, Alayash AI. Effects of cross-linking and zero-link polymerization on oxygen transport and redox chemistry of bovine hemoglobin. Biochim Biophys Acta. 2009;1794:1234–42. doi: 10.1016/j.bbapap.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 146.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit: effect of cell-free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol. 2003;285:H1600–8. doi: 10.1152/ajpheart.00077.2003. [DOI] [PubMed] [Google Scholar]
- 147.Zal F, Green BN, Lallier FH, Vinogradov SN, Toulmond A. Quaternary structure of the extracellular haemoglobin of the lugworm Arenicola marina: a multi-angle-laser-light-scattering and electrospray-ionisation-mass-spectrometry analysis. European Journal of Biochemistry / FEBS. 1997;243(1-2):85–92. doi: 10.1111/j.1432-1033.1997.85_1a.x. [DOI] [PubMed] [Google Scholar]
- 148.Rousselot M, Delpy E, Drieu La Rochelle C, Lagente V, Pirow R, Rees JF, et al. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol J. 2006;1(3):333–45. doi: 10.1002/biot.200500049. [DOI] [PubMed] [Google Scholar]
- 149.Thuillier R, Dutheil D, Trieu MT, Mallet V, Allain G, Rousselot M, et al. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. Am J Transplant. 2011;11:1845–60. doi: 10.1111/j.1600-6143.2011.03614.x. [DOI] [PubMed] [Google Scholar]
- 150.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–45. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wilcox CS, Pearlman A. Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacological Reviews. 2008;60:418–69. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buehler PW, Baek JH, Lisk C, Connor I, Sullivan T, Kominsky D, et al. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am J Physiol Lung Cell Mol Physiol. 2012;303:L312–26. doi: 10.1152/ajplung.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kentner R, Safar P, Behringer W, Wu X, Henchir J, Ma L, et al. Small volume resuscitation with tempol is detrimental during uncontrolled hemorrhagic shock in rats. Resuscitation. 2007;72:295–305. doi: 10.1016/j.resuscitation.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 154.Hsia CJ, Ma L. A hemoglobin-based multifunctional therapeutic: polynitroxylated pegylated hemoglobin. Artif Organs. 2012;36:215–20. doi: 10.1111/j.1525-1594.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hsia CJC, Ma L. A Hemoglobin-Based Multifunctional Therapeutic: Polynitroxylated Pegylated Hemoglobin. Artificial Organs. 2012;36:215–20. doi: 10.1111/j.1525-1594.2011.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nho K, Glower D, Bredehoeft S, Shankar H, Shorr R, Abuchowski A. PEG-bovine hemoglobin: safety in a canine dehydrated hypovolemic-hemorrhagic shock model. Biomater Artif Cells Immobilization Biotechnol. 1992;20:511–24. doi: 10.3109/10731199209119677. [DOI] [PubMed] [Google Scholar]
- 157.Kida Y, Maeda M, Iwata S, Iwashita Y, Goto K, Nishi K. Effects of pyridoxalated hemoglobin polyoxyethylene conjugate and other hemoglobin-related substances on arterial blood pressure in anesthetized and conscious rats. Artif Organs. 1995;19:117–28. doi: 10.1111/j.1525-1594.1995.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 158.Manjula BN, Tsai A, Upadhya R, Perumalsamy K, Smith PK, Malavalli A, et al. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem. 2003;14:464–72. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 159.Young MA, Riddez L, Kjellstrom BT, Bursell J, Winslow F, Lohman J, et al. MalPEG-hemoglobin (MP4) improves hemodynamics, acid-base status, and survival after uncontrolled hemorrhage in anesthetized swine. Crit Care Med. 2005;33:1794–804. doi: 10.1097/01.ccm.0000172648.55309.13. [DOI] [PubMed] [Google Scholar]
- 160.Bjorkholm M, Fagrell B, Przybelski R, Winslow N, Young M, Winslow RM. A phase I single blind clinical trial of a new oxygen transport agent (MP4), human hemoglobin modified with maleimide-activated polyethylene glycol. Haematologica. 2005;90:505–15. [PubMed] [Google Scholar]
- 161.Wettstein R, Tsai AG, Erni D, Lukyanov AN, Torchilin VP, Intaglietta M. Improving microcirculation is more effective than substitution of red blood cells to correct metabolic disorder in experimental hemorrhagic shock. Shock. 2004;21:235–40. doi: 10.1097/01.shk.0000114301.36496.ea. [DOI] [PubMed] [Google Scholar]
- 162.Winslow RM. Targeted O2 delivery by low-p50 hemoglobin: a new basis for hemoglobin-based oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:1–12. doi: 10.1081/bio-200046634. [DOI] [PubMed] [Google Scholar]
- 163.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, et al. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–34. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 164.Lui FE, Dong P, Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–80. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 165.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–94. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 166.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–67. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 168.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–47. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roche CJ, Dantsker D, Samuni U, Friedman JM. Nitrite reductase activity of sol-gel-encapsulated deoxyhemoglobin. Influence of quaternary and tertiary structure. J Biol Chem. 2006;281:36874–82. doi: 10.1074/jbc.M603914200. [DOI] [PubMed] [Google Scholar]
- 170.Roche CJ, Dantsker D, Alayash AI, Friedman JM. Enhanced nitrite reductase activity associated with the haptoglobin complexed hemoglobin dimer: functional and antioxidative implications. Nitric Oxide. 2012;27:32–9. doi: 10.1016/j.niox.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang X, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, et al. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci U S A. 2004;101:11477–82. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Katz LM, Manning JE, McCurdy S, Sproule C, McGwin G, Jr., Moon-Massat P, et al. Nitroglycerin attenuates vasoconstriction of HBOC-201 during hemorrhagic shock resuscitation. Resuscitation. 2010;81:481–7. doi: 10.1016/j.resuscitation.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 173.Wegria R, Nickerson JL, Case RB, Holland JF. Effect of nitroglycerine on the cardiovascular system of normal persons. Am J Med. 1951;10:414–8. doi: 10.1016/0002-9343(51)90286-0. [DOI] [PubMed] [Google Scholar]
- 174.Arnaud F, Scultetus AH, Kim B, Haque A, Saha B, Nigam S, et al. Dose response of sodium nitrite on vasoactivity associated with HBOC-201 in a swine model of controlled hemorrhage. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:195–205. doi: 10.3109/10731199.2010.533126. [DOI] [PubMed] [Google Scholar]
- 175.Moon-Massat P, Scultetus A, Arnaud F, Brown A, Haque A, Saha B, et al. The effect HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury. 2012;43:638–47. doi: 10.1016/j.injury.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 176.Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM. Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology. 2009;110:113–22. doi: 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, et al. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112:586–94. doi: 10.1097/ALN.0b013e3181cd7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991;34:3242–7. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 179.Maragos CM, Wang JM, Hrabie JA, Oppenheim JJ, Keefer LK. Nitric oxide/nucleophile complexes inhibit the in vitro proliferation of A375 melanoma cells via nitric oxide release. Cancer Res. 1993;53:564–8. [PubMed] [Google Scholar]
- 180.Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis. 2011;203:1454–63. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gotshall RW, Hamilton KL, Foreman B, van Patot MC, Irwin DC. Glutaraldehyde-polymerized bovine hemoglobin and phosphodiesterase-5 inhibition. Crit Care Med. 2009;37:1988–93. doi: 10.1097/CCM.0b013e3181a00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49–56. doi: 10.1152/ajpheart.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM. Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med. 2010;49:530–8. doi: 10.1016/j.freeradbiomed.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem. 2002;277:9637–40. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 185.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. 2002;33:1590–6. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 187.Romeo AA, Filosa A, Capobianco JA, English AM. Metal chelators inhibit S-nitrosation of Cys beta 93 in oxyhemoglobin. J Am Chem Soc. 2001;123:1782–3. doi: 10.1021/ja005612y. [DOI] [PubMed] [Google Scholar]
- 188.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–8. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 189.Lui FE, Kluger R. Enhancing nitrite reductase activity of modified hemoglobin: bis-tetramers and their PEGylated derivatives. Biochemistry. 2009;48:11912–9. doi: 10.1021/bi9014105. [DOI] [PubMed] [Google Scholar]
- 190.Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, et al. Nitrite Regulates Hypoxic Vasodilation via Myoglobin-Dependent Nitric Oxide Generation. Circulation. 2012;126:325–34. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh M, Arya A, Kumar R, Bhargava K, Sethy NK. Dietary nitrite attenuates oxidative stress and activates antioxidant genes in rat heart during hypobaric hypoxia. Nitric Oxide. 2012;26:61–73. doi: 10.1016/j.niox.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 192.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem. 2005;99:237–46. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 193.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an s-nitrosothiol-based signal. Circ Res. 2008;103:545–53. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Burke TK, Teng X, Patel RP, Baldwin AL. Effects of S-nitrosation on hemoglobin-induced microvascular damage. Antioxid Redox Signal. 2006;8:1093–101. doi: 10.1089/ars.2006.8.1093. [DOI] [PubMed] [Google Scholar]
- 195.Nakai K, Togashi H, Yasukohchi T, Sakuma I, Fujii S, Yoshioka M, et al. Preparation and characterization of SNO-PEG-hemoglobin as a candidate for oxygen transporting material. Int J Artif Organs. 2001;24:322–8. [PubMed] [Google Scholar]
- 196.Winslow RM, Intaglietta M. Red cell age and loss of function: advance or SNO-job? Transfusion. 2008;48:411–4. doi: 10.1111/j.1537-2995.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 197.Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34:622–34. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 198.Riess JG. Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:567–80. doi: 10.1080/10731190600973824. [DOI] [PubMed] [Google Scholar]
- 199.Riess JG. Fluorocarbon-based in vivo oxygen transport and delivery systems. Vox Sang. 1991;61:225–39. doi: 10.1111/j.1423-0410.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 200.Zarif L, Postel M, Septe B, Trevino L, Riess JG, Mahe AM, et al. Biodistribution of mixed fluorocarbon-hydrocarbon dowel molecules used as stabilizers of fluorocarbon emulsions: a quantitative study by fluorine nuclear magnetic resonance (NMR) Pharm Res. 1994;11:122–7. doi: 10.1023/a:1018914215345. [DOI] [PubMed] [Google Scholar]
- 201.Giraudeau C, Djemai B, Ghaly MA, Boumezbeur F, Meriaux S, Robert P, et al. High sensitivity 19F MRI of a perfluorooctyl bromide emulsion: application to a dynamic biodistribution study and oxygen tension mapping in the mouse liver and spleen. NMR Biomed. 2012;25:654–60. doi: 10.1002/nbm.1781. [DOI] [PubMed] [Google Scholar]
- 202.Glogar DH, Kloner RA, Muller J, DeBoer LW, Braunwald E, Clark LC., Jr. Fluorocarbons reduce myocardial ischemic damage after coronary occlusion. Science. 1981;211:1439–41. doi: 10.1126/science.7466402. [DOI] [PubMed] [Google Scholar]
- 203.Cabrales P, Carlos Briceno J. Delaying blood transfusion in experimental acute anemia with a perfluorocarbon emulsion. Anesthesiology. 2011;114:901–11. doi: 10.1097/ALN.0b013e31820efb36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Clark LC, Jr., Wesseler EP, Miller ML, Kaplan S. Ring versus straight chain perfluorocarbon emulsions for perfusion media. Microvasc Res. 1974;8:32040. doi: 10.1016/s0026-2862(74)80007-5. [DOI] [PubMed] [Google Scholar]
- 205.Clark LC, Jr., Wesseler EP, Kaplan S, Miller ML, Becker C, Emory C, et al. Emulsions of perfluorinated solvents for intravascular gas transport. Fed Proc. 1975;34:1468–77. [PubMed] [Google Scholar]
- 206.Riess JG. Oxygen carriers ("blood substitutes")--raison d’etre, chemistry, and some physiology. Chem Rev. 2001;101:2797–920. doi: 10.1021/cr970143c. [DOI] [PubMed] [Google Scholar]
- 207.Nolte D, Pickelmann S, Lang M, Keipert P, Messmer K. Compatibility of different colloid plasma expanders with perflubron emulsion: an intravital microscopic study in the hamster. Anesthesiology. 2000;93:1261–70. doi: 10.1097/00000542-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 208.Lowe KC. Perfluorocarbons as oxygen-transport fluids. Comp Biochem Physiol A. 1987;87:825–38. doi: 10.1016/0300-9629(87)90001-6. [DOI] [PubMed] [Google Scholar]
- 209.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–79. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 210.Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol. 2004;287:H320–30. doi: 10.1152/ajpheart.01166.2003. [DOI] [PubMed] [Google Scholar]
- 211.Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–63. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- 212.McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92:103–17. doi: 10.1016/s0301-4622(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 213.Faithfull NS. Oxygen delivery from fluorocarbon emulsions--aspects of convective and diffusive transport. Biomater Artif Cells Immobilization Biotechnol. 1992;20:797–804. doi: 10.3109/10731199209119721. [DOI] [PubMed] [Google Scholar]
- 214.Cabrales P, Tsai AG, Intaglietta M. Perfluorocarbon in microcirculation during ischemia reperfusion. J Am Coll Surg. 2007;204:225–35. doi: 10.1016/j.jamcollsurg.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 215.Cleman M, Jaffee CC, Wohlgelernter D. Prevention of ischemia during percutaneous transluminal coronary angioplasty by transcatheter infusion of oxygenated Fluosol DA 20. Circulation. 1986;74:555–62. doi: 10.1161/01.cir.74.3.555. [DOI] [PubMed] [Google Scholar]
- 216.Lutz J, Herrmann G. Perfluorochemicals as a treatment of decompression sickness in rats. Pflugers Arch. 1984;401:174–7. doi: 10.1007/BF00583878. [DOI] [PubMed] [Google Scholar]
- 217.Spiess BD, Cochran RP. Perfluorocarbon emulsions and cardiopulmonary bypass: a technique for the future. J Cardiothorac Vasc Anesth. 1996;10:83–9. doi: 10.1016/s1053-0770(96)80182-0. quiz 9-90. [DOI] [PubMed] [Google Scholar]
- 218.Venkatesh AK, Zhao L, Balamore D, Jolesz FA, Albert MS. Evaluation of carrier agents for hyperpolarized xenon MRI. NMR Biomed. 2000;13:245–52. doi: 10.1002/1099-1492(200006)13:4<245::aid-nbm635>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 219.Pollack GL, Kennan RP, Holm GT. Solubility of inert gases in PFC blood substitute, blood plasma, and mixtures. Biomater Artif Cells Immobilization Biotechnol. 1992;20:1101–4. doi: 10.3109/10731199209119768. [DOI] [PubMed] [Google Scholar]
- 220.Tremper KK, Zaccari J, Cullen BF, Hufstedler SM. Liquid-gas partition coefficients of halothane and isoflurane in perfluorodecalin, fluosol-DA, and blood/fluosol-DA mixtures. Anesth Analg. 1984;63:690–2. [PubMed] [Google Scholar]
- 221.Wall TC, Califf RM, Blankenship J, Talley JD, Tannenbaum M, Schwaiger M, et al. TAMI 9 Research Group Intravenous Fluosol in the treatment of acute myocardial infarction. Results of the Thrombolysis and Angioplasty in Myocardial Infarction 9 Trial. Circulation. 1994;90:114–20. doi: 10.1161/01.cir.90.1.114. [DOI] [PubMed] [Google Scholar]
- 222.Habler O, Kleen M, Hutter J, Podtschaske A, Tiede M, Kemming G, et al. IV perflubron emulsion versus autologous transfusion in severe normovolemic anemia: effects on left ventricular perfusion and function. Res Exp Med (Berl) 1998;197:301–18. doi: 10.1007/s004330050079. [DOI] [PubMed] [Google Scholar]
- 223.Keipert PE. Use of Oxygent, a perfluorochemical-based oxygen carrier, as an alternative to intraoperative blood transfusion. Artif Cells Blood Substit Immobil Biotechnol. 1995;23:381–94. doi: 10.3109/10731199509117954. [DOI] [PubMed] [Google Scholar]
- 224.Spahn DR, van Brempt R, Theilmeier G, Reibold JP, Welte M, Heinzerling H, et al. European Perflubron Emulsion Study Group Perflubron emulsion delays blood transfusions in orthopedic surgery. Anesthesiology. 1999;91:1195–208. doi: 10.1097/00000542-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 225.Cuignet OY, Baele PM, Van Obbergh LJ. A second-generation blood substitute (perflubron emulsion) increases the blood solubility of modern volatile anesthetics in vitro. Anesth Analg. 2002;95:368–72. doi: 10.1097/00000539-200208000-00023. table of contents. [DOI] [PubMed] [Google Scholar]
- 226.Rafikova O, Sokolova E, Rafikov R, Nudler E. Control of plasma nitric oxide bioactivity by perfluorocarbons: physiological mechanisms and clinical implications. Circulation. 2004;110:3573–80. doi: 10.1161/01.CIR.0000148782.37563.F8. [DOI] [PubMed] [Google Scholar]
- 227.Rafikova O, Rafikov R, Nudler E. Catalysis of S-nitrosothiols formation by serum albumin: the mechanism and implication in vascular control. Proc Natl Acad Sci U S A. 2002;99:5913–8. doi: 10.1073/pnas.092048999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lundgren CE, Bergoe GW, Tyssebotn IM. Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:473–86. doi: 10.1080/10731190600769271. [DOI] [PubMed] [Google Scholar]
- 229.Lundgren CE, Bergoe GW, Tyssebotn I. The theory and application of intravascular microbubbles as an ultra-effective means of transporting oxygen and other gases. Undersea Hyperb Med. 2004;31:105–6. [PubMed] [Google Scholar]
- 230.Lundgren C, Bergoe G, Olszowka A, Tyssebotn I. Tissue nitrogen elimination in oxygen-breathing pigs is enhanced by fluorocarbon-derived intravascular micro-bubbles. Undersea Hyperb Med. 2005;32:215–26. [PubMed] [Google Scholar]
- 231.Schneider M, Broillet A, Tardy I, Pochon S, Bussat P, Bettinger T, et al. Use of intravital microscopy to study the microvascular behavior of microbubble-based ultrasound contrast agents. Microcirculation. 2012;19:245–59. doi: 10.1111/j.1549-8719.2011.00152.x. [DOI] [PubMed] [Google Scholar]
- 232.Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Prog Cardiovasc Dis. 2001;44:13–31. doi: 10.1053/pcad.2001.26440. [DOI] [PubMed] [Google Scholar]
- 233.Winslow RM. Blood substitutes. Current status. Transfusion. 1989;29:753–4. doi: 10.1046/j.1537-2995.1989.29990070175.x. [DOI] [PubMed] [Google Scholar]
- 234.Kluger R. Red cell substitutes from hemoglobin--do we start all over again? Curr Opin Chem Biol. 2010;14:538–43. doi: 10.1016/j.cbpa.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 235.Buerk DG, Tsai AG, Intaglietta M, Johnson PC. In vivo tissue pO2 measurements in hamster skinfold by recessed pO2 microelectrodes and phosphorescence quenching are in agreement. Microcirculation. 1998;5:219–25. [PubMed] [Google Scholar]
- 236.Kaufman AG, Intaglietta M. Automated diameter measurement of vasomotion by cross-correlation. Int J Microcirc Clin Exp. 1985;4:45–53. [PubMed] [Google Scholar]
- 237.Kerger H, Tsai AG, Saltzman DJ, Winslow RM, Intaglietta M. Fluid resuscitation with O2 vs. non-O2 carriers after 2 h of hemorrhagic shock in conscious hamsters. Am J Physiol. 1997;272:H525–37. doi: 10.1152/ajpheart.1997.272.1.H525. [DOI] [PubMed] [Google Scholar]
- 238.Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol Heart Circ Physiol. 2004;287:H320–30. doi: 10.1152/ajpheart.01166.2003. [DOI] [PubMed] [Google Scholar]
- 239.Tsai AG, Friesenecker B, Mazzoni MC, Kerger H, Buerk DG, Johnson PC, et al. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci U S A. 1998;95:6590–5. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, et al. Molecular dimensions of Hb-based O(2) carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol. 2000;279:H908–15. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 241.Johnson PC. Autoregulation of blood flow. Circ Res. 1986;59:483–95. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- 242.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 243.Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03774.x. [DOI] [PubMed] [Google Scholar]
- 244.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285:H1411–9. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 245.Sauaia A, Moore EE, Banerjee A. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300:1297. doi: 10.1001/jama.300.11.1297-a. author reply 8-9. [DOI] [PubMed] [Google Scholar]
- 246.Zhou JL, Li G, Hai Y, Guan L, Huang XL, Sun P. Protection of carbon monoxide-releasing molecule against lung injury induced by limb ischemia-reperfusion. Chin J Traumatol. 2009;12:71–6. [PubMed] [Google Scholar]
- 247.Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46–55. doi: 10.1080/08941930802709470. [DOI] [PubMed] [Google Scholar]
- 248.Mourouzis I, Dimopoulos A, Saranteas T, Tsinarakis N, Livadarou E, Spanou D, et al. Ischemic preconditioning fails to confer additional protection against ischemia-reperfusion injury in the hypothyroid rat heart. Physiol Res. 2009;58:29–38. doi: 10.33549/physiolres.931387. [DOI] [PubMed] [Google Scholar]
- 249.Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol. 2006;4:215–27. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- 250.Milsom AB, Patel NS, Mazzon E, Tripatara P, Storey A, Mota-Filipe H, et al. Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric Oxide. 2010;22:141–8. doi: 10.1016/j.niox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 251.Cabrales P. Examining and Mitigating Acellular Hemoglobin Vasoactivity. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]