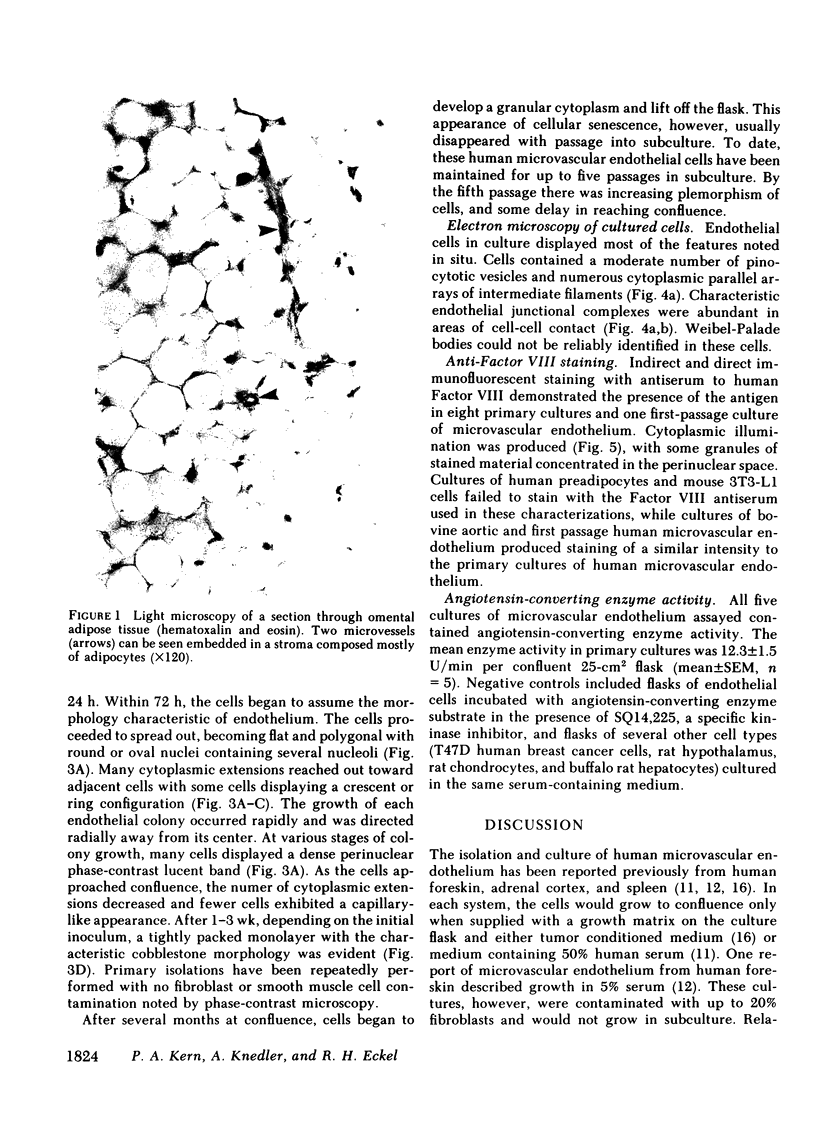
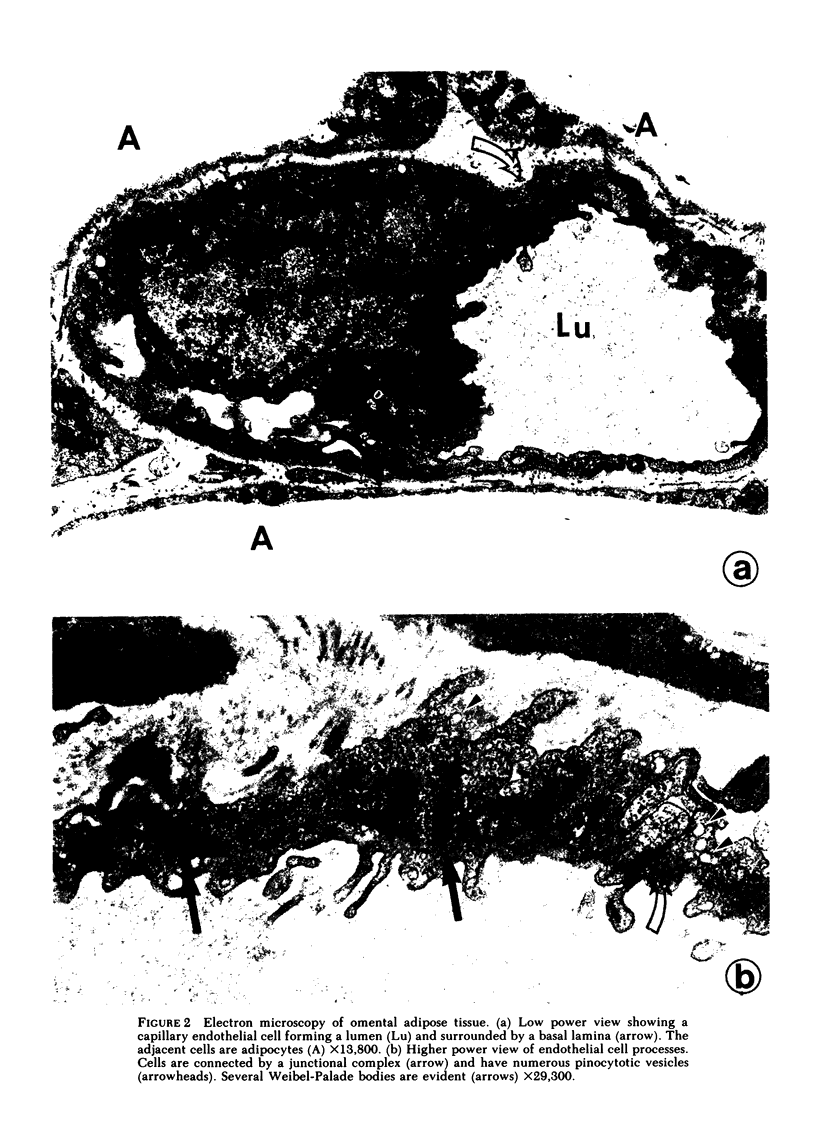
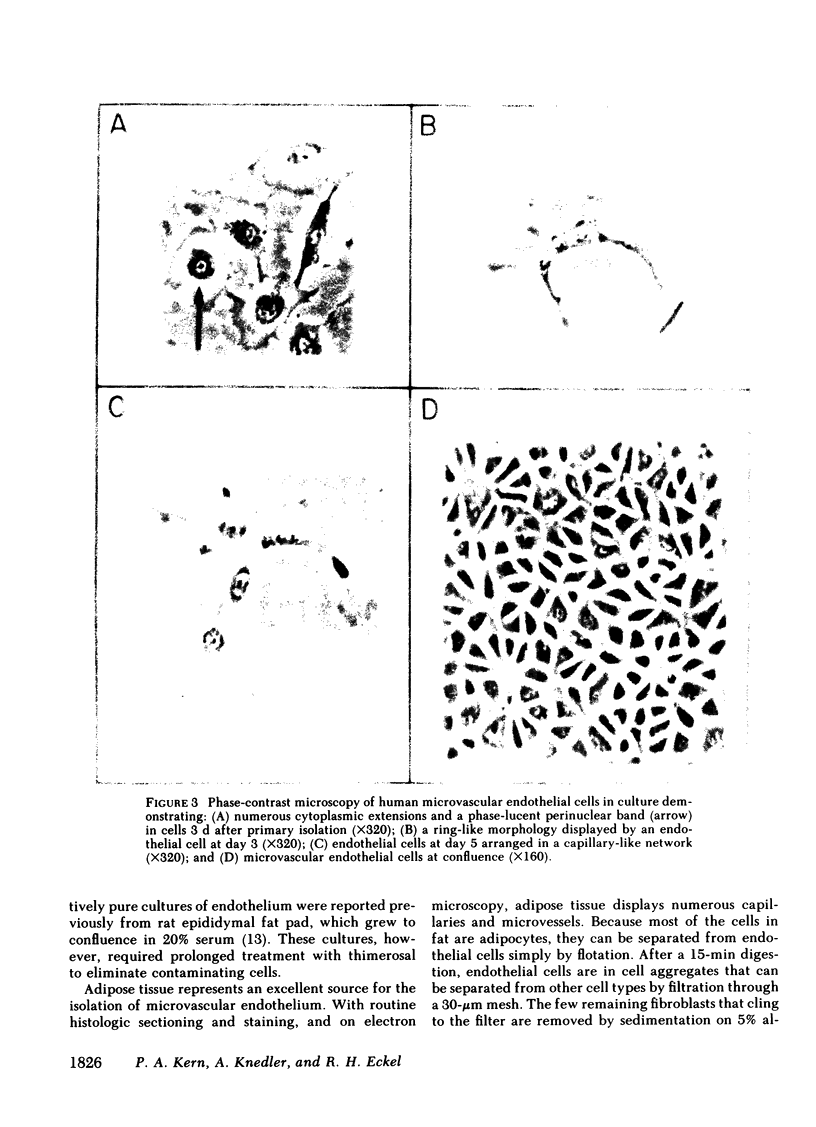
Abstract
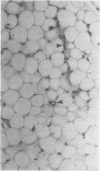
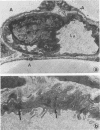
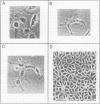
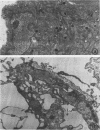
The study of human endothelial cells in tissue culture has been previously limited to umbilical vein, a large vessel source, and microvascular endothelium from human foreskin, spleen, and adrenal. Microvascular endothelium cultured from these sources have required matrix-coated culture flasks, tumor-conditioned medium, or 50% human serum for growth and subcultivation. To obtain cultures of microvascular endothelium with less stringent growth requirements, human adipose tissue was digested with collagenase and endothelial cells were separated from other stromal elements by sequential filtration and layering cells onto 5% albumin. Using standard medium containing 10% fetal calf serum, these cells grew readily to confluence and survived serial passages. When the cultures were subconfluent, cytoplasmic extensions and a capillary-like morphology were observed. Confluent cultures displayed the "cobblestone" appearance characteristic of other endothelial preparations. Electron microscopy demonstrated the presence of characteristic tight junctions and pinocytotic vesicles. Immunofluorescent staining for Factor VIII was positive, and cultures contained angiotensin-converting enzyme activity. Thus, cultures of human microvascular endothelium were readily obtained from adipose tissue and required only standard medium with 10% serum for growth and subcultivation. This system can be used to study human endothelial cell biology and may prove useful in the study of pathologic states such as diabetic microvasculopathy and tumor angiogenesis.
Full text
PDF

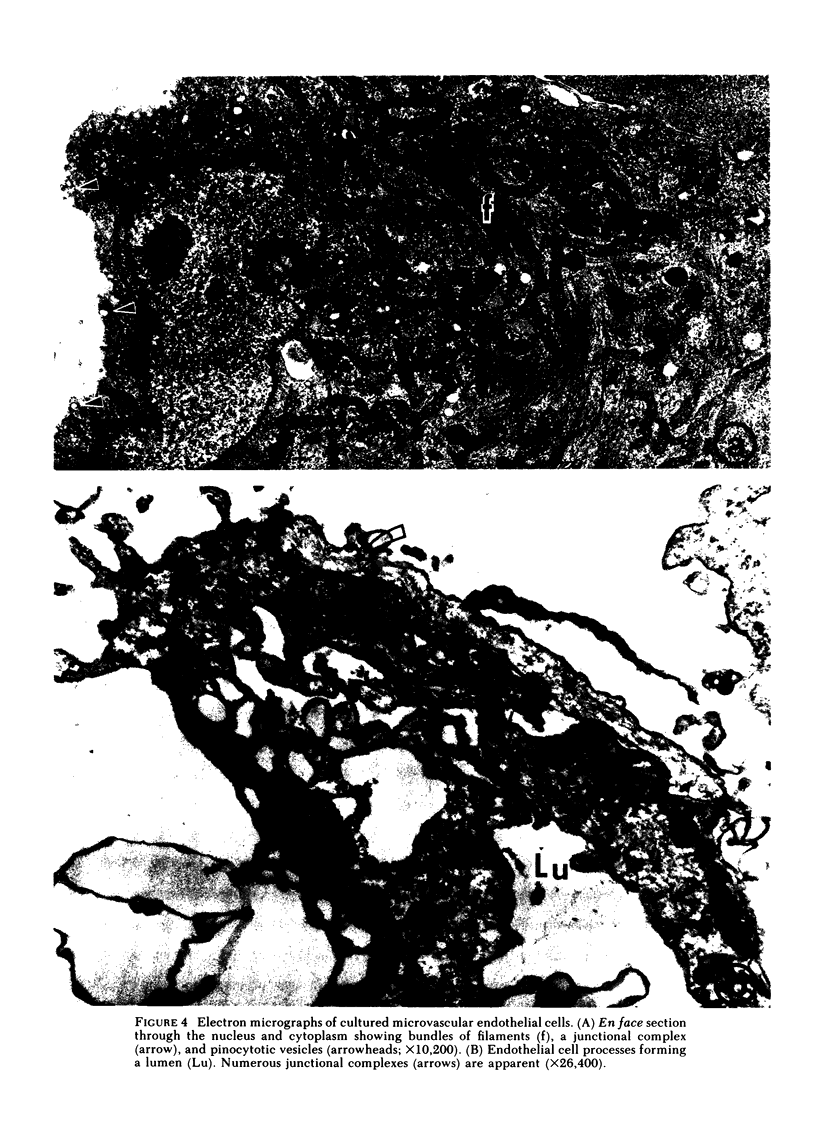


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björntorp P., Hansson G. K., Jonasson L., Pettersson P., Sypniewska G. Isolation and characterization of endothelial cells from the epididymal fat pad of the rat. J Lipid Res. 1983 Feb;24(2):105–112. [PubMed] [Google Scholar]
- Blose S. H., Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J Cell Biol. 1976 Aug;70(2 Pt 1):459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H., Shelanski M. L., Chacko S. Localization of bovine brain filament antibody on intermediate (100 A) filaments in guinea pig vascular endothelial cells and chick cardiac muscle cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):662–665. doi: 10.1073/pnas.74.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman P. D., Betz A. L., Ar D., Wolinsky J. S., Penney J. B., Shivers R. R., Goldstein G. W. Primary culture of capillary endothelium from rat brain. In Vitro. 1981 Apr;17(4):353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Buzney S. M., Massicotte S. J. Retinal vessels: proliferation of endothelium in vitro. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1191–1195. [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Davison P. M., Bensch K., Karasek M. A. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol. 1980 Oct;75(4):316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- Finley J. L., Clark R. A., Colvin R. B., Blackman R., Noe J., Rosen S. Immunofluorescent staining with antibodies to factor VIII, fibronectin, and collagenous basement membrane protein in normal human skin and port wine stains. Arch Dermatol. 1982 Dec;118(12):971–975. [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Heinemann H. O., Ryan J. W., Ryan U. S. Is the lung a para-endocrine organ? Am J Med. 1977 Oct;63(4):595–603. doi: 10.1016/0002-9343(77)90205-4. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttner I., Boutet M., More R. H. Studies on protein passage through arterial endothelium. I. Structural correlates of permeability in rat arterial endothelium. Lab Invest. 1973 Jun;28(6):672–677. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977 Apr 22;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Chung A., Martin L. C., Ryan U. S. New substrates for the radioassay of angiotensin converting enzyme of endothelial cells in culture. Tissue Cell. 1978;10(3):555–562. doi: 10.1016/s0040-8166(16)30348-2. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Sherer G. K., Fitzharris T. P., Faulk W. P., LeRoy E. C. Cultivation of microvascular endothelial cells from human preputial skin. In Vitro. 1980 Aug;16(8):675–684. doi: 10.1007/BF02619197. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N. Isolation and characterization of endothelial cells from the heart microvasculature. Microvasc Res. 1978 Nov;16(3):426–452. doi: 10.1016/0026-2862(78)90074-2. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y., Eisenberg S. A radioautographic study of the transport of 125 I-labeled serum lipoproteins in rat aorta. Z Zellforsch Mikrosk Anat. 1973 Mar 29;138(2):223–237. doi: 10.1007/BF00306609. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]
- Weinberg K. S., Douglas W. H., MacNamee D. R., Lanzillo J. J., Fanburg B. L. Angiotensin I-converting enzyme localization on cultured fibroblasts by immunofluorescence. In Vitro. 1982 Apr;18(4):400–406. doi: 10.1007/BF02796341. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kilo C. Vascular complications in diabetes mellitus. N Engl J Med. 1980 Feb 14;302(7):399–400. doi: 10.1056/NEJM198002143020710. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980 May 1;285(5759):41–43. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. The endothelial cells of large and small blood vessels. Diabetes. 1981;30(Suppl 2):24–28. doi: 10.2337/diab.30.2.s24. [DOI] [PubMed] [Google Scholar]