Abstract
Adherence to treatment for hepatitis C virus (HCV) maximizes treatment efficacy. Missed doses and failing to persist on treatment are two patient-level processes that are rarely defined or analyzed separately from other factors affecting treatment adherence. We evaluated the prevalence and patterns of missed doses and nonpersistence, and identified patient characteristics associated with these outcomes. Missed doses of ribavirin (RBV) and peginterferon (PEG), measured prospectively in Virahep-C using electronic monitoring technology, were analyzed using generalized estimating equations. Cox proportional hazards models analyzed time to nonpersistence from baseline to week 24 (N=401) and from week 24–48 in Responders (N=242). Average proportion of PEG and RBV missed doses increased over time from 5 to 15% and 7 to 27%, respectively. Patients who were younger, African American, unemployed, or unmarried were at greater risk of missing PEG from week 0–24; higher baseline depression predicted missing PEG from weeks 24–48. Patients who were younger or African American were more likely to miss daily RBV from weeks 0–24; and those without private insurance or employment were more likely to miss RBV from weeks 24–48. Fifty-two patients failed to persist on treatment for patient-driven deviations. Predictors of nonpersistence from weeks 0–24 included younger age, lower education, public or no insurance, or worse baseline headaches. In conclusion electronic monitoring and the prospective Virahep-C design afforded a unique opportunity to evaluate missing doses and nonpersistence separately, and identify patients at risk for nonadherence. These processes will be important to investigate as the dosing schedules of antiviral regimens become increasingly complex.
Keywords: compliance, dose, interferon, liver, medication adherence
Nonadherence to prescribed treatment regimens for hepatitis C viral (HCV) infection has been a major impediment to treatment success during peginterferon and ribavirin (PEG/RBV) regimens (1, 2). Treatment effectiveness of the new triple therapy regimens will continue to be hindered by nonadherence given the greater complexity of the daily dosing schedules, requisite nutritional intake, and additional side effects of the protease inhibitors (3). “Adherence to the prescribed treatment protocol” as it has been described in the HCV literature, usually refers to: 1) a combination of the proportion of actual doses taken compared to the total amount of prescribed doses with 2) the proportion of actual days on treatment compared to the ideal prescribed treatment duration. Patients who are maintained on greater than 80% of PEG and 80% of RBV for 80% of the treatment duration (the 80/80/80 standard) have greater chances of achieving a sustained virological response (SVR) (1, 4).
Nonadherence to the prescribed regimens occurs for myriad reasons including premature treatment discontinuations, dose reductions, and patients missed doses that are not well differentiated in the literature because these deviations from the protocol are often combined into one overall composite “treatment adherence” variable. Unfortunately, composite adherence variables (e.g., the 80/80/80 standard) obscure our ability to distinguish medically-necessary protocol deviations from patient-driven deviations. Importantly, the latter may be modified through interventions, thereby increasing the number of patients who can be treated successfully.
Two patient-driven protocol deviations that are useful to define and measure individually are “execution of dosing” and “persistence” (5–7). Execution of dosingoften referred to as “medication adherence,” is defined as the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen (5). Poor execution of dosing is often termed “missed doses.” While execution of dosing is infrequently measured among patients with HCV, studies indicate that patients report problems taking their HCV medications and that inadequate dosing may impede virologic response and SVR (2, 8–13). To date, there is a lack of understanding as to why patients miss taking their medications as prescribed.
In contrast, medication persistence is defined as the total length of time a patient takes a medication, from first dose to last dose(5, 7). In the broader medication adherence literature which seeks to understand and improve patient’s medication-taking behavior and emphasizes patient adherence “nonpersistence” generally refers to all behaviors or decisions to stop a medication regimen early which are judged to be under the patient’s control. In these settings, examples of nonpersistence include, but are not limited to, intolerance of unpleasant but not medically dangerous side effects, not refilling prescriptions, being lost to follow-up, and noncompliance with physician recommendations (5–7). Typically, medically-necessary or physician-recommended deviations from medication regimens are not described in medication adherence studies, not because they do not occur, but because these deviations are not considered “patient adherence” issues(14). The field of HCV has historically used the term “premature treatment discontinuation” to capture all reasons for premature discontinuation, without making any distinction between patient-driven nonpersistence and medically-necessary provider-driven discontinuations, both of which can occur during HCV treatment(15). Since both patient-driven and medically-necessary discontinuations occur during HCV treatment but will ultimately require different types of interventions, it is prudent for the field to disentangle these processes(15). While this differentiation is not well-described in the HCV literature, adoption of such lexicon would promote a more fine-grained analysis and understanding of these interrelated but distinct phenomena which would better serve intervention development to target precise issues that jeopardize treatment success. Also, aligning the HCV adherence literature with the broader adherence literature would help to inform our conceptualization and understanding of these distinct processes, and vice versa contribute to the larger empirical literature on adherence.
A better understanding of these phenomena and adoption of such definitions would allow researchers: 1) to apply a consistent lexicon for future HCV adherence research; 2) identify the largest threats to HCV treatment efficacy; 3) to develop precise interventions to address each type of protocol deviation (e.g., behavioral reminder systems for missed doses; side effect management for nonpersistence); and 4) to identify subgroups of patients in need of further monitoring and intervention.
The objective of the current study was to examine the prevalence and patterns of the missed doses and nonpersistence (i.e, patient-driven treatment discontinuations), and to identify patient characteristics associated with these two patient-driven protocol deviations. The specific aims were as follows: 1) To prospectively examine the proportion of patients who missed doses of weekly PEG and daily RBV and failed to persist on PEG/RBV treatment; and 2) To identify patient characteristics associated with missed doses and nonpersistence.
MATERIALS AND METHODS
Virahep-C Design
We used data from the NIDDK-funded Virahep-C study, which stratified sampling to enroll 196 African American (AA) and 205 Caucasian American (CA) adults with chronic hepatitis C, genotype 1 infection at eight U.S. medical centers to study factors associated with differential response to antiviral therapy between racial groups (12). In brief, the results of the main study demonstrated SVR rates of 28% in AAs compared to 52% in CAs, racial differences in viral response were found early as early as treatment week 4, and these differences were not explained by disease characteristics, baseline viral load, or amount of drug taken. One of the specific aims of Virahep-C was to evaluate pre-treatment and on-treatment factors that may account for differential treatment responses, therefore adherence to the treatment protocol was rigorously measured as a potential predictive variable. Participants were treated with PEG/RBV for a minimum of 24 weeks. Those with detectable viremia at week 24 (i.e.“Nonresponders”) were discontinued from therapy, whereas patients with undetectable or indeterminate HCV RNA by 24 weeks were continued for 48 weeks (“TW24 Responders”). Patients attended a baseline visit and then follow-ups at treatment weeks 2, 4, 8, 12 and then monthly up to 48 weeks. SVR was defined as undetectable viremia (HCV RNA < 50 IU/mL) 24 weeks after stopping therapy.
Virahep-C Patient Education and Adherence Program
To optimize treatment efficacy, all participants received numerous adherence-enhancing interventions throughout the study, including a modified version of the Medication Adherence Training Interview (16), informational-exchange, skills development, social support enlistment, and educational videos. Details of the Virahep-C protocol can be found at https://www.niddkrepository.org/niddk/jsp/public/dataset.jsp#VIRAHEP-C.
Measures
Baseline Sociodemographic and Clinical Characteristics
Baseline data were collected via self-report and medical chart reviews. Sociodemographic variables included: age, gender, race, marital status, education level, employment status, and health insurance status. Medical variables included: fibrosis level, smoking status, alcohol consumption, number of medications taken for comorbid conditions, source of HCV infection, and presence of baseline antidepressant use.
Baseline Psychosocial Instruments
Depressive Symptoms
The Center for Epidemiologic Studies-Depression (CES-D) scale was used to measure depressive symptoms (17). The CES-D total score ranges from 0 to 63, with higher scores indicative of more severe symptomatology.
Visual Analog Symptom Scales
Six visual analog scales (VASs) were used to assess: (1) overall symptomatology (VAS-ALL), (2) fatigue (VAS-FAT), (3) headaches (VAS-HEAD), (4) muscle/joint aches and pains (VAS-ACHE), (5) irritability (VAS-IRRIT), and (6) depression (VAS-DEP). Participants marked a computerized line via a touch screen computer which was converted to a continuous 0 (“none”) to 10 (“worst ever”), with higher scores indicative of worse symptoms in the week preceding a clinic visit.
Health-Related Quality of Life
The SF-36 Health Survey was used to measure health-related quality of life, and yields a mental composite score (MCS) and physical composite score (PCS) (18).
Social support
Social support were assessed using the MOS Social Support Survey (19). The overall social support score, calculated as a continuous variable, ranged from 0–100% with higher scores indicative of more social support.
Outcome Measures
Execution of PEG and RBV Dosing
Dosing of weekly PEG and daily RBV was measured by the Medication Event Management System (MEMS). MEMS uses a computer chip in the cap of a medication vial that records the precise date and time the vial was opened, and presumably, when the drug was taken. Study participants were instructed in proper use of the MEMs caps before and during each clinic visit. MEMS caps provide a highly accurate, reliable and objective measure of drug-taking behavior when used correctly(20). A MEMS cap was placed on the RBV vial and five PEG syringes were placed inside a large opaque prescription bottle that accommodated the MEMS cap. MEMs data from computer chips were downloaded at each study visit.
Using the MEMs data, execution of dosing was defined as the number of prescribed doses actually taken weekly for PEG (0,1) and daily for RBV (0,1,2). Patients were expected to inject PEG once every 7 days; for these analyses, PEG needed to occur 6–8 days from the previous PEG dose to be coded as 1 (i.e. “adherent). Participants were expected to ingest the prescribed dose of RBV twice within a 24 hour window; for these analyses, RBV dosing had to occur twice within each 24-hour period (3a.m. to 3a.m.) to be coded as 2 (i.e. “fully adherent”) or once in 24 hours to be coded as 1 (i.e. “partially adherent”) for that day. If a subject failed to open the MEMs cap during these prespecified windows, they were coded as 0 (i.e. nonadherent). When a subject was instructed to dose reduce to 0 for either PEG or RBV, and did not open the MEMS cap during this time, s/he was considered fully adherent. Time 0 (first day/opening) was excluded for all subjects because this generally occurred during training in MEMS cap use.
Reasons for Missed Doses
If patients endorsed missing any of their medications in the 4 weeks preceding a study visit, they were administered an adapted 14-item Barriers to Adherence questionnaire (21, 22). For each reason, patients indicated how often they missed taking their study medications on a 4-point Likert scale (Never, Rarely, Sometimes, Often).
Nonpersistence
Nonpersistence was defined as time to patient-driven premature study or medication discontinuation at any time during the study, excluding nonresponse. Nonpersistent events included: drop out; intolerance to side effects; patient preference; nonadherence to study protocol; patient withdrawal; loss to follow-up or refusal. Serious adverse events and deaths were censored events since these were considered out of the patients’ control. For the 0–24 week analyses, all patients who persisted to week 24 were censored at day 168. For TW24 Responders, the clock was reset to 0, and nonpersistence was analyzed from week 25 to 48.
Analytic and Statistical Plan
Dosing execution was summarized weekly for RBV and in 4 week blocks for PEG by calculating the proportion of doses taken (number of doses actually taken divided by the expected number of doses to be taken). Data are presented along with 95% confidence intervals. Proportions and frequencies are used to describe reasons for missed doses and nonpersistence over the 48 week treatment period. We employed generalized estimating equations to model poor execution of dosing (“missed doses”) over time using type 3 score statistics to calculate p-values. The multinomial distribution with the cumlogit link function was used for daily RBV to model the probabilities of levels of n having lower order values (i.e. comparison of fully adherent, n=2, to partially adherent or nonadherent (n=1 and 0), and partially adherent (n=1) compared to nonadherent (n=0)). The logit link function was used for weekly PEG. We explored the bivariate association between execution of dosing and each of the baseline sociodemographic, clinical and psychosocial characteristics. Any relationships significant at the 0.20 level were included in a multivariate model. To avoid potential multicollinearity issues, the VAS-ALL and VAS-DEP were excluded from the initial multivariate model due to moderate to strong correlations with other VASs and baseline CES-D, respectively. Effects significant in the multivariate model at the 0.05 level after backwards selection were retained in the final model. Models were run on the full sample (N=401) for treatment week 0 to 24. Conditional on being a TW24 Responder and continuing on treatment, models were repeated for weeks 24 to 48 (N=242). Baseline CES-D was excluded from the initial multivariate model due to its high correlation with VAS-DEP and its bivariate association with missing PEG doses not being as strong.
Patient-driven nonpersistence
was analyzed as time to event data. Kaplan Meier curves were used to plot the raw data. Cox proportional hazards models were used to explore variables associated with nonpersistence: (1) from baseline to week 24; and (2) conditional on being a TW24 Responder, from week 24–48, with the clock reset to time 0. Characteristics significant at the 0.20 level were included in multivariate models. Variables significant at the 0.05 level after backwards selection were retained in the final model. Owing to lack of power due to a small number of nonpersistent events from weeks 24–48, multivariate analyses were limited to weeks 0–24 only. Analyses were conducted using SAS v9.2 (Cary, NC).
RESULTS
Missed Doses
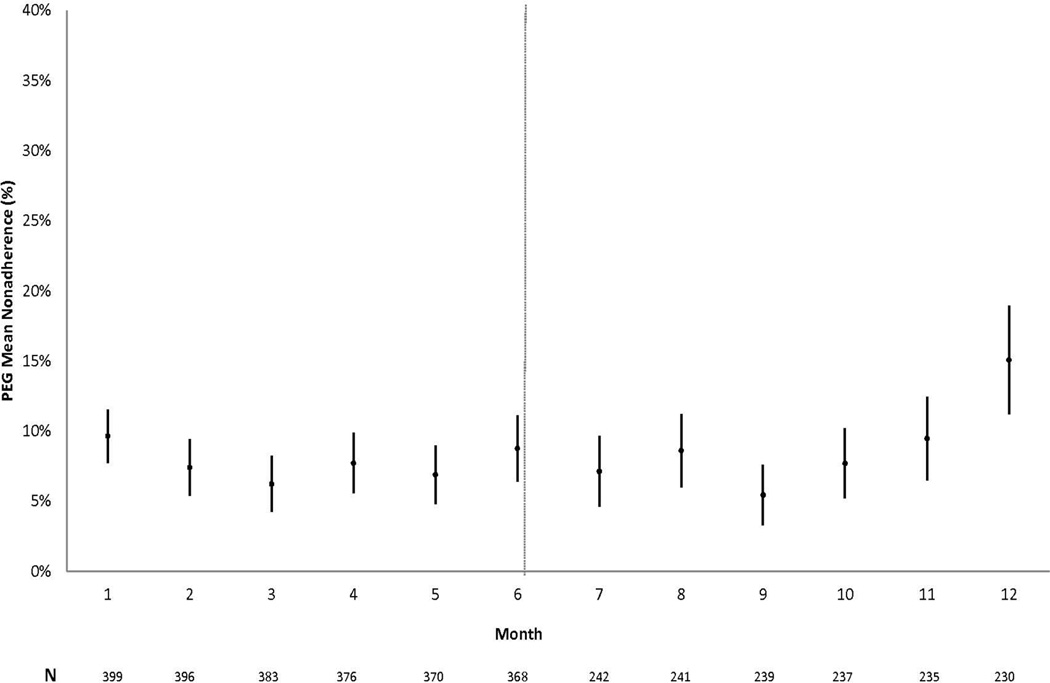
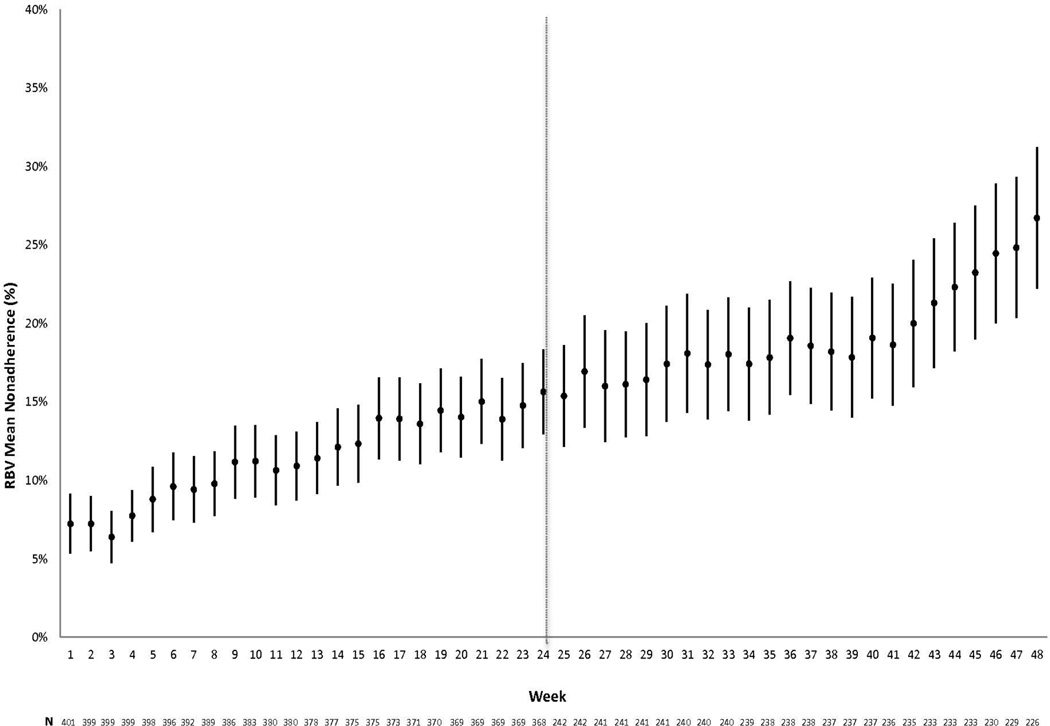
Patient characteristics for the 401 Virahep-C participants are described elsewhere (12). The panel in Figure 1 shows the average proportions of missed doses plotted over the course of the 48 week treatment period, in 4 week blocks for PEG (Figure 1A) and weekly for RBV (Figure 1B). The proportion of missed doses of PEG at each week was low, remaining below 10% throughout the study, with the exception of the last month. In contrast, there was a steady increase in missing doses of RBV over time, with approximately 15% of doses missed by patients at week 24, and 25% of doses missed by week 48.
Figure 1.
a: Average proportion of missed PEG doses over the course of 48 weeks. Each month shown on the X-axis is the proportion of missed doses for the preceding 4 weeks. PEG dosing needed to occur 6–8 days after the previous dose to be coded as adherent for each week. Sample sizes at each time frame are indicated below.
b: Average proportion of missed RBV doses over the course of 48 weeks, shown in weekly intervals. For each of the preceding 7 days that comprised each week on the X-axis, RBV dosing had to occur twice within each 24-hour day to be coded as fully adherent or once in 24 hours to be coded as partially adherent for that day. Sample sizes are indicated below.
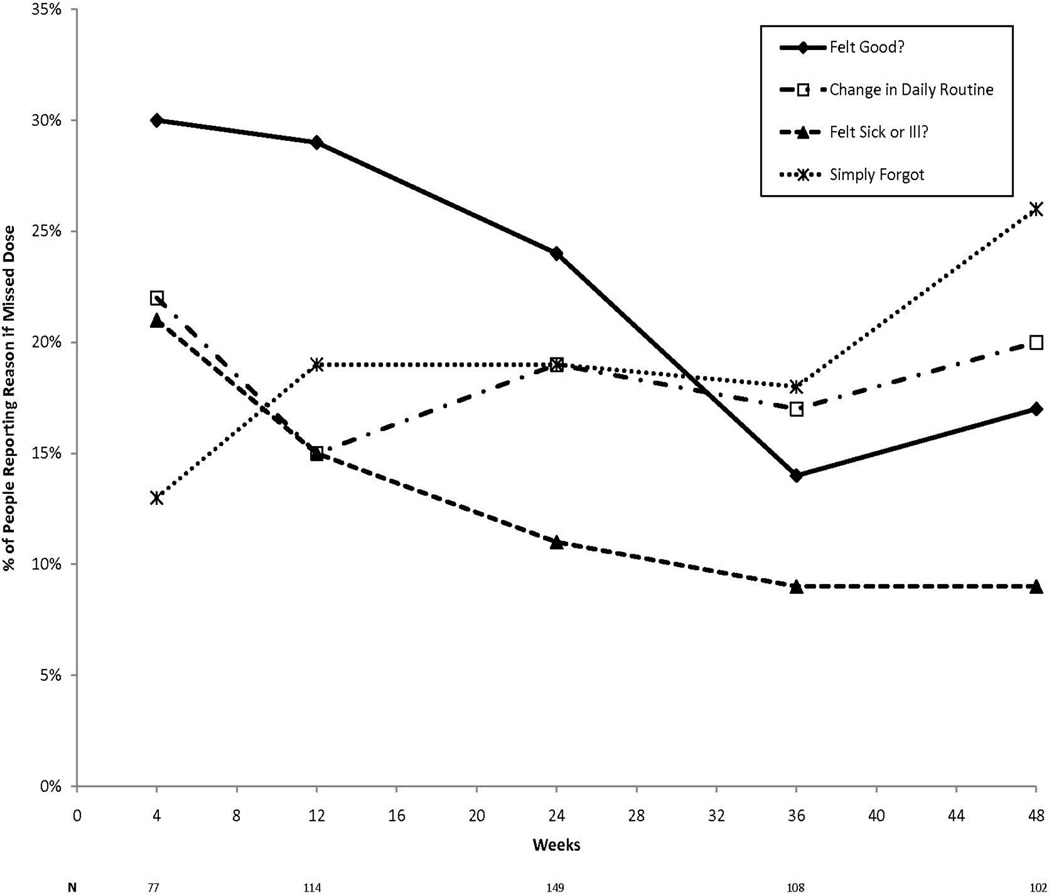
For those who reported missing doses, the most common reasons endorsed by more than 20% of respondents at any time point included: feeling good; change in daily routine; felt sick or ill; and simply forgot (Figure 2). The reason for missing medications that received the highest response rate during the first 24 weeks was “feeling good" (average=28%), and among TW24 Responders, the reason with the highest response rate from week 24–48 was because they “simply forgot” (21%).
Figure 2.
Graph shows the most common reasons for missed doses of PEG or RBV (i.e., endorsed by at least 20% of participants at any one time point) by participants who reported missing any doses in preceding 4 weeks.
Patient Characteristics Associated with Missing PEG and RBV Doses
Baseline to Week 24
Patient characteristics predictive of longitudinally missing doses of weekly PEG at p<0.20 are shown in bivariate analyses displayed in Table 1. The final multivariate model, after backwards selection, included: age; race; employment status; and marital status. Participants who were younger, African-American (AA), unemployed, or unmarried (i.e., not in a relationship) had higher odds of missing their weekly dose of PEG. For continuous variables such as age, the unit change represented by the odds ratio is given; for example, for every 5 year decrease in age, the odds of missing PEG increased by 24% in the multivariate model (OR=0.76).
Table 1.
Bivariate and Multivariate Models of Patient Characteristics Associated with Missing Doses of PEG
| Weeks 0–24 (N=401) | Weeks 24–48 (N=242) | |||||
|---|---|---|---|---|---|---|
| Bivariate Associationsa | Final Multivariatea | Bivariate Associationsa | ||||
| OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | |
| Age Δ=5yrc | 0.85 (0.74, 0.98) | 0.046 | 0.76 (0.66, 0.88) | 0.003 | 0.91 (0.80, 1.03) | 0.126 |
| Gender | 0.878 | -- | -- | 0.694 | ||
| Male | 1.00 (Ref) | 1.00 (Ref) | ||||
| Female | 1.03 (0.68, 1.58) | 0.91 (0.58, 1.43) | ||||
| Race | 0.003 | <0.001 | 0.669 | |||
| Caucasian | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||
| African American | 1.88 (1.25, 2.83) | 2.22 (1.51, 3.27) | 1.10 (0.71, 1.70) | |||
| HX Antidepressant Use | 0.508 | -- | -- | 0.232 | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes | 1.28 (0.66, 2.50) | 1.42 (0.80, 2.53) | ||||
| Fibrosis Score | 0.793 | -- | -- | 0.894 | ||
| 0–3 | 1.00 (Ref) | 1.00 (Ref) | ||||
| 4–6 (Severe) | 1.07 (0.63, 1.82) | 1.06 (0.45, 2.49) | ||||
| Social Support Δ=10% | 0.93 (0.83, 1.05) | 0.272 | -- | -- | 0.96 (0.84, 1.09) | 0.541 |
| Source of Infection | 0.371 | -- | -- | 0.718 | ||
| Other | 1.00 (Ref) | 1.00 (Ref) | ||||
| Illicit Drug Use | 1.73 (0.82, 3.66) | 0.74 (0.35, 1.59) | ||||
| Incidental Exposure | 1.69 (0.79, 3.62) | 0.79 (0.33, 1.87) | ||||
| Unknown | 2.01 (0.82, 4.91) | 1.13 (0.42, 3.01) | ||||
| Education | 0.237 | -- | -- | 0.556 | ||
| ≥ High School | 1.00 (Ref) | 1.00 (Ref) | ||||
| < High School | 1.36 (0.85, 2.19) | 1.19 (0.68, 2.08) | ||||
| Insurance | <0.001 | -- | -- | 0.083 | ||
| Private | 1.00 (Ref) | 1.00 (Ref) | ||||
| Public | 2.78 (1.80, 4.29) | 1.73 (1.05, 2.85) | ||||
| Uninsured | 2.10 (1.14, 3.87) | 1.63 (0.89, 2.97) | ||||
| Employment | <0.001 | <0.001 | 0.788 | |||
| Employed | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||
| Unemployed | 2.53 (1.69, 3.78) | 2.57 (1.68, 3.92) | 1.07 (0.67, 1.71) | |||
| Marital Status | 0.003 | 0.029 | 0.485 | |||
| Married/Relationship | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||
| Never Married | 2.21 (1.30, 3.77) | 1.46 (0.89, 2.42) | 1.20 (0.74, 1.95) | |||
| Divorced/Widow/Separated | 2.07 (1.32, 3.24) | 2.00 (1.23, 3.28) | 1.36 (0.82, 2.27) | |||
| Alcohol Consumption | 0.374 | -- | -- | 0.868 | ||
| ≤2 drinks/week | 1.00 (Ref) | 1.00 (Ref) | ||||
| >2 drinks/week | 0.79 (0.46, 1.36) | 0.96 (0.57, 1.61) | ||||
| Baseline CES-DΔ=5pt | 1.15 (1.04, 1.28) | 0.022 | -- | -- | 1.12 (1.01, 1.24) | 0.076 |
| Current Smoker | 0.143 | -- | -- | 0.694 | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes | 1.42 (0.89, 2.28) | 1.11 (0.67, 1.83) | ||||
| Baseline PCSΔ=10pt | 0.78 (0.66, 0.94) | 0.016 | -- | -- | 0.86 (0.67, 1.10) | 0.266 |
| Baseline MCSΔ=10pt | 0.92 (0.73, 1.15) | 0.463 | -- | -- | 0.95 (0.75, 1.21) | 0.708 |
| Baseline VAS-DEPΔ=1ptc | 1.08 (1.01, 1.16) | 0.045 | -- | -- | 1.09 (1.02, 1.17) | 0.048* |
| Baseline VAS-FATΔ=1pt | 1.08 (0.999, 1.17) | 0.069 | -- | -- | 1.03 (0.95, 1.12) | 0.479 |
| Baseline VAS-HEADΔ=1pt | 1.12 (1.03, 1.22) | 0.03 | -- | -- | 1.03 (0.94, 1.13) | 0.527 |
| Baseline VAS-IRRITΔ=1pt | 1.10 (1.02, 1.19) | 0.031 | -- | -- | 1.07 (0.997, 1.15) | 0.096 |
| Baseline VAS-ACHEΔ=1pt | 1.06 (0.99, 1.13) | 0.121 | -- | -- | 1.01 (0.94, 1.08) | 0.869 |
| Baseline VAS-ALLΔ=1pt | 1.07 (0.99, 1.17) | 0.14 | -- | -- | 1.05 (0.97, 1.14) | 0.246 |
| Baseline # of MedsΔ=1Med | 1.02 (0.90, 1.16) | 0.768 | -- | -- | 1.02 (0.87, 1.19) | 0.845 |
CES-D=Center for Epidemiologic Studies-Depression; PCS=Physical Composite Score; MCS=Mental Composite Score; VAS-DEP = Visual Analog Scale (VAS) for Depression; VAS-FAT=VAS for Fatigue; VAS-HEAD=VAS for Headache; VAS-IRRIT=VAS for Irritability; VAS-ACHE=VAS for Muscle/joint aches; VAS-ALL= VAS for overall symptoms
Modeling probability of missing doses of PEG
Calculated using Score Statistics for Type 3 GEE
For every 5 year decrease in age, the odds of missing PEG significantly increased by 15% and 24%, respectively. For every 1 point increase on the VAS depression scale (range 0–10), the odds of missing PEG increased 8% and 9%, respectively.
Only VAS-DEP significant at p<.05 in multivariate models
Bivariate associations with missing doses of RBV at p<0.20 are shown in Table 2. In the final multivariate model, after backwards selection, participants had higher odds of missing more daily doses of RBV if they were younger and AA.
Table 2.
Bivariate and Multivariate Models of Patient Characteristics Associated with Missing Doses of RBV
| Weeks 0–24 (N=401) | Weeks 24–48 (N=242) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate Associationsa | Final Multivariatea | Bivariate Associationsa | Final Multivariatea | |||||
| OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | OR (95% CI) | pb | |
| AgeΔ=5yrc | 0.82 (0.75, 0.91) | <0.001 | 0.80 (0.72, 0.88) | <0.001 | 0.89 (0.79, 1.005) | 0.069 | -- | -- |
| Gender | 0.471 | -- | -- | 0.844 | -- | -- | ||
| Male | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Female | 0.89 (0.64, 1.23) | 1.04 (0.69, 1.56) | ||||||
| Race | <0.001 | <0.001 | 0.117 | -- | -- | |||
| Caucasian | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| African American | 1.83 (1.34, 2.50) | 2.01 (1.48, 2.73) | 1.38 (0.93, 2.06) | |||||
| HX Antidepressant Use | 0.400 | -- | -- | 0.627 | -- | -- | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Yes | 1.22 (0.79, 1.88) | 1.16 (0.66, 2.01) | ||||||
| Fibrosis Score | 0.112 | -- | -- | 0.131 | -- | -- | ||
| 0–3 | 1.00 (Ref) | 1.00 (Ref) | ||||||
| 4–6 (Severe) | 0.74 (0.50, 1.10) | 0.64 (0.34, 1.22) | ||||||
| Social Support Δ=10% | 0.98 (0.91, 1.05) | 0.517 | -- | -- | 0.98 (0.89, 1.08) | 0.754 | -- | -- |
| Source of Infection | 0.321 | -- | -- | 0.583 | -- | -- | ||
| Other | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Illicit Drug Use | 0.95 (0.53, 1.71) | 0.59 (0.25, 1.41) | ||||||
| Incidental Exposure | 0.82 (0.44, 1.54) | 0.72 (0.28, 1.82) | ||||||
| Unknown | 0.62 (0.31, 1.27) | 0.52 (0.19, 1.44) | ||||||
| Education | 0.445 | -- | -- | 0.420 | -- | -- | ||
| ≥ High School | 1.00 (Ref) | 1.00 (Ref) | ||||||
| < High School | 1.17 (0.79, 1.75) | 1.26 (0.74, 2.14) | ||||||
| Insurance | 0.108 | -- | -- | 0.183 | 0.027 | |||
| Private | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Public | 1.46 (1.03, 2.06) | 1.59 (0.98, 2.57) | 2.32 (1.33, 4.02) | |||||
| Uninsured | 1.01 (0.64, 1.59) | 0.99 (0.62, 1.58) | 1.23 (0.76, 1.97) | |||||
| Employment | 0.814 | -- | -- | 0.043 | 0.002 | |||
| Employed | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| Unemployed | 0.96 (0.70, 1.32) | 0.67 (0.45, 0.996) | 0.79 (0.30, 0.78) | |||||
| Marital Status | 0.046 | -- | -- | 0.406 | -- | -- | ||
| Married/Relationship | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Never Married | 1.82 (1.19, 2.78) | 1.40 (0.80, 2.46) | ||||||
| Divorced/Widow/Separated | 1.17 (0.82, 1.67) | 1.25 (0.82, 1.91) | ||||||
| Alcohol Consumption | 0.606 | -- | -- | 0.758 | -- | -- | ||
| ≤2 drinks/week | 1.00 (Ref) | 1.00 (Ref) | ||||||
| >2 drinks/week | 0.91 (0.63, 1.31) | 1.09 (0.65, 1.83) | ||||||
| Baseline CES-DΔ=5pt | 1.06 (0.97, 1.14) | 0.205 | -- | -- | 1.10 (0.998, 1.21) | 0.098 | -- | -- |
| Current Smoker | 0.015 | -- | -- | 0.093 | -- | -- | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||||
| Yes | 1.55 (1.09, 2.19) | 1.44 (0.94, 2.20) | ||||||
| Baseline PCSΔ=10pt | 1.01 (0.88, 1.15) | 0.915 | -- | -- | 0.99 (0.83, 1.20) | 0.952 | -- | -- |
| Baseline MCSΔ=10pt | 0.95 (0.82, 1.10) | 0.503 | -- | -- | 0.94 (0.78, 1.14) | 0.543 | -- | -- |
| Baseline VAS-DEPΔ=1pt | 1.02 (0.96, 1.08) | 0.504 | -- | -- | 1.02 (0.95, 1.10) | 0.616 | -- | -- |
| Baseline VAS-FATΔ=1pt | 1.005 (0.95, 1.06) | 0.857 | -- | -- | 1.03 (0.97, 1.11) | 0.343 | -- | -- |
| Baseline VAS-HEADΔ=1pt | 1.01 (0.95, 1.08) | 0.706 | -- | -- | 1.02 (0.94, 1.11) | 0.573 | -- | -- |
| Baseline VAS-IRRITΔ=1pt | 1.002 (0.94, 1.07) | 0.9475 | -- | -- | 1.02 (0.94, 1.11) | 0.637 | -- | -- |
| Baseline VAS-ACHEΔ=1pt | 1.01 (0.96, 1.07) | 0.648 | -- | -- | 1.002 (0.94, 1.07) | 0.951 | -- | -- |
| Baseline VAS-ALLΔ=1pt | 1.03 (0.97, 1.10) | 0.355 | -- | -- | 1.07 (0.99, 1.15) | 0.111 | -- | -- |
| Baseline # of Meds=1Med | 1.03 (0.93, 1.13) | 0.622 | -- | -- | 1.09 (0.95, 1.26) | 0.257 | -- | -- |
CES-D=Center for Epidemiologic Studies-Depression; PCS=Physical Composite Score; MCS=Mental Composite Score; VAS-DEP = Visual Analog Scale (VAS) for Depression; VAS-FAT=VAS for Fatigue; VAS-HEAD=VAS for Headache; VAS-IRRIT=VAS for Irritability; VAS-ACHE=VAS for Muscle/joint aches; VAS-ALL= VAS for overall symptoms
Modeling probabilities of levels of number of doses having higher values (i.e. being non-adherent compared to more adherent)
Calculated using Score Statistics for Type 3 GEE
For every 5 year decrease in age, the odds of missing PEG significantly increased by 18% and 20%, respectively.
Week 24 to 48
Conditional on being a TW24 Responder, bivariate associations were conducted and are presented in Table 1. Baseline CES-D was excluded from the initial multivariate model due to its high correlation with VAS-DEP and its bivariate association with missing PEG doses not being as strong. The only variable retained in the multivariate model was VAS-DEP (p=.048). Individuals with higher scores at baseline on the VAS-DEP were more likely to miss doses of weekly PEG, such that every 1 point increase on the 10-point depression scale increases the odds of missing PEG by 9%.
Conditional on being a TW24 Responder, bivariate associations with missed doses of RBV at p<0.20 are shown in Table 2. After backwards selection, insurance and employment status were significant at 0.05; those employed or with public or no insurance (compared to private) had greater odds of missing daily RBV doses.
Nonpersistence (i.e., Patient-DrivenTreatment Discontinuations
Of the 401 initial participants, 50 discontinued PEG for various reasons (39 due to nonpersistence; 8 due to serious adverse events; 3 due to death) during the first 24 weeks of treatment, resulting in the probability of persisting to 24 weeks of 90.2% (95% CI: 86.8%-92.7%) for PEG. The 39 patients who failed to persist for patient-driven reasons did so due to intolerance to the medication (41%; n=16); noncompliance with study protocol (28%; n=11); patient preference (28%; n=11); and other (3%; n=1). Almost identical numbers were observed for RBV (data not shown).
After Nonresponders were discontinued from therapy, an additional 16 TW24 Responders discontinued PEG/RBV (13 due to patient-driven nonpersistence; 3 due to serious adverse events) from week 24 to 48. This resulted in a conditional probability of persisting to week 48 of 94.5% (95% CI: 90.8%-96.8%) for PEG. The 13 Responders who failed to persist on treatment did so due to patient preference (46%; n=6); intolerance to medications (31%; n=4); and noncompliance with study protocol (23%; n=3).
Out of the 66 treatment discontinuations for all causes, 52 (78.8%) were considered patient-driven nonpersistence events that could be amenable to intervention.
Patient Characteristics Associated with Nonpersistence
Baseline to Week 24
Bivariate associations at p<0.20 with patient-driven nonpersistence up to week 24 are shown in Table 3. For reasons described above, baseline VAS-DEP and VAS-ALL were excluded from the initial multivariate model. The final model, after backwards selection, included: age; education; insurance; and baseline VAS-HEAD. Participants had a greater risk of being nonpersistent if they were younger, had less than a high school education, had public or no insurance and had worse headaches at baseline. Results for RBV nonpersistence were nearly identical since nonpersistence resulted in discontinuation of both study medications at approximately the same time. For every 5 year decrease in age, the risk of nonpersistence significantly increases by 24% and for every 1 point increase on the 10-point headache scale, the risk of nonpersistence increases 16%.
Table 3.
Characteristics Associated with PEG Nonpersistence
| Treatment Weeks 0–24 | Treatment Weeks 24–48 | |||||
|---|---|---|---|---|---|---|
| Bivariate | Final Multivariate | Bivariate | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age Δ=5 years a | 0.76 (0.63, 0.91) | 0.003 | 0.76 (0.62, 0.92) | 0.005 | 0.66 (0.48, 0.90) | 0.01 |
| Gender | 0.230 | -- | -- | 0.964 | ||
| Male | 1.00 (Ref) | 1.00 (Ref) | ||||
| Female | 1.47 (0.78, 2.78) | 1.03 (0.34, 3.14) | ||||
| Race | 0.191 | -- | -- | 0.071 | ||
| Caucasian | 1.00 (Ref) | 1.00 (Ref) | ||||
| African American | 1.53 (0.81, 2.90) | 2.80 (0.92, 8.56) | ||||
| HX Antidepressant Use | 0.578 | -- | -- | 0.308 | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes | 0.75 (0.27, 2.10) | 1.96 (0.54, 7.11) | ||||
| Fibrosis Score | 0.372 | -- | -- | -- | -- | |
| 0–3 | 1.00 (Ref) | |||||
| 4–6 (Severe) | 0.65 (0.26, 1.67) | |||||
| Social Support Δ=10% | 1.05 (0.89, 1.25) | 0.550 | -- | -- | 1.004 (0.76, 1.32) | 0.980 |
| Source of Infection | 0.510 | -- | -- | 0.640 | ||
| Other | 1.00 (Ref) | 1.00 (Ref) | ||||
| Illicit Drug Use | 1.00 (0.24, 4.22) | 0.37 (0.04, 3.18) | ||||
| Incidental Exposure | 0.51 (0.10, 2.51) | 0.77 (0.09, 6.57) | ||||
| Unknown | 0.97 (0.20, 4.79) | 0.62 (0.06, 6.81) | ||||
| Education | <0.001 | 0.034 | 0.973 | |||
| ≥ High School | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||
| < High School | 4.01 (2.11, 7.60) | 2.11 (1.06, 4.19) | 1.03 (0.23, 4.63) | |||
| Insurance | <0.001 | 0.002 | 0.577 | |||
| Private | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||
| Public | 7.05 (3.10, 16.00) | 4.77 (2.01, 11.30) | 0.94 (0.25, 3.46) | |||
| Uninsured | 4.35 (1.72, 11.01) | 2.51 (0.95, 6.63) | 0.33 (0.04, 2.62) | |||
| Employment | 0.003 | -- | -- | 0.400 | ||
| Employed | 1.00 (Ref) | 1.00 (Ref) | ||||
| Unemployed | 2.60 (1.37, 4.93) | 0.57 (0.16, 2.09) | ||||
| Marital Status | <0.001 | -- | -- | -- | -- | |
| Married/Relationship | 1.00 (Ref) | |||||
| Never Married | 4.87 (2.15, 11.03) | |||||
| Divorced/Widow/Separated | 2.29 (0.99, 5.29) | |||||
| Alcohol Consumption | 0.778 | -- | -- | 0.854 | ||
| ≤2 drinks/week | 1.00 (Ref) | 1.00 (Ref) | ||||
| >2 drinks/week | 1.12 (0.51, 2.43) | 0.87 (0.19, 3.92) | ||||
| Baseline CES-DΔ=5 pt | 1.25 (1.09, 1.43) | 0.002 | -- | -- | 1.23 (0.97, 1.55) | 0.089 |
| Current Smoker | 0.037 | -- | -- | 0.776 | ||
| No | 1.00 (Ref) | 1.00 (Ref) | ||||
| Yes | 2.25 (1.05, 4.80) | 0.84 (0.26, 2.76) | ||||
| Baseline PCSΔ=10 pt | 0.73 (0.57, 0.94) | 0.015 | -- | -- | 0.96 (0.59, 1.56) | 0.862 |
| Baseline MCSΔ=10 pt | 0.63 (0.48, 0.83) | 0.001 | -- | -- | 0.65 (0.41, 1.04) | 0.074 |
| Baseline VAS-DEPΔ=1 pt | 1.18 (1.07, 1.31) | <0.001 | -- | -- | 1.14 (0.95, 1.37) | 0.166 |
| Baseline VAS-FATΔ=1 pt | 1.12 (1.01, 1.25) | 0.037 | -- | -- | 1.17 (0.98, 1.41) | 0.090 |
| Baseline VAS-HEADΔ=1 pta | 1.21 (1.09, 1.34) | <0.001 | 1.16 (1.04, 1.29) | 0.008 | 1.04 (0.83, 1.30) | 0.742 |
| Baseline VAS-IRRITΔ=1 pt | 1.20 (1.07, 1.35) | <0.001 | -- | -- | 1.21 (1.00, 1.46) | 0.053 |
| Baseline VAS-ACHEΔ=1 pt | 1.12 (1.02, 1.23) | 0.022 | -- | -- | 1.10 (0.93, 1.30) | 0.269 |
| Baseline VAS-ALLΔ=1 pt | 1.12 (1.00, 1.26) | 0.048 | -- | -- | 1.07 (0.87, 1.31) | 0.508 |
| Baseline # of Meds Δ=1 med | 0.99 (0.80, 1.24) | 0.950 | -- | -- | 1.02 (0.69, 1.51) | 0.926 |
Table highlights bivariate and multivariate models of characteristics associated with PEG nonpersistence from baseline to week 24 and week 24–48, and bivariate models of characteristics associated with PEG nonpersistence from baseline to week 24 for treatment week 24 responders; AA= African American; CA=Caucasian American; SS=Social Support; CES-D=Center for Epidemiologic Studies-Depression; PCS=Physical Composite Score; MCS=Mental Composite Score; VAS-DEP = Visual Analog Scale (VAS) for Depression; VAS-FAT=VAS for Fatigue; VAS-HEAD=VAS for Headache; VAS-IRRIT=VAS for Irritability; VAS-ACHE=VAS for Muscle/joint aches; VAS-ALL= VAS for overall symptoms.
For every 5 year decrease in age, the risk of nonpersistence significantly increases by 24%, 24%, and 34%, respectively. For every 1 point increase in the VAS-HEAD scale (range 0–10), the risk of nonpersistence increases 16%.
Week 24 to 48
Characteristics found to be associated at p<0.20 with PEG nonpersistence in TW24 Responders included: age; race; CES-D; MCS; VAS-DEP; VAS-FAT; and VAS-IRRIT (Table 3). Multivariate analyses were not conducted due to the small number of nonpersistent events from 24–48 weeks.
DISCUSSION
Triple therapy will bring new and even more challenging barriers to adherence to HCV treatment regimens than seen during dual therapy. The current study expands the knowledge base of adherence-related processes by a) measuring and analyzing missing doses and nonpersistence (i.e., patient-driven discontinuations) as separate processes; b) using a large sample size (n=401); c) utilizing MEMs caps, a highly objective, state-of-the-art electronic monitoring technology to investigate daily dosing; and d) identifying several baseline patient characteristics associated with missing doses and nonpersistence.
While weekly dosing of PEG injections was excellent (90%) in this study, patients demonstrated more difficulty adhering to twice daily dosed RBV. Despite intensive adherence interventions delivered throughout the study, 7% of RBV doses were missed in the first week of treatment, 15% at week 24, and 25% at week 48. Using MEMS allowed us to capture a much higher rate of missed RBV doses than has been previously reported by studies that relied upon self-report which tends to underestimate missed doses (4, 10, 11, 23). These data are concerning given that RBV adherence during the first 24 weeks of antiviral therapy is critical to viral response (2, 24).
This is the first study to provide insight into why HCV patients miss taking medications. These data demonstrate that patients miss medications during the first 24 weeks primarily because they “feel good.” This seems to reflect a deliberate decision on behalf of patient to consciously refrain from taking their medications when they feel good, and may be surprising to clinicians who assume that missed doses occur most often due to forgetfulness. Clinicians should be aware of this phenomenon particularly during the early phase of treatment. These data highlight the changing etiology of missing doses from early to late treatment phases, and suggests that drastically different dialog during patient-provider interactions may be needed to optimize dose-taking behaviors.
Overall the proportion of patients persisting on treatment, censoring medically-necessary discontinuations, was quite good. A total of 66 (16.5%) patients were discontinued from treatment prematurely, 52 (79%) of whom did so due to patient-driven nonpersistence. Most patients discontinued treatment due to intolerance of treatment-related side effects, while others failed to persist due to noncompliance with the study protocol or patient preference. These results have implications for the development of pharmacological and nonpharmacological interventions to manage side effects. For example, efficacious cognitive and behavioral interventions that help HIV and cancer patients cope with treatment-related side effects may provide coping skills to help HCV patients tolerate unpleasant side effects and persist on therapy (25, 26).
We identified several characteristics of patients for whom additional support will be needed. Patients who were younger, African-American, unemployed, or those not in a relationship were at greater risk for missing PEG and/or RBV during the first 24 weeks. Additionally, younger, less educated patients, with public or no insurance, and more headaches before treatment were less likely to persist to week 24. The association between lower educational status and nonpersistence is consistent with a recent study in HCV+ drug users (4). Some of the characteristics associated with missed doses and nonpersistence are perceived of as social determinants of health by the Centers for Disease Control (i.e., insurance coverage; employment; education; access to resources), which have implications for health policy (27). Clinicians ought to be aware of these correlates of treatment adherence, and future research could examine more intensive counseling and education, case management, or multidisciplinary models to improve treatment adherence in at-risk groups.
Historically, there has been reluctance to treat HCV patients with premorbid psychological disorders owing to safety and adherence concerns (28, 29). However, studies showing an association between psychological disturbances and premature treatment discontinuations are equivocal; PEG/RBV registration trials found that depression is a common cause of all-cause discontinuations (30), while other studies have not (31). Moreover, the few studies that have investigated the impact of psychological disturbances on missed doses have found no associations (4, 13, 32). The current study demonstrates that this controversial topic is even more complex than previously recognized. We found that baseline depressive symptoms were not associated with missing PEG or RBV or failing to persist during the first 24 weeks, but did predict missing PEG doses from weeks 24 to 48.
Perhaps equally important are the factors that did not engender risk for missed doses or nonpersistence in this study. No statistically significant gender differences were found for execution of dosing or persistence. Clinical characteristics such as fibrosis level, history of drug use, other medications, alcohol use at screening, smoking status, antidepressants, social support, and quality of life, were not associated with execution of dosing or persistence in final multivariate models. With regard to symptomatology measured via visual analog scales, baseline irritability, fatigue, and muscle/joint pain were not threats to dosing or persistence. Notably, these conclusions need to be interpreted with caution since study methodology (e.g., exclusion criteria), sample size, and lack of power could have precluded statistically significant associations from being found.
A few limitations are noted. These findings may not generalize to dissimilar patient populations (e.g., patients with active drug or alcohol use, comorbid psychiatric illness) or clinical settings (e.g., non-research clinical practice). In fact, it is likely that these results reflect the best possible adherence rates for HCV treatment given the sample characteristics and intensive adherence strategies employed. Clinicians should anticipate even higher rates of missed doses and nonpersistence under less favorable conditions, and during triple therapy which requires dosing 3–4 times a day, which has an expected dosing rate of only 51–65% (3). Another limitation includes low power to detect significant associations in the survival models for nonpersistence, owing to the small number of events. Finally, MEMs caps ascertain when the medication vials are opened, but not whether the medication was actually taken. Despite this limitation, the use of MEMs caps was a significant strength of the study, overcoming less objective adherence measurements like self-report, which are subject to both recall and reporting bias.
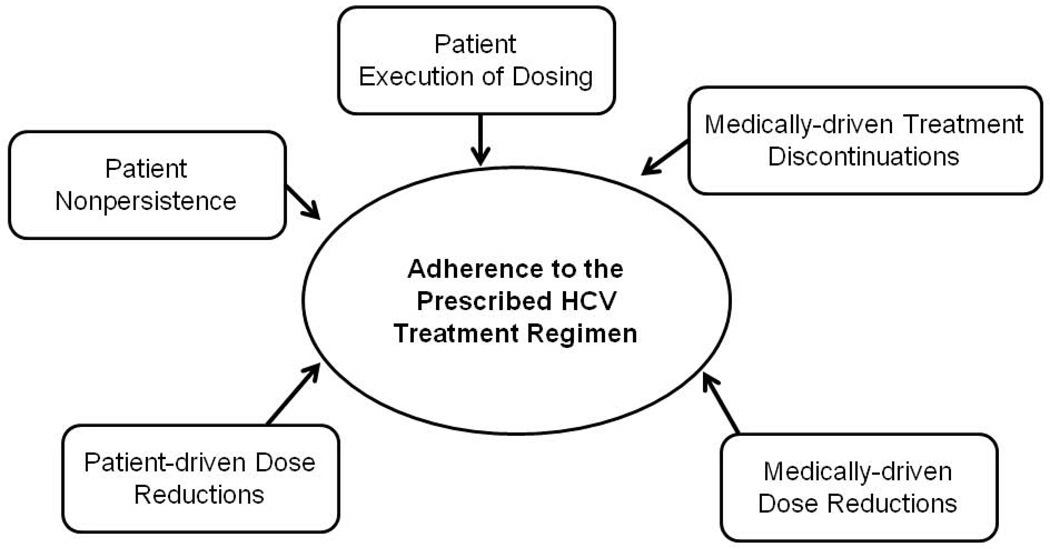
As previously stated, future research should aim to differentiate between medically-driven and patient-driven processes that affect adherence to treatment protocols (see Figure 4). We found that the majority of treatment discontinuations (79%) result from patient-driven reasons, thereby providing support for properly referring to this process as “nonpersistence” in order to better align with the broader adherence literature. Medically-driven and patient-driven discontinuations should be further distinguished from poor execution of dosing and not combined into one overall adherence variable. Investigations of adherence to HCV treatment will move the field forward more rapidly if these processes are measured and analyzed separately, as they are related to different patient characteristics, may have different clinical consequences, and will require markedly different interventions. The consequences of nonpersistence and missed doses on viral mutation and treatment response need to be evaluated. Electronic monitoring devices or other objective measures should be used to prospectively measure dosing execution in studies where adherence is a primary outcome. Finally, future studies need to examine the proportion of missed doses during triple therapy regimens, and ideally would include assessment of dose-timing intervals (i.e., every 8 hours) as dose-timing errors have been associated with viral resistance and worse treatment outcomes in other treatment populations (33).
While the current study was conducted using data collected during the era of dual therapy, these findings have implications for more complex therapeutic regimens, including interventions that may optimize execution of dosing and persistence. To improve execution of dosing, behavioral reminder systems and educational and counseling intervention, which have been efficacious in HIV adherence research, should be examined (34). Innovative technology and adherence intervention delivery systems, such as smart-phone technology, interactive voice response technology, and smart phone video-capture of medication taking may be useful strategies to investigate (35, 36). In contrast, failing to persist on treatment could be mitigated through more extensive pre-treatment education, case management, adjunct pharmacotherapy, and cognitive and behavioral interventions that have proven efficacious in HIV and cancer care (37, 38).
Figure 3.
Conceptual model of HCV adherence-related processes, which differentiates between various medically-driven and patient-driven factors that can each attenuate overall adherence to a prescribed HCV treatment regimen.
Acknowledgements
The VIRAHEP-C study was conducted by multiple investigators and supported by the National Institute of Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with the investigators of Virahep-C and does not necessarily reflect the opinions or views of the Virahep-C study or the NIDDK. The authors would like to thank the Virahep-C investigators, coordinators, and study participants who contributed to Virahep-C.
Declaration of Funding Interests: This study was supported, in part, by National Institutes of Health Award Number K23DK089004-02 (Evon); the UNC Clinical and Translational Science Award (UL1RR025747; Esserman, Bonner, & Rao); a mentoring grant (K24 DK066144-01; Fried), and a Center for Aids Research grant (P30-AI50410; Golin).
Footnotes
Statement of Interests
Declaration of Personal Interests: Donna M. Evon served as an ad hoc consultant to Vertex in the past 12 months. Michael W. Fried receives research grant funding from Genetech, Vertex, Tibotec, Janssen, Gilead, Abbott, BristolMyers Squibb, and Anadys. Dr. Fried also serves as ad hoc consultant to Genetech/Roche, Vertex, Merck, Tibotec, Janssen, BristolMyers Squibb, Novartis, and Gilead. All other authors have nothing to disclose.
Specific Author Contributions: Drs. Evon, Bonner, Fried, and Golin were responsible for conceptualizing, writing, and editing of the manuscript. Dr. Esserman and Ms. Rao were responsible for data analysis, writing and editing.
Reference List
- 1.McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastro. 2002 Oct;123(4):1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 2.Lo Re V, III, Amorosa VK, Localio AR, O'Flynn R, Teal V, Dorey-Stein Z, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin Infect Dis. 2009 Jan 15;48(2):186–193. doi: 10.1086/595685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001 Aug;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 4.Grebely J, Matthews GV, Hellard M, Shaw D, van B, I, Petoumenos K, et al. Adherence to treatment for recently acquired hepatitis C virus (HCV) infection among injecting drug users. J Hepatol. 2011 Jul;55(1):76–85. doi: 10.1016/j.jhep.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008 Jan;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Urquhart J, Vrijens B. New findings about patient adherence to prescribed drug dosing regimens: An introduction to pharmionics. Euro J Hosp Pharm Sci. 2005;11(5):103–106. [Google Scholar]
- 7.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008 May 17;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam I, Stainbrook T, Cecil B, Kistler KD. Enhanced adherence to HCV therapy with higher dose ribavirin formulation: final analyses from the ADHERE registry. Aliment Pharmacol Ther. 2010 Aug;32(4):535–542. doi: 10.1111/j.1365-2036.2010.04381.x. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub P, Ouzan D, Melin P, Lang JP, Rotily M, Fontanges T, et al. Patient education improves adherence to peg-interferon and ribavirin in chronic genotype 2 or 3 hepatitis C virus infection: a prospective, real-life, observational study. World J Gastroenterol. 2008 Oct 28;14(40):6195–6203. doi: 10.3748/wjg.14.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumaz CR, Munoz-Moreno JA, Ballesteros AL, Paredes R, Ferrer MJ, Salas A, et al. Influence of the type of pegylated interferon on the onset of depressive and neuropsychiatric symptoms in HIV-HCV coinfected patients. AIDS Care. 2007 Jan;19(1):138–145. doi: 10.1080/09540120600645539. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JJ, Bhatti L, Dieterich DT, Edlin BR, Fishbein DA, Goetz MB, et al. Hepatitis C patients' self-reported adherence to treatment with pegylated interferon and ribavirin. Aliment Pharmacol Ther. 2008 Aug 1;28(3):289–293. doi: 10.1111/j.1365-2036.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006 Aug;131(2):470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Lo Re V, III, Teal V, Localio AR, Amorosa VK, Kaplan DE, Gross R. Relationship between adherence to hepatitis C virus therapy and virologic outcomes: a cohort study. Ann Intern Med. 2011 Sep 20;155(6):353–360. doi: 10.1059/0003-4819-155-6-201109200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosworth HB, Oddone EZ, Weinberger M, editors. Patient Treatment Adherence: Concepts, Interventions, and Measurement. Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc.; 2006. [Google Scholar]
- 15.Weiss JJ, Brau N, Stivala A, Swan T, Fishbein D. Review article: adherence to medication for chronic hepatitis C - building on the model of human immunodeficiency virus antiretroviral adherence research. Aliment Pharmacol Ther. 2009 Jul;30(1):14–27. doi: 10.1111/j.1365-2036.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pherson-Baker S, Jones D, Duran RE, Klimas N, Schneiderman N. Development and implementation of a medication adherence training instrument for persons living with HIV: the MATI. Behav Modif. 2005 Mar;29(2):286–317. doi: 10.1177/0145445504272604. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1(3):385–401. [Google Scholar]
- 18.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 19.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001 May 15;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000 Jun;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 22.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003 Jun;50(2):187–199. doi: 10.1016/s0738-3991(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 23.Rodis JL, Kibbe P. Evaluation of medication adherence and quality of life in patients with hepatitis C virus receiving combination therapy. Gastroenterol Nurs. 2010 Sep;33(5):368–373. doi: 10.1097/SGA.0b013e3181f443cb. [DOI] [PubMed] [Google Scholar]
- 24.Hiramatsu N, Oze T, Yakushijin T, Inoue Y, Igura T, Mochizuki K, et al. Ribavirin dose reduction raises relapse rate dose-dependently in genotype 1 patients with hepatitis C responding to pegylated interferon alpha-2b plus ribavirin. J Viral Hepat. 2009 Aug;16(8):586–594. doi: 10.1111/j.1365-2893.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- 25.Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009 Jul;23(5):580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004 Sep 1;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan Ramirez LK, Baker EA, Metzler M. Promoting Health Equity: A Resource to Help Communities Address Social Determinants of Health. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 28.Evon DM, Verma A, Dougherty KA, Batey B, Russo M, Zacks S, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007 Nov;52(11):3251–3258. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 29.Muir AJ, Provenzale D. A descriptive evaluation of eligibility for therapy among veterans with chronic hepatitis C virus infection. J Clin Gastroenterol. 2002 Mar;34(3):268–271. doi: 10.1097/00004836-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002 Nov;36(5 Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 31.Schaefer M, Hinzpeter A, Mohmand A, Janssen G, Pich M, Schwaiger M, et al. Hepatitis C treatment in "difficult-to-treat" psychiatric patients with pegylated interferon-alpha and ribavirin: Response and psychiatric side effects. Hepatology. 2007 Aug 1; doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 32.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007 Sep;19(9):741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Miller LG, Golin CE, Hays RD, Wu T, Wenger NS, et al. Repeated measures analyses of dose timing of antiretroviral medication and its relationship to HIV virologic outcomes. Stat Med. 2007 Feb 28;26(5):991–1007. doi: 10.1002/sim.2592. [DOI] [PubMed] [Google Scholar]
- 34.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012 Mar 5; doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder KE, Johnson CJ, Wiebe JS. Interactive Voice Response Technology applied to sexual behavior self-reports: a comparison of three methods. AIDS Behav. 2007 Mar;11(2):313–323. doi: 10.1007/s10461-006-9145-z. [DOI] [PubMed] [Google Scholar]
- 36.Swendeman D, Rotheram-Borus MJ. Innovation in sexually transmitted disease and HIV prevention: internet and mobile phone delivery vehicles for global diffusion. Curr Opin Psychiatry. 2010 Mar;23(2):139–144. doi: 10.1097/YCO.0b013e328336656a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen BL, Farrar WB, Golden-Kreutz D, Emery CF, Glaser R, Crespin T, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007 Oct;21(7):953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003 Sep;6(3):173–188. doi: 10.1080/1025389031000156727. [DOI] [PubMed] [Google Scholar]