Abstract
Poly-unsaturated fatty acids (PUFAs) alter the function of many membrane proteins, whereas monounsatured fatty acids generally are inert. We previously showed that docosahexaenoic acid (DHA) at pH 7 decreases the bilayer stiffness, consistent with an amphiphile-induced increase in elasticity, but not with a negative change in curvature; oleic acid (OA) was inert (Bruno, Koeppe and Andersen, Proc. Natl. Acad. Sci. USA 104:9638–9643, 2007). To further explore how PUFAs and other amphiphiles may alter lipid bilayer properties, and thus membrane protein function, we examined how changes in acyl chain unsaturation, and head group charge and size, alter bilayer properties, as sensed by bilayer-spanning gramicidin A (gA) channels of different lengths. Compared to DHA, the neutral DHA-methyl ester has reduced effects on bilayer properties, and 1-palmitoyl-2-docosahexaenoyl-phosphatidylcholine (PDPC) forms bilayers that are softer than dioleoylphosphatidylcholine (DOPC). The changes in channel function are larger for the short gA channels, indicating that changes in elasticity dominate over changes in curvature. We altered the fatty acid protonation by titration: docosahexaenoic acid (DHA) is more potent at pH 9 (relative to pH 7) and is inert at pH 4; OA, which was inert at pH 7, becomes a potent modifier of bilayer properties at pH 9. At both pH 7 and 9, DHA and OA produced larger changes in the lifetimes of the short gA channels, demonstrating that they increase lipid bilayer elasticity when deprotonated—though OA promotes the formation of inverted hexagonal phases at pH 7. The positively charged oleylamine (OAm), which has a small head-group and therefore should be a negative curvature promoter, inhibited gA channel function, with similar reductions in the lifetimes of the short and long gA channels, indicating a curvature-dominated effect. Monitoring the single-channel conductance, we find that the negatively charged fatty acids increase the conductance by increasing the local negative charge around the channel, whereas the positively charged OAm has no effect. These results suggest that deprotonated fatty acids increase bilayer elasticity by reversibly adsorbing at the bilayer/solution interface.
INTRODUCTION
Fatty acids (FAs), including the poly-unsaturated fatty acids (PUFAs), are important components of fat and membrane phospholipids, and are as such involved in body energy metabolism and the regulation of many different cell function, for recent reviews see [1–3]. PUFAs, in particular, have been implicated as important precursors for cell signaling molecules and as regulators of membrane protein function [4, 5]. Given the many different biological processes that are modulated by PUFAs, they have both beneficial and harmful effects [6, 7]. The regulation of membrane protein function occurs, at least in part, through what appears to be membrane effects, as opposed to effects mediated through direct FA-membrane protein interactions or cellular signaling processes ([8], see also Table 1 in [9]). Using the gramicidin (gA) channels as probes, we previously reported that docosahexaenoic acid (DHA), an important PUFA in fish oil, alters bilayer properties in a manner that may account for some of DHA’s acute effects on ion channel function, whereas mono-unsaturated oleic acid OA was inert [9]. This pattern is similar to what has been observed for other ion channels, and membrane-active compounds like Triton X-100 can elicit similar changes in cellular excitability as DHA [8], suggesting that these changes indeed are due to altered bilayer properties as opposed to direct fatty acid-membrane protein interactions.
Our previous study [9] left open the question why OA was inert. The partition coefficient of OA into lipid bilayers is higher than that of DHA, so bilayer-incorporated OA must somehow interact differently with the lipid bilayer (membrane lipids) than DHA. It was in this context striking that OA caused little change in the single-channel channel currents, as compared to DHA [9]. The DHA-induced changes in current could arise because the adsorption of DHA imparts a negative surface charge, thereby increasing the interfacial cation concentration and thus the single-channel conductance; it also might reflect a local enrichment of DHA adjacent to the channels, which would increase the density of the more polar double bonds and thereby conceivably increase the single-channel conductance through a reduction of the electrostatic barrier for ion movement through the channel, cf. [10]. The latter mechanism could explain why OA is inert, but it is not clear that changes in the electrostatic barrier would be sufficient to account for the observed conductance changes—nor is it clear to what extent the DHA-induced changes in bilayer properties that were reported by the gA channels reflect simply the presence of poly-unsaturated acyl chain, which would be expected to alter both intrinsic curvature and bilayer elasticity, or whether the head group charge is important as well. (The poly-unsaturated acyl chains may alter also the bilayer “fluidity”; we consider this issue in the Discussion.)
We therefore explored the relative importance of acyl unsaturation and carboxylate charge fatty acids’ ability to modify lipid bilayer properties—as probed using gA channels, which in this context is a generic bilayer-spanning protein. The role of acyl chain unsaturation was probed in experiments where we added poly-unsaturated acyl chains, keeping the charge invariant—by either adding the polyunsaturated acyl chain as the neutral methyl ester derivative of DHA (DHA-ME), or by incorporating it into the phospholipid per se, in the form of 1-palmitoyl-2-docosahexaenoyl- phosphatidylcholine (PDPC). The role of charge was probed in experiments with DHA or OA in which we varied the pH of the electrolyte solution.
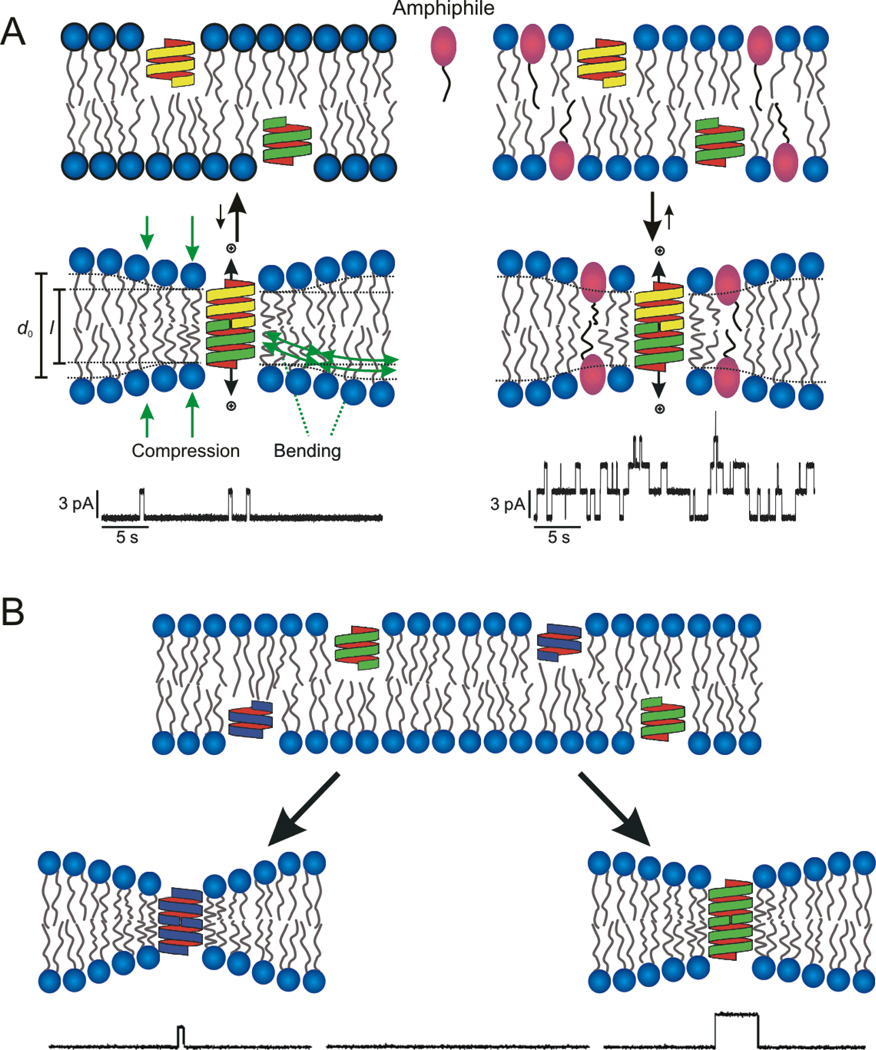
The principles underlying using gA channels as probes to detect changes in bilayer properties, as sensed by a bilayer-spanning channel, are illustrated in Figure 1.
Figure 1.
Gramicidin channels as probes of changes in bilayer properties: A) Bilayer-spanning gA channels form by transmembrane dimerization of two β6.3-helical subunits; channel formation is reported as changes in the current through the bilayer. Left: Because the channel length (l) is less than the unperturbed bilayer thickness (d0), the bilayer will adapt to the hydrophobic mismatch to minimize the exposure of hydrophobic residues to water. This adaptation involves local compression of the bilayer hydrophobic core and bending of the bilayer/solution interface. Channel formation thus will incur an energetic cost, meaning that the energetic cost of channel formation—and the single-channel appearance frequency and lifetime—varies with changes in lipid bilayer properties. Right: In bilayers that have been modified by the adsorption of amphiphiles at the bilayer/solution interface, the bilayer has additional degrees of freedom in terms of how it adapts to a channel-bilayer hydrophobic mismatch because the amphiphiles may redistribute between the membrane and the aqueous phases, which will tend to reduce the magnitude of the such that the gA monomer↔dimer equilibrium will tend to be shifted toward the conducting dimers. B) It is possible to use gA channels of different lengths, which can be distinguished by the magnitude of the single-channel current transitions for each channel type, to explore the relative contributions of changes in intrinsic curvature and bilayer elasticity because the hydrophobic mismatch-dependent contribution to Fdis (Eq. 3) will differ for channels of different length. (For practical reasons, these experiments are done with gramicidin analogues of different helix sense; this precludes the formation of heterodimers, which would complicate the analysis)
As is the case for other membrane-spanning proteins, gA channels will locally reorganize the surrounding bilayer [11, 12]. This local bilayer deformation has an associated energetic cost, the bilayer deformation energy (), that varies as a function of the hydrophobic mismatch between the channel length (l) and the bilayer thickness (d0), the channel radius and the bilayer material properties (thickness, intrinsic curvature, c0, and the associated elastic compression and bending moduli). The difference between the bilayer deformation energy associated with the two monomers that will form the channel () and with the conducting dimer () becomes the bilayer contribution () to the total energetic cost of the monomer↔dimer transition, and the equilibrium distribution between the non-conducting monomers (M) and conducting dimers (D) will be given by:
| (1) |
where is the “intrinsic” energetic cost of channel formation (e.g., due to formation of the interface between the two subunits, which is stabilized by six hydrogen bonds), and kB and T are Boltzmann’s constant and temperature in Kelvin
The bilayer deformation energy (ΔGdef) associated with a local bilayer thinning can be expressed as :
| (2) |
where HB, HX and HC are elastic coefficients that are functions of the bilayer thickness, elastic moduli and channel radius (and include contributions from the energetic cost of redistributing the different components in a multi-component membrane, including the redistribution of the decane in our planar bilayer experiments), and c0 the intrinsic monolayer curvature. Eq. 2 can be derived from the theory of elastic bilayer deformations [13]; a more general derivation, which is not subject to the assumptions used in the theory of elastic bilayer deformation, is obtained from a series expansion of in (l − d0) and c0 [14].
The bilayer responds to the deformation by imposing a disjoining force (Fdis) on the channel [12, 15]
| (3) |
Changes in Fdis—due, for example, to the adsorption of FAs at the bilayer/solution interface, which for thermodynamic reasons will tend to increase bilayer elasticity [16, 17]—will be observable as changes in τ. Moreover, because the hydrophobic mismatch-dependent and curvature-dependent contributions to Fdis are additive (Eq. 3), the gA channels not only become probes of changes in bilayer properties, it becomes possible to examine the relative importance of changes in curvature (c0) and elasticity (the H coefficients) by doing experiments with gA channels of different lengths (Fig 1B).
MATERIALS AND METHODS
Materials
The naturally occurring mixture of gramicidins from B brevis (gD, for R. Dubos who discovered gramicidin [18]), which is ~80% [Val1]gA [19], was from Sigma (St. Louis, MO). The gA analogues gA(13) (formyl-l-Ala-d-Leu-l-Ala-d-Val-l-Val-d-Val-l-Trp-d-Leu-l-Trp-d-Leu-l-Trp-d-Leu-l-Trp-ethanolamine) and AgA−(15) (formyl-d-Ala-Gly-d-Ala-l-Leu-d-Ala-l-Val-d-Val-l-Val-d-Trp-l-Leu-d-Trp-l-Leu-d-Trp-l-Leu-d-Trp-ethanolamine) were synthesized and purified as described previously [20]. (The two analogues form channels that have opposite helix sense: gA(13) forms right-handed channels, AgA−(15) forms left-handed channels.) Stock solutions (~10−6 M) were stored in methanol at −40 °C; they were diluted to 10−8 M to 10−9 M in absolute ethanol. Dioleoylphosphatidylcholine (DOPC), 1-palmitoyl-2-docosahexaenoyl-phosphatidylcholine (PDPC) and 1,2-dierucoyl-phosphatidylcholine (DC22:1PC) from Avanti Polar Lipids (Alabaster, AL) were used as provided and stored in chloroform at −40 °C until needed. Docosahexaenoic acid (DHA, ≥98% pure), docosahexaenoic acid methyl ester (DHA-ME, ≥98% pure) and oleic acid (OA, ~99% pure) were from Sigma; oleylamine (OAm, 80 – 90% C18 content) was from Acros Organics (Geel, Belgium); they were used without further purification. n-decane, 99.9% pure, was from Chemsampco (Trenton, NJ), 8-aminonaphthalene-1,3,6-trisulfonate, disodium salt (ANTS) was from Invitrogen (Eugene, OR). Stock solutions of DHA, DHA-ME, OA and OAm (0.5 to 5 mM plus an equimolar amount of NaOH in the case of DHA and OA) were made in absolute ethanol, stabilized with 25 µM butylated hydroxytoluene (BHT) and stored under argon. They were stable against oxidation for two to three weeks and used within two weeks. Water was deionized Millipore Corp. Milli-Q water (Bedford, MA).
Bilayer formation and single-channel measurements
Single-channel measurements were done as described previously [20] using 20 mg/mL solutions of DOPC or PDPC in n-decane across a ~1.6 mm diameter hole in a 0.1 mm thick Teflon partition separating two compartments filled with 1 M NaCl and 10 mM HEPES (Sigma), and buffered to pH 4.0 (with 10 mM glycyl-glycine), 7.0 (with 10 mM HEPES) or pH 9.0 (with 10 mM Na2HPO4). The current signal was recorded with an AxoPatch 1C patch clamp (Axon Instruments, Foster City, CA), filtered at 2000 Hz, digitized at 20 kHz and digitally filtered to between 100 and 500 Hz. Single-channel current transitions were detected as described previously [21].
The gA analogues were added to both sides of the bilayer. To examine how changes in bilayer composition alter lipid bilayer properties, we used pairs of analogues having different sequence lengths, to vary the channel-bilayer hydrophobic mismatch, and opposite helical sense to prevent heterodimerization. After recording the channel activity in the absence of the bilayer modifier, DHA, DHA-ME, OA or OAm were added to both sides of the bilayer; the solutions were stirred for five min after addition and equilibrated for an additional 10 min. (The final [BHT] was < 0.1 µM; 0.5 µM BHT has no effect on channel function, results not shown.)
Single-channel current transitions were detected as described previously [21] using software written in VisualBasic. The current transitions for each channel type appear as a single peak in current transition amplitude histograms. For each channel type, lifetime histograms were constructed for channels with current transitions falling within the characteristic peak in the amplitude histogram (e.g. Figure 2). The average channel lifetimes, τ, were determined by fitting a single exponential distribution:
| (4) |
where N(t) is the number of channels with lifetimes longer than time t, to the lifetime distributions. The reported results are averages from at least 3 independent experiments, each with 300 to 1000 channel events.
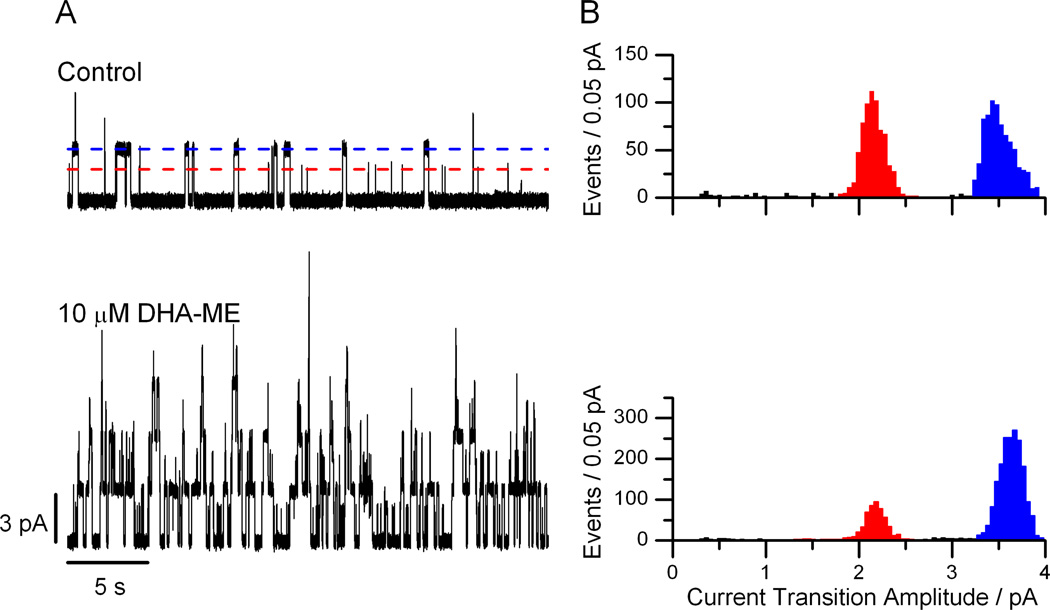
Figure 2.
DHA-methyl ester is a bilayer modifier. A: Current traces before and after the addition of 10 µM DHA-ME to both sides of the DOPC/n-decane bilayer incubated with gA(13) and AgA−(15). The current amplitudes of gA(13) and AgA−(15) channels are indicated by the red and blue lines, respectively. B. Current transition amplitude histograms of gA(13) (red peak) and AgA−(15) (blue peak) channels in the absence (top) and presence (bottom) of 10 µM DHA-ME. The current transition amplitudes for the gA(13) and AgA−(15) channels are (mean ± SD): 2.16 ± 0.13 pA and 3.51 ± 0.16 pA in the absence of DHA-ME and 2.17 ± 0.14 pA and 3.63 ± 0.12 pA in the presence of DHA-ME. DOPC, 1 M NaCl, 10 mM HEPES (pH 7), 200 mV.
Fluorescence quench experiments
Large unilamellar vesicles (LUVs), loaded with the membrane-impermeable water-soluble fluorophore ANTS, were made of DC22:1PC using a four-step process of sonication, freeze-thawing, extrusion and elution following [22, 22]. The LUVs were doped with gD and incubated for 24 h at 12.5 °C before incubating the vesicles for 10 min at 25 °C in the absence or presence of either DHA or OA at the desired concentration and pH to measure the FA-induced changes in the fluorescence quench rate. The ANTS fluorescence ANTS is quenched by Tl+, which crosses the bilayer slowly, whereas the conducting gramicidin channels are very Tl+ permeable, meaning that the Tl+ influx rate (the fluorescence quench rate) becomes a measure of the number of bilayer-spanning, conducting gramicidin channels in the LUV membranes. To measure the fluorescence quench rate, the ANTS-loaded LUVs were mixed with the Tl+ quencher using a stopped-flow spectrofluorometer (Applied Photophysics, SX.20) and the fluorescence quench rate was determined by fitting a stretched exponential
| (5) |
where F(t) is fluorescence intensity at time t, β a measure of sample dispersity (0<β≤1), and τ0 a parameter with units of time [23], and evaluating the time-dependent quench rate k(t)
| (6) |
at 2 ms. The reported results are averages from at least 3 independent experiments.
31P Nuclear magnetic resonance sample preparation
Phospholipid membranes with or without OA were prepared using previously described procedure [24]. A lipid film consisting of 33 µmole of DOPC:DOPE (1:3) with or without 1 mole percent OA was prepared from a stock solution in CHCl3 after removal of solvent on a rotavapor and further drying under vacuum. The lipid was hydrated for 1 h with 0.5 mL of 0.1 M buffer (titrated to the desired pH), vortexed until the lipid film was fully suspended, and centrifuged at 14,600 g for 120 min at 4 °C. The supernatant was removed, the pellet flushed with N2, the tube was closed and the 31P NMR spectrum was recorded as described [24].
Surface potential measurements
The surface potential of lipid monolayers spread at the air/water interface was measured using the ionizing electrode technique [25]. The lipid phase was 5 µL of a 20 mg/ml DOPC solution in hexadecane and the subphase was 250 mL 1 M NaCl, 1 mM MOPS, 1 mM glycyl-glycine, 1 mM sodium citrate, pH 4.0. The subphase electrode was a saturated KCl calomel electrode and the ionizing electrode was made from the polonium component of a Staticmaster brush (Model 1C200, Nuclear Products Co., El Monte, CA) soldered to a BNC cable and held ~0.25 cm from the interface. The potential was measured using a high-resistance electrometer (Keithley, Model 616).
The surface potential was measured before and after the monolayer was spread across the surface. The surface was cleaned by repeated suction of the surface to remove impurities. Before spreading the monolayer, a stable potential between +250 and +300 mV was considered to indicate a clean surface. 3 µM FA then was added to the subphase under constant stirring. The change in potential was recorded after 15 min. The pH of the subphase then was increased from pH 4 to pH 10 by incremental additions of 25 µL 1 N NaOH.
LogP
LogP was estimated using the ACD/Labs LogP algorithm at http://www.chemspider.com.
RESULTS
Changing Acyl Chain Unsaturation
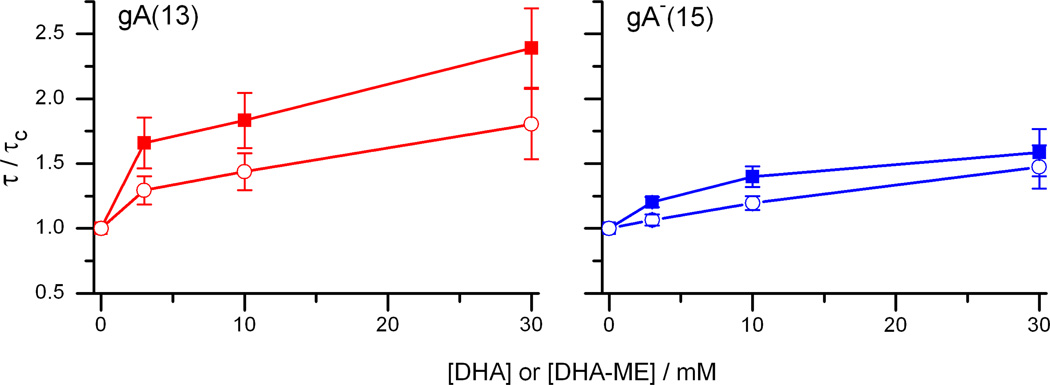
To examine the relative importance of acyl chain unsaturation and charge on lipid bilayer properties, as sensed by bilayer-spanning channels (membrane proteins), we first examined the effects of the uncharged DHA-ME on gA(13) and AgA−(15) channels in DOPC/n-decane bilayers. Like DHA [9], DHA-ME increases channel appearance rates in DOPC bilayers, as seen in the current traces in Figure 2A. Unlike DHA, DHA-ME does not increase the single-channel current transition amplitudes (Figure 2B), suggesting that it is the charge on the carboxylate that is responsible for the DHA-induced current changes. Yet DHA-ME increases the single-channel lifetimes in a dose-dependent manner (Figure 3), though to a lesser extent than DHA—and, as was found for DHA, the relative changes in τ of the shorter gA(13) channels, τ13, were larger than those of the longer AgA−(15) channels, τ15 (Figure 3), indicating that DHA-ME increases bilayer elasticity, cf. [9, 14, 26].
Figure 3.
DHA-methyl ester is a less potent bilayer modifier than DHA. Single-channel lifetimes of gA(13) and AgA−(15) channels as functions of [DHA] (closed symbols) and [DHA-ME] (open symbols). Experimental conditions as in Fig. 2.
This conclusion was examined further in experiments with PDPC bilayers, which has a palmitoyl chain at the sn-1 position and a DHA at the sn-2 position. DOPC and PDPC have similar phosphate peak-to-peak differences (~35 Å) [27, 28], meaning that it is possible to compare gA channel function in the two membranes without introducing significant changes in thickness (though the present experiments were done in n-decane containing bilayers).
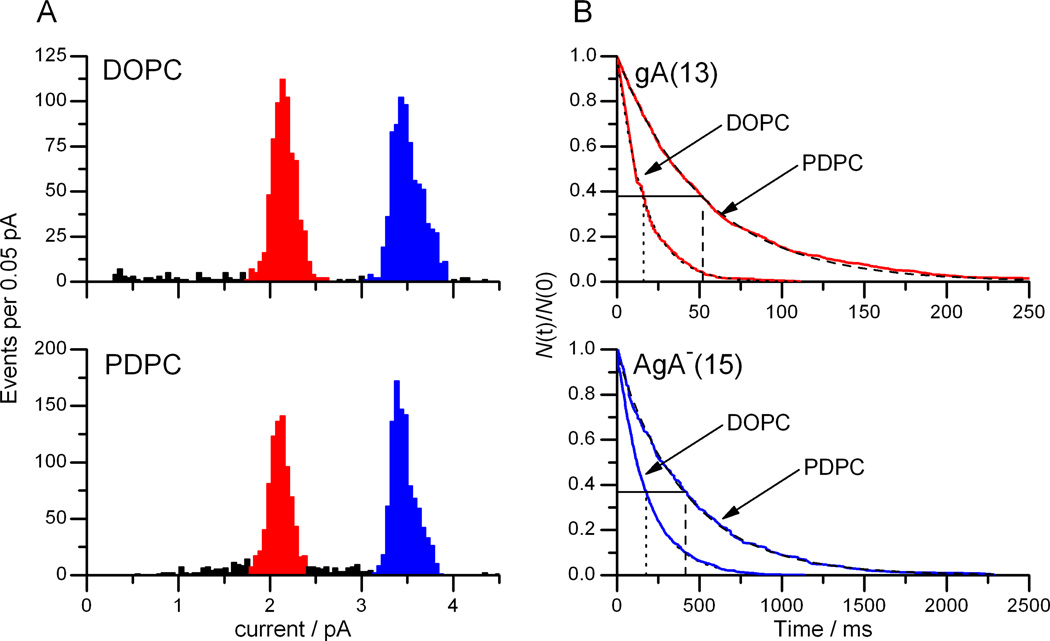
The single-channel current transition amplitudes of gA(13) and AgA−(15) channels are similar in DOPC and PDPC bilayers (Figure 4A), whereas the lifetimes of the gA(13) and AgA−(15) channels in PDPC membranes are three- and two-fold, respectively, longer than in DOPC membranes (Figure 4B), see also Table 1. Going from DOPC to PDPC, the bilayer-dependent changes in channel function are larger for the channel with the greater hydrophobic mismatch. It is not possible to interpret directly the changes in channel appearance rates between DOPC and PDPC membranes, but we needed to add five-fold less of either gA analogue to the PDPC/n-decane bilayers to achieve channel appearance rates comparable to those in the DOPC/n-decane bilayers—indicating that the dimerization constants are about 50-fold higher in the PDPC membranes, as compared to the DOPC membranes.
Figure 4.
Comparison of gA single-channel current transition amplitudes and lifetimes in DOPC and PDPC bilayers A: Current transition amplitude histograms of gA(13) (red peak) and AgA−(15) (blue peak) channels in DOPC or PDPC bilayers. B: Normalized single-channel survivor histograms fitted with single exponential distributions for gA(13) channels (top, τDOPC = 16 ms, τPDPC = 53 ms) and AgA−(15) channels (bottom, τDOPC=177 ms, τPDPC=414 ms). 1 M NaCl, 10 mM HEPES (pH 7), 200 mV.
Table 1.
Changes in gA channel lifetimes by changes in acyl chain unsaturation
| τDOPC | τDHA/τDOPC | τDHA-ME/τDOPC | τPDPC | |
|---|---|---|---|---|
| gA(13) channels | 17 ± 1 ms | 2.2 ± 0.2 | 1.5 ± 0.2 | 49 ± 8 ms |
| AgA−(15) channels | 175 ± 7 ms | 1.46 ± 0.08 | 1.17 ± 0.06 | 385 ± 30 ms |
| τ13/τ15 | 0.097 ± 0.003 | 1.5 ± 0.1 | 1.3 ± 0.10 | 0.13 ± 0.01 |
The subscripts denote lipid type or channel. τ13/τ15 denotes the ratio of the relative changes in the lifetime of gA(13) channels relative to the changes observed for the AgA−(15) channels
These results suggests that the single-channel current changes observed upon addition of DHA [9] primarily are due to the adsorption of the negatively charged DHA at the bilayer/solution interface, which produces a negative surface potential, rather than to changes in bilayer dielectric constant, which would lower the barrier for ion movement through the channel [10]. (Changes in the dielectric constant of the bilayer core do cause modest increases in single-channel conductances [29]. The similar conductances in DOPC and PDPC bilayers may suggest the dual effect of replacing two mono-unsaturated acyl chains by a saturated and a poly-unsaturated.) These results further suggest that the increases in single-channel lifetimes and bilayer elasticity are due, at least in part, to the poly-unsaturated docosahexaenoyl chain—though the lifetime changes produced by DHA-ME are less than those produced by DHA (the partition coefficient for DHA-ME, LogP 8.4, is likely to be higher than that of DHA, LogP 6.8), meaning that we cannot rule out a role for the carboxyl group charge.
Changing head group charge
To explore the possible role of charge on the DHA-induced changes in bilayer properties (as sensed by gA channels), we examined whether the effects of DHA vary as a function of pH. The pK of bilayer-adsorbed fatty acids adsorbed is about 2.0 – 2.5 pH units higher than their bulk pH of 4.5 – 5.0 [30–32]. We therefore examined the effect of DHA at pH 4, where DHA should be fully protonated, and pH to 9, where DHA should be fully deprotonated. At pH 4, DHA is as inert as OA is at pH 7—it increases neither the gA single-channel current transition amplitudes, appearance rates nor lifetimes (Figure 5).
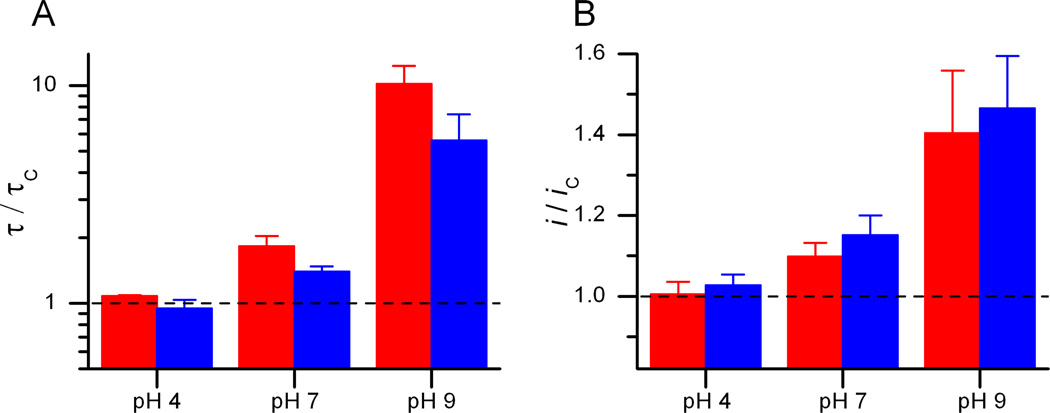
Figure 5.
The bilayer-modifying activity of DHA varies as function of pH. Changes in i (A) and τ (B) for gA(13) channels (red) and AgA−(15) channels (blue) produced by 3 µM DHA at pH 4, 7 or 9. DOPC, 1 M NaCl, 10 mM Gly-Gly (pH 4), 10 mM HEPES (pH 7) or 10 mM Na2HPO4 (pH 9). 200 mV.
At pH 9, DHA is a much more potent modifier of gA channel function than at pH 7—indeed, it was difficult to obtain recordings at DHA concentrations higher than 3 µM because of poor bilayer stability, high channel appearance frequencies and very long lifetimes. The DHA-induced changes in i and τ were several-fold larger than at pH 7, demonstrating that the charge on the carboxylate is important for the ability of DHA to increase both i and τ and, perhaps, for its ability to modulate other membrane proteins.
Does this mean that OA could modify gA channel function (lipid bilayer properties) if it were more fully deprotonated? (OA has little effect on i at pH 7, suggesting that it is more protonated than DHA when adsorbed at the bilayer/solution interface [9].) We therefore examined whether OA alters gA channel function at pH 9 (Figure 6).
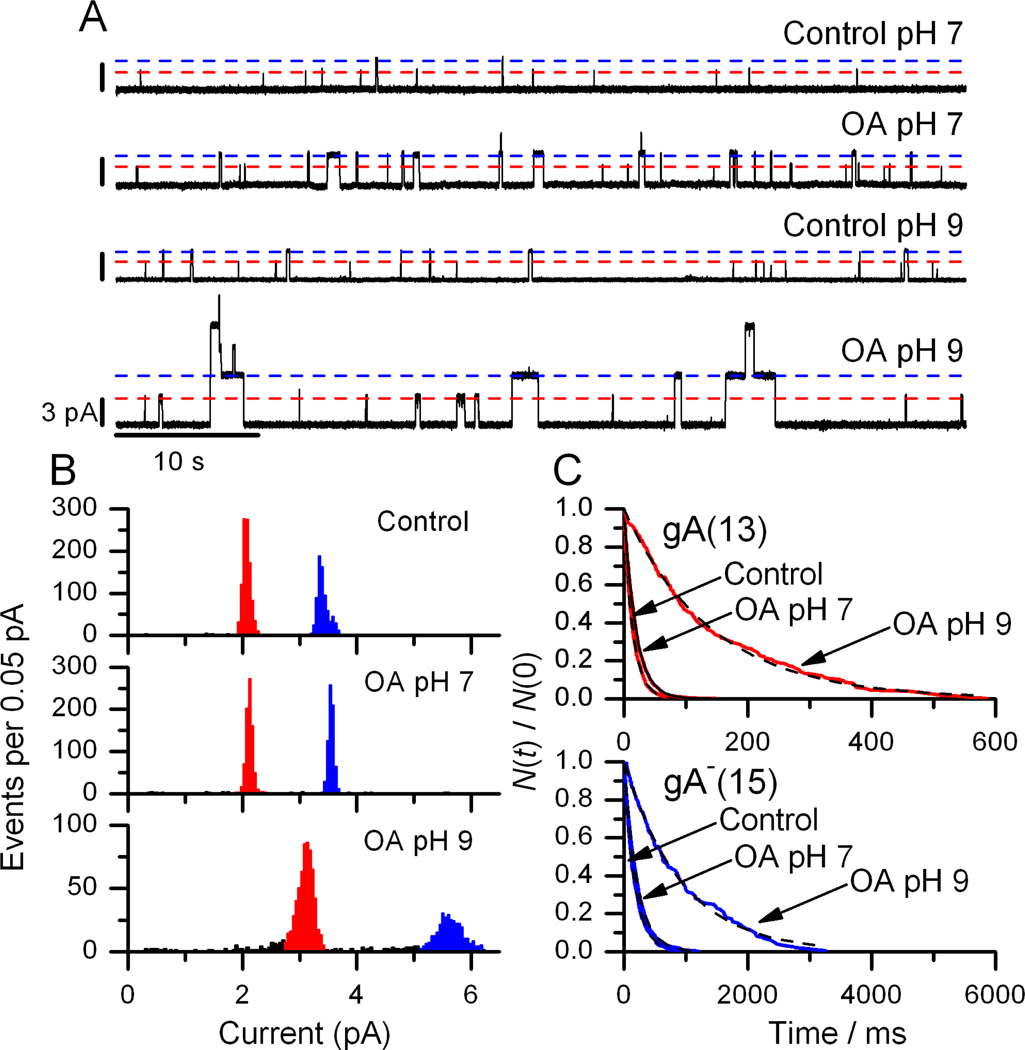
Figure 6.
OA increases gA single-channel current transition amplitudes and lifetimes at pH 9 but not at pH 7. A: Current traces obtained before and after the addition of 10 µM OA to both sides of DOPC/n-decane bilayers doped with gA(13) and AgA−(15) at pH 7 (top two traces) or pH 9 (bottom two traces). Interrupted lines denote the current levels for gA(13) (red) and AgA−(15) (blue) channels. B: Current transition amplitude histograms of gA(13) (red peak) and AgA−(15) (blue peak) in the absence or presence of 10 µM OA at pH 7 or 9. C: Single-channel survivor histograms fitted with single exponential distributions for gA(13) channels (top) (τc,7 = 17 ms, τOA,7 = 20 ms; τc,9 = 21 ms, τOA,9 = 141 ms) and AgA−(15) channels (bottom) (τc=187 ms, τOA,7 = 220 ms; τc,9 = 217 ms, τOA,9 = 917 ms). DOPC, 1 M NaCl, 10 mM HEPES (pH 7) or 10 mM Na2HPO4 (pH 9), 200 mV.
Compared to pH 7, where OA is inert (Fig. 6A), OA produced robust changes in single-channel currents and lifetimes at pH 9 (Figure 6B and 6C). The τ-[OA] dose-response curve (Figure 7) shows that OA at pH 9 is a more potent bilayer-modifier than DHA at pH 7 (Figure 3).
Figure 7.
Dose-response curves for OA at pH 7 and 9. Changes in i (A) and τ(B) for gA(13) channels (red) and AgA−(15) channels (blue) as functions of [OA]. Circles denote results at pH 7; squares denote results at pH 9. DOPC, 1 M NaCl, 10 mM HEPES (pH 7) or 10 mM Na2HPO4 (pH 9), 200 mV.
Fatty acids are negative curvature promoters [33]. But, whatever the pH, neither DHA nor OA alter gA single-channel properties in a manner that is consistent with a primary role of negative changes in curvature in the regulation of gA channel function—and the OA-induced changes in gA channel function do not results from changes in channel structure, as CD spectroscopy on [Val1]gA incorporated into DOPC vesicles (following [20]) show that gA retains its characteristic helical structure at pH 7 and 9 in both the absence and presence of OA (H. Gu and R. E. Koeppe II, unpublished results).
Negative changes in curvature would be expected to decrease channel appearance rates and lifetimes [12, 34], contrary to what we observed. To explore this question further, we examined the effect of oleylamine (OAm) on gA channel function. The volume of the amine group in OAm, ~13 Å3 [35], is less than that of the carboxyl group in OA, ~31 Å3 [35] and OAm, like OA, would be expected to promote a negative curvature. Indeed, OAm promotes membrane fusion [36], which often is considered to be characteristic of molecules having negative intrinsic curvature [37], and forms inverse phases [38]. The amine group should be fully protonated at both pH 7 and 9 [31], meaning that adsorption of OAm would be expected to decrease the single-channel currents.
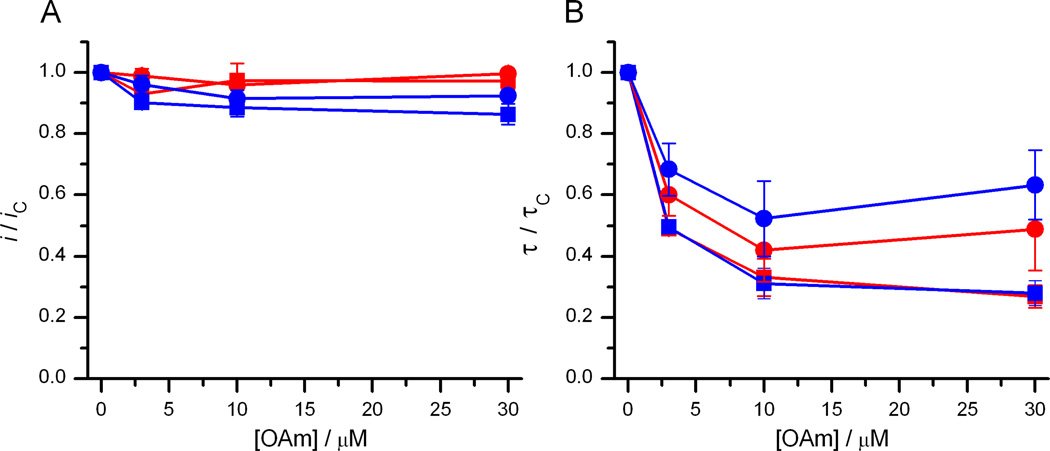
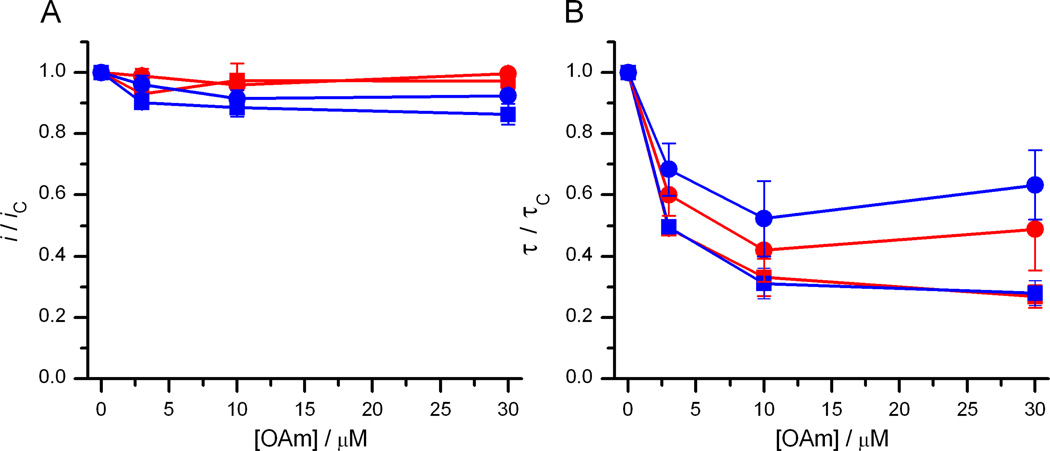
Figure 8 shows the dose-response curve for OAm on gA(13) and AgA−(15) single-channel current transition amplitudes and lifetimes.
Figure 8.
Dose-response curves for OAm at pH 7 and 9. Changes in i (A) and τ(B) for gA(13) channels (red) and AgA−(15) channels (blue) as functions of [OAm] . Circles denote results at pH 7; squares denote results at pH 9. DOPC, 1 M NaCl, 10 mM HEPES (pH 7) or 10 mM Na2HPO4 (pH 9), 200 mV.
Rather than increase i and τ, OAm decreased both i and τ. The changes in τ are consistent with both negative changes in curvature and decreases in elasticity. Unlike OA (at pH 9) and DHA (at pH 7 and 9), however, the OAm-induced changes in τ are similar for the shorter gA(13) and the longer AgA−(15) channels—and identical at pH 9 (Figure 8B), which following Eq. 3 suggests that the decreases in τ (increases in Fdis) result primarily from changes in curvature (c0), not from changes in elasticity (the H coefficients). The modest changes in i further suggests that there is little enrichment of OAm in the vicinity of the channels, cf. [9].
Fluorescence quenching experiments in phospholipid vesicles
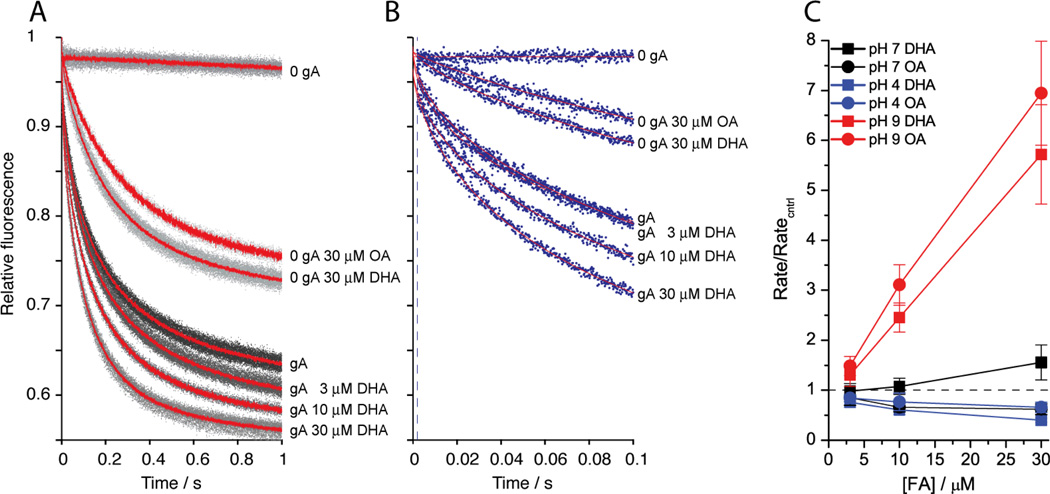
For technical reasons, our planar lipid bilayer experiments are done on membranes formed using DOPC/n-decane suspensions. To explore whether similar results are obtained in hydrocarbon-free membranes, we also explored the bilayer-modifying effects of DHA and OA using a gA-based fluorescence-quench assay [22, 39], which monitors the rate of quenching of the fluorescent reporter ANTS by Tl+ that enters the vesicle though gA channels in the vesicle membrane. Changes in the rate of fluorescence quenching thus becomes a measure of the number of bilayer-spanning gA channels, which will vary with changes in gramicidin monomer↔dimer equilibrium—with changes in lipid bilayer properties, as sensed by a bilayer-spanning channel. The results show that both OA and DHA are more potent bilayer modifiers at pH 9 than at 7—and that DHA inhibits channel function at pH 4, whereas OA is an inhibitor of gA channel function at both pH 7 and pH 4 (Figure 9).
Figure 9.
DHA and OA alter gA channels function in hydrocarbon-free lipid vesicles. A: Effects of DHA and OA in the time course of the Tl+-induced fluorescence quenching of ANTS encapsulated in LUVs (at pH 7). ANTS-containing DC22:1PC vesicles doped with gA were incubated for 10 min at 25 °C with increasing concentration of DHA (bottom four traces) or 30 µM OA (middle two traces) in the absence or presence of gA; the top trace was recorded in the absence of FA. Gray dots denote results from all repeats (n > 5 per experimental condition); red lines denote the average of all repeats for each condition. B: the first 100 ms from a single repeat for each condition; gray dots denote results, red lines are stretched exponential fits (2 – 100 ms) to these repeats. The stippled line denotes the 2 ms mark, the time at which the rate of quenching is determined. C: Normalized changes in fluorescence quench rates for DHA and OA at pH 4, 7 and 9 as functions of [DHA] or [OA]. The horizontal stippled line denotes no changes in the rate of quenching.
These results show that the key finding in the planar bilayer experiments, that OA and DHA at pH 9 shift the equilibrium distribution between non-conducting monomers and conducting dimers toward the conducting dimers (channels) is not due to the hydrocarbon used in the electrophysiological experiments.
Effects of fatty acids on the phospholipid phase preference
To further explore the effects of OA on the intrinsic lipid curvature, we examined how OA altered the lamellar↔inverse hexagonal (HII) phase preference at pH 4 and 7 (it was not possible to get results at pH 9 due to lipid hydrolysis) using 31P NMR (Figure 10).
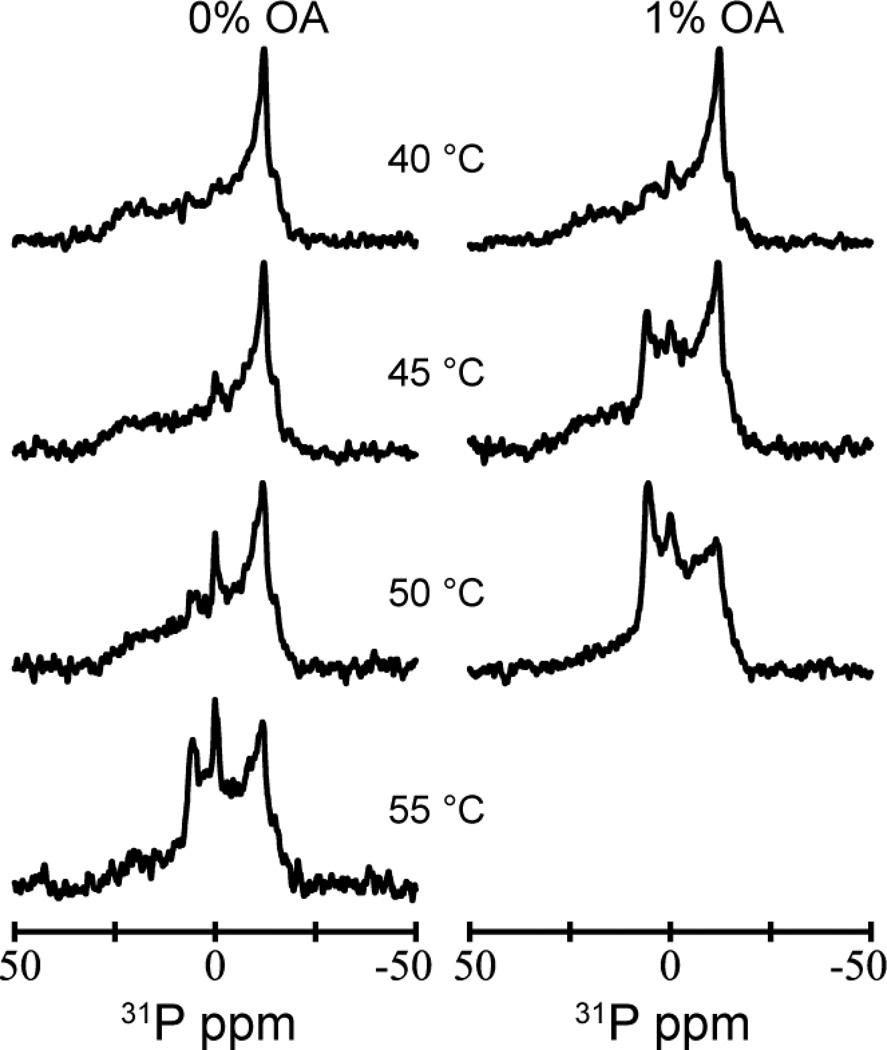
Figure 10.
Effect of OA on the lipid phase preference. 31P NMR spectra of DOPC:DOPE (1:3) liposomes in the absence and presence of 1 mole percent OA at pH 7, measured at 40, 45, 50 and 55 °C (no OA) and 40, 45 and 50 °C (1 mole percent OA).
The 40 °C (and 0% OA) 31P NMR spectrum is typical of the lamellar phase. As the temperature is raised, the left-most peak in the spectra (Fig 10) is characteristic for the presence of an HII phase, and the central peak is characteristic of an isotropic phase. In the presence of 1% OA, the new peaks appear at lower temperatures than in the absence of OA. Table 2 summarizes our results.
Table 2.
Estimated transition temperature for the onset of the HII phase
| pH | 0% Oleic Acid | 1% Oleic Acid | ~ ΔT |
|---|---|---|---|
| 4 | 316 – 318 K | 315–317 K | −1 K |
| 7 | 322 – 324 K | 312 – 314 K | −10 K |
The transition temperature was estimated from linear interpolation of spectra similar to those in Figure 10.
These changes in the propensity to form non-bilayer phases is observed at just 1 mole percent OA, demonstrating that lipid bilayers respond to even minor amounts of amphiphile, consistent with the X-ray and NMR studies of Keller et al. [40], the mechanical studies of [41] and our electrophysiological studies [9, 14, 42]. Interestingly, the population of the isotropic phase appears to be less when OA is present.
Titrating bilayer-adsorbed fatty acids
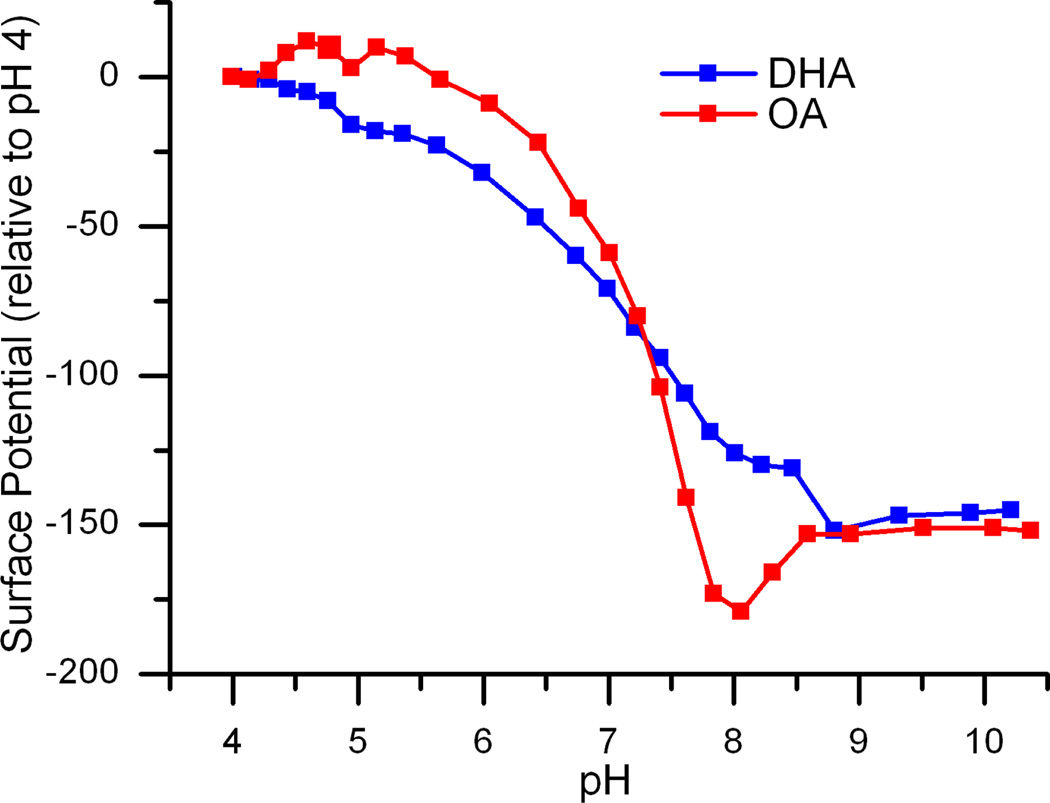
If the changes in single-channel currents result from the charge on the FA carboxyl, which causes a negative bilayer surface charge, and the changes in single-channel lifetime also are governed by the protonation state of the FA, why does OA have so little effect on i and τ at pH 7, whereas DHA increases both [9]? To ascertain whether the pKa of bilayer-adsorbed OA could be higher than that of bilayer-adsorbed DHA, we measured the changes in surface potential of DOPC monolayers in the presence of either 3 µM OA or DHA as function of pH (Figure 11).
Figure 11.
Titration of fatty acids in DOPC monolayers. Changes in the surface potential of DOPC monolayers in the presence of 3 µM DHA or OA as functions of pH. The midpoint potential for each titration curve was determined from linear interpolation between the adjacent points.
The midpoints of the titration curves, which do not correspond to the pKas of FAs adsorbed at the bilayer/solution interface (cf. McLaughlin and Harary [43]), are 7.5 ± 0.12 and 7.1 ± 0.05 for OA and DHA, respectively (mean ± S.D.). DHA is likely to be more deprotonated than OA at pH 7. Given the surprisingly large changes in potential, which reflect not only changes in FA protonation but also in the mole percent of FA in the monolayer, we did not pursue this further.
DISCUSSION
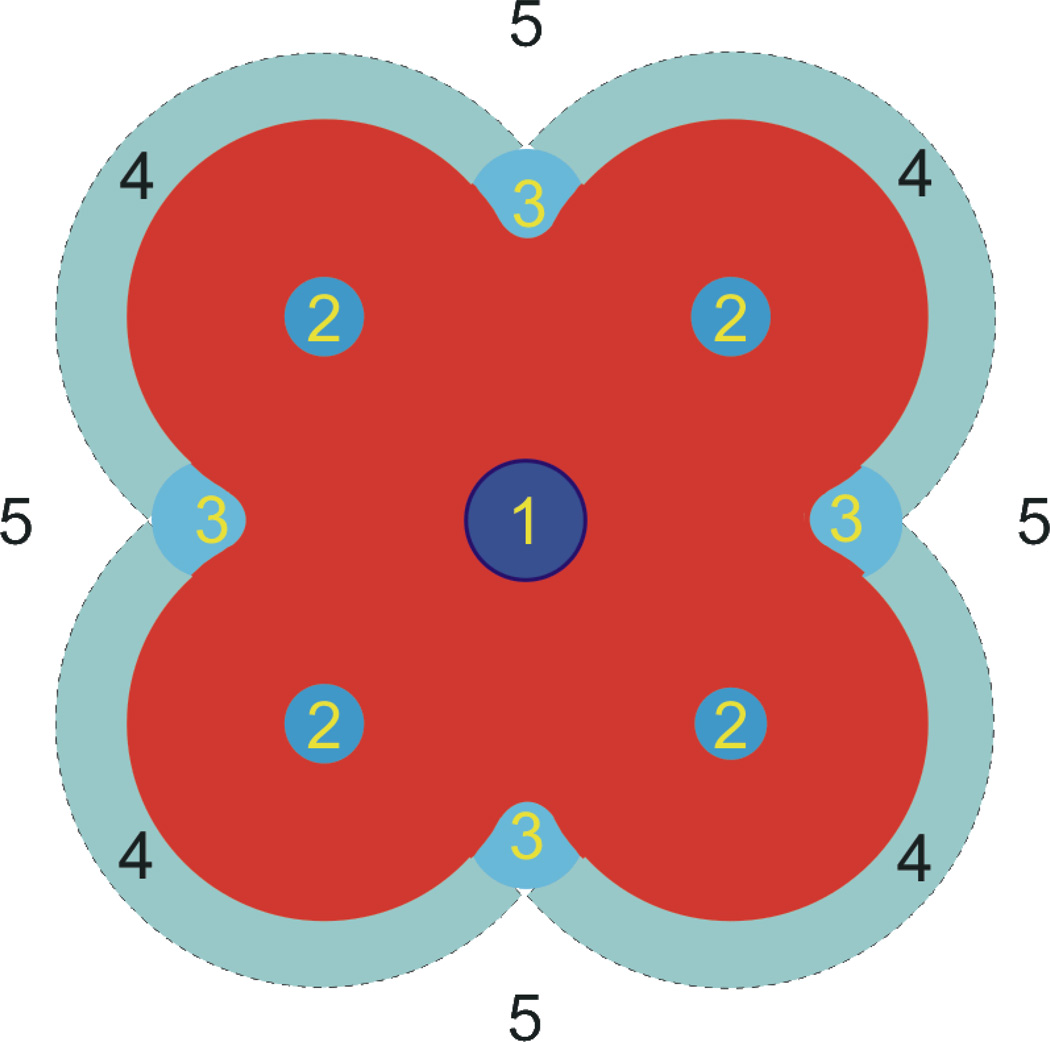
There is a long history of membrane effects of drugs and other amphiphiles, dating back at least to Meyer and Overton in the early 1900s (reviewed in [44]); for recent compilations of small-molecule effects on diverse membrane proteins see [9, 14, 42, 45–48]). Amphiphiles can, in principle, alter membrane protein function by many different mechanisms, ranging from binding to specific sites to altering the material properties of the host bilayer (Figure 12). In this study we examined a rather simple case of how amphiphiles alter lipid bilayer properties, as sensed by bilayer-spanning channels, as a prototypical example of membrane protein modulation by small molecules.
Figure 12.
Mechanisms by which drugs and other amphiphiles can inhibit membrane protein function: 1) occluding the pore or substrate binding site to block function; 2) binding to sites formed by the protein, to alter function by altering the free energy difference between different conformations , cf. Eq. 1; 3) binding to selective sites at the protein/bilayer interface, which may both , cf. Eq. 1; 4) non-specific accumulation at the protein/bilayer interface to alter the local lipid packing and 5) partitioning at the bilayer/solution interface to alter lipid bilayer material properties, which both will alter , cf. Eq. 1 the bilayer contribution to the conformational equilibrium (Modified after [73]).
The simplicity arises because the structure of the gA channel effectively excludes specific binding. The phospholipid ester and phosphate oxygens may form (transient) hydrogen bonds with the indole NHs [49], but there are no stereospecific interactions between gA channels and their host bilayer [50]. We therefore discuss our results in the context of mechanisms 4 and 5 in Figure 12.
Amphiphiles as bilayer modifiers
An amphiphile’s polar surface area to non-polar volume ratio usually will differ from that of the bilayer-forming lipids, meaning that the profile of intermolecular interactions along the bilayer normal (and the acyl chain dynamics) will be perturbed when amphiphiles adsorb at the bilayer/solution interface—leading to changes in bilayer properties such as: thickness, intrinsic curvature, the associated elastic compression and bending moduli, surface charge and interfacial dipole potential, and fluidity. Numerous studies show that small amphiphiles alter the bilayer bending [41, 51, 52] and compression [16, 17, 53] moduli. These changes in bilayer elasticity occurs at amphiphile mole fractions in the membrane that may be as low as 0.001 [41]. The physical basis for the amphiphile-induced decrease in bilayer elastic moduli is that the amphiphile adsorption into the bilayers varies as a function of the stress that is imposed on the bilayer [16], which increases the degrees available for the bilayer to pack around the channel and thus gives rise to a thermodynamic softening of the bilayer [17]. In the case of the area-compression modulus, the apparent elasticity modulus (Kap) in the limit of zero tension can be expressed as [17]:
| (7) |
where KA denotes the area-compression of the unmodified bilayer, n the amphiphile mole fraction in the membrane, Ka the apparent area compression modulus of the amphiphile, and a the cross sectional area of the amphiphile (which for simplicity is set equal to that of the membrane-forming lipid). A key assumption in the derivation of Eq. 7 is that the chemical potential of the amphiphile in the aqueous phase is constant, which should be valid for experiments with isolated bilayers and lipid vesicles where the volume-fraction of the lipid phase is 10−3 or less.
Because the changes in bilayer properties result (at least in part, see below) from the altered profile of intermolecular interactions, one should expect that a change in any one bilayer property (e.g., curvature) is associated with changes also in other properties (e.g., thickness or elastic moduli—and acyl chain dynamics or “fluidity”, as well as changes in interfacial dipole potential). It thus becomes difficult to identify the key features of an amphiphile that cause (as opposed to correlate with) the observed changes in bilayer properties and protein function. In the case of fatty acids, the contributions from acyl chain flexibility, charge and head-group size cannot be easily separated—and the lateral interactions between the phospholipid acyl chains and hydrophobic part of the amphiphile may, in principle, decrease or increase the elastic moduli independent of the thermodynamic softening (Eq. 7) [54].
Before proceeding, we note that a causal role of bilayer fluidity can be excluded because the FAs alter the equilibrium distribution between non-conducting monomers and conducting dimers by increasing the rate constant for channel formation and decreasing the rate constant for dissociation (cf. Figs. 6 and 9 and [9]), see also Lee [55]. (The lateral diffusion coefficient of bilayer-incorporated peptides varies little with changes in hydrophobic mismatch [56], and should have little effect on the relative changes in the single-channel lifetimes of channels of different length.)
Because many amphiphiles are dipolar, they also may alter the interfacial dipole potential [57], which in its own right may alter membrane protein function. It has been proposed that gA single-channel lifetimes are directly modulated by changes in the interfacial dipole potential due to the adsorption of amphiphiles [58]. The relative importance of the changes in dipole potential and the thermodynamic softening (Eq. 7) is difficult to determine, however, because reversibly adsorning amphiphiles that alter the interfacial dipole potential, such as phloretin [57] also will soften the bilayer (reduce Kap, Eq. 7). Indeed, amphiphiles such as Triton X-100 and lysophosphatidylcholine, which are known to shift the gramicidin monomer↔dimer equilibrium toward the conducting dimers [59, 60], similar to phloretin [61], produce positive changes in the interfacial dipole potential as opposed to the negative changes observed with phloretin (D. B. Sawyer and O. S. Andersen, unpublished results)
Acyl unsaturation and gramicidin channel function
The experiments with neutral DHA-ME and zwitterionic PDPC show that introducing the poly-unsaturated DHA chain, whether by the adsorption of DHA-ME or by incorporation into the bilayer-forming phospholipid, increases single-channel lifetimes—with the larger changes in τ being observed for the short gA(13), as compared to the long AgA−(15) channels (Figs. 2 – 4, Table 1). The changes in channel function thus vary with hydrophobic mismatch, meaning that DHA reduces the 2 · HB · (d0 − l0) contribution to Fdis (cf. Eq. 3). The amphiphile-induced changes in HB can be estimated as [12, 14]
| (8) |
where the subscripts 13 and 15 denote the two channel types. δ ≈ 0.16 nm and l15 − l13 ≈ 0.32 nm [26, 62]. Using Eq. 8 we find that 10 µM DHA-ME (nominal concentration; the actual aqueous concentration is ~100 nM, estimated following [9], see below) decreases the elastic coefficient HB by 2.3 ± 0.7 kBT/nm2 (6 ± 2 kJ/(mole·nm2), a 10% decrease from the control value of 56 kJ/(mole·nm2) [12]. The decrease may seem modest, but is sufficient to decrease the HB · (d0 − l0)2 contribution to by 7.5 kBT (for l = 2.2 nm [11, 63] and d0 = 4.0 nM [26]). Similarly one can estimate ΔHB going from DOPC to PDPC membranes to be −2.8 ± 0.7 kBT/nm2. The increased flexibility of the poly-unsaturated DHA chain [64] increases bilayer elasticity, consistent with the finding that multiple double bonds decrease the bilayer bending modulus [65].
The changes in single-channel lifetimes are not related to changes in ion permeability because the single-channel current amplitudes in PDPC and DHA-ME membranes were similar to those in DOPC membranes (Figs. 2 – 4); see also [34]. These results further suggest that the increased conductance produced by DHA [9] is primarily due to the negative charge on the (at least partly) deprotonated DHA, not an increase in the dielectric constant in the bilayer hydrophobic core due to the incorporation of double bonds (cf. [29]).
Carboxyl group protonation state and gramicidin channel function
The charge on the fatty acid is important also for the changes in bilayer properties because DHA at pH 7 produced greater changes in τ than DHA-ME. The relative changes in τ13 were again larger than those in τ15, and 10 µM DHA (nominal concentration) decreases HB by 4.2 ± 0.9 kBT/nm2, a 20% decrease relative to control (DOPC). The mole fractions of DHA-ME and DHA in the membrane were similar, ~0.03, meaning that DHA is more bilayer-modifying than DHA-ME. (The mole fractions were estimated from bilayer partition coefficients following [9], using the ADIFAB method [66]. The method fails for DHA-ME, which has no carboxyl group, but the neutral, more hydrophobic DHA-ME would be expected to have a higher partition coefficient than DHA. As already ~99% of the added DHA is in the membrane [9], the mole fraction of DHA-ME will be similar to that of DHA.) Per molecule in the membrane, DHA is the more potent bilayer modifier—at least at pH 7, where it is partly deprotonated. The changes in membrane properties produced by 10 µM DHA (nominal concentration) are even larger than the difference between DOPC and PDPC membranes (Table 1), even though the mole fraction of DHA chains in PDPC membranes is 0.5, some 30-fold higher than in DOPC membranes doped with 10 µM DHA.
To explore further the importance of the FA protonation state on bilayer properties, as sensed by gA channels, we explored the bilayer-modifying potency of DHA at pH 4, where it had little effect on i or τ (Fig. 5). Indeed, there was a slight decrease in τ15. This surprising result motivated us to examine how DHA alters gA channel function at pH 9, where it was much more active than at pH 7. It was again possible to estimate the changes in the elastic coefficient HB; at 10 µM DHA, ΔHB was −2.0 ± 0.6 kBT/nm2 at pH 4 and −10 ± 2 kBT/nm2 at pH 9. As the DHA carboxyl group becomes deprotonated, DHA becomes an increasingly potent bilayer modifier. Even OA, which is inert at pH 7, becomes a potent modifier of bilayer properties at pH 9 (Figs. 6 and 7). As was the case for DHA, OA increases bilayer elasticity at high pH where the carboxyl group should be fully deprotonated; at 10 µM OA, ΔHB was indistinguishable from 0 at pH 7 and −10 ± 1 kBT/nm2 at pH 9.
The single-channel experiments were done using hydrocarbon-containing planar bilayers, but similar results were obtained in hydrocarbon-free lipid vesicles (Fig. 9). Both DHA and OA increase gramicidin channel activity at pH 9, as compared with pH 7, and both DHA and OA decrease the activity at pH 4. (These experiments were done with gD, the naturally occurring mix of 15 amino acid gA analogues, and are thus concordant with the pH 4 results for DHA; Fig. 5.) The reduction in gramicidin channel activity, however, are less than those produced by long-chain alcohols [54], suggesting that the relative lack of effect may result from a combination of the thermodynamic softening (Eq. 7) and acyl chain interactions that increase acyl chain order (cf. [67]).
Fatty acids promote lipid phases with negative curvature, e.g., [33]. It is thus surprising that DHA at pH 4 and OA at pH 7 are as inert as they are—especially given the negative shift in the lamellar→HII phase transition temperature at pH 7 (Fig. 10), which raises the possibility that mixed ionization states may be important because the pKas of adsorbed FAs will be ~2 pH units higher than their bulk pKa [31, 32, 68].
To further explore the role of curvature, we examined the effect of OAm, a putative HII phase promoter. Contrary to OA, OAm decreases τ, with similar changes in τ13 and τ15 (Fig. 8 and Table 2), meaning that OAm does not alter the hydrophobic mismatch-dependent term in Eq. 3 and, by exclusion, that OAm inhibits gA channel function by inducing a negative change in curvature. At pH 9, the changes in i are less and the changes in τ are greater than at pH 7, suggesting that OAm may be somewhat deprotonated at pH 9, which would be expected to reduce head group volume and hydration—such that the deprotonated OAm may produce more negative changes in curvature than the protonated form.
Finally, to further explore why DHA is a more effective bilayer modifier than OA at pH 7, we explored the surface titration of DHA and OA. In agreement with previous studies [30–32, 68], we find that the midpoint of the titration curves is shifted ~2.5 pH units positive compared to the bulk pKa. (We do not, however, understand the basis for the magnitude of the potential change.) The shift in the titration curve for DHA is less than that of OA, but it seems unlikely that the small difference in the curves, per se, could account for the rather dramatic difference in channel modulation. A possible alternative mechanism, based on the unusual shape of the OA titration curve, is that the difference in titration curves reflect different intermolecular interactions, different acyl chain flexibility, of DHA and OA. The increased flexibility of the poly-unsaturated DHA chain would be expected to increase its limiting area in the monolayer (and bilayer). (The limiting areas of steric acid (18:0), OA (18:1) and linoleic acid (18:2) are 21 Å2, 42 Å2 and 48 Å2, respectively [69]; the limiting area of DHA is likely to be larger than that of linoleic acid.) Increasing the limiting area would increase the intermolecular distances between carboxylic acid head groups, or between the head group and the phosphate of the phospholipid, meaning that mono-unsaturated OA is likely to have stronger interactions with neighboring phospholipids and other OAs, as compared to the poly-unsaturated DHA, which could produce a larger shift in pKa. If OA can pack more tightly at lower pH (< 8), two OAs may be able to share a proton. Such a “double-chained” FA would be expected to have a higher partition coefficient, which could account for the more pronounced negative-going change in the titration curve for OA as compared to DHA.
We have no independent evidence for this proposal, but note that the existence of such non-covalently linked doublets would be consistent with titration studies on FAs at high concentrations [70, 71], where FAs form micelles at pH >9 and an oil phase at pH < 8, with a mixed bilayer and oil phase between pH 8 and 9. If such doublets exist, and partition less well into the perturbed bilayer adjacent to a gA channel, it would explain why OA at pH 7 is inert, as sensed by gA channels, even though it partitions strongly into the bilayer as judged from the changes in surface potential and its partition coefficient [9, 72]. At pH > 8, when OA is more fully deprotonated (and the oil phase disappears), these doublets would disappear and OA would be expected to behave more like DHA—which seems to be the case, as judged from the changes in gA channel function and from the titration experiments. In effect, the non-covalently linked doublet, with a small polar surface area as compared to the hydrophobic volume (cross-sectional area) would be expected to have a strong propensity to form HII phases, whereas the fully protonated OA would have a weaker propensity—thus accounting for the pH-dependent changes in OA’s ability to promote the formation of HII phases (Fig. 10 and Table 2). The deprotonated OA, would be expected to promote a positive curvature, meaning that it could partition more readily into the perturbed bilayer adjacent to a gA channel, thereby increasing τ by a combination of an increase in the local bilayer elasticity and a more positive curvature, and increasing i by adding a local negative surface charge.
ACKNOWLEGMENTS
This work was supported by NIH grants NIH grants GM021342 and ARRA Supplement GM021342-35S1 and RR015569. We thank Lawrence G. Palmer for suggesting that we explore the possible importance of the carboxylate charge, and Hong Gu for assistance with the circular dichroism experiments.
References
- 1.Schmitz G, Ecker J. Prog. Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Biomed. Pap. 2011;155:117–130. doi: 10.5507/bp.2011.038. [DOI] [PubMed] [Google Scholar]
- 3.Kremmyda LS, Tvrzicka E, Stankova B, Zak A. Biomed. Pap. 2011;155:195–218. doi: 10.5507/bp.2011.052. [DOI] [PubMed] [Google Scholar]
- 4.Hulbert AJ, Turner N, Storlien LH, Else PL. Biol. Rev. Camb. Philos. Soc. 2005;80:155–169. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- 5.Adkins Y, Kelley DS. J. Nutr. Biochem. 2010;21:781–792. doi: 10.1016/j.jnutbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 6.McMurray DN, Bonilla DL, Chapkin RS. Chem Phys Lipids. 2011 doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serini S, Fasano E, Piccioni E, Cittadini AR, Calviello G. Chem. Res. Toxicol. 2011;24:2093–2105. doi: 10.1021/tx200314p. [DOI] [PubMed] [Google Scholar]
- 8.Leaf A, Xiao YF. J. Membrane Biol. 2001;184:263–271. doi: 10.1007/s00232-001-0095-0. [DOI] [PubMed] [Google Scholar]
- 9.Bruno MJ, Koeppe RE, II, Andersen OS. Proc. Natl. Acad. Sci. USA. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen TW, Andersen OS, Roux B. Proc. Natl. Acad. Sci. USA. 2004;101:117–122. doi: 10.1073/pnas.2635314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang HW. Biophys. J. 1986;50:1061–1070. doi: 10.1016/S0006-3495(86)83550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundbæk JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS. J. R. Soc. Interface. 2010;7:373–395. doi: 10.1098/rsif.2009.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen C, Andersen OS. Biophys. J. 2000;79:2583–2604. doi: 10.1016/S0006-3495(00)76498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusinova R, Herold KF, Sanford RL, Greathouse DV, Hemmings HC, Jr, Andersen OS. J. Gen. Physiol. 2011;138:249–270. doi: 10.1085/jgp.201010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen OS, Koeppe RE., II Annu. Rev. Biophys. Biomol. Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 16.Evans E, Rawicz W, Hofmann AF. In: Bile Acids in Gastroenterology: Basic and Clinical Advances. Hofmann AF, Paumgartner G, Stiehl A, editors. Dordrecht: Kluwer Academic Publishers; 1995. pp. 59–68. [Google Scholar]
- 17.Zhelev DV. Biophys. J. 1998;75:321–330. doi: 10.1016/S0006-3495(98)77516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubos RJ. J. Exp. Med. 1939;70:1–10. doi: 10.1084/jem.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abo-Riziq A, Crews BO, Callahan MP, Grace L, de Vries MS. Angew. Chem. Int. Ed. Engl. 2006;45:5166–5169. doi: 10.1002/anie.200601516. [DOI] [PubMed] [Google Scholar]
- 20.Greathouse DV, Koeppe RE, II, Providence LL, Shobana S, Andersen OS. Meth. Enzymol. 1999;294:525–550. doi: 10.1016/s0076-6879(99)94031-4. [DOI] [PubMed] [Google Scholar]
- 21.Andersen OS. Biophys. J. 1983;41:119–133. doi: 10.1016/S0006-3495(83)84414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingólfsson HI, Andersen OS. Assay Drug Dev. Technol. 2010;8:427–436. doi: 10.1089/adt.2009.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berberan-Santos MN, Bodunov EN, Valeur B. Chem. Phys. 2005;315:171–182. [Google Scholar]
- 24.van der Wel PC, Pott T, Morein S, Greathouse DV, Koeppe RE, II, Killian JA. Biochemistry. 2000;39:3124–3133. doi: 10.1021/bi9922594. [DOI] [PubMed] [Google Scholar]
- 25.Gaines GL., Jr . Insoluble Monolayers at Liquid-Gas Interfaces. New York: Interscience; 1966. [Google Scholar]
- 26.Lundbæk JA, Koeppe RE, II, Andersen OS. Proc. Natl. Acad. Sci. USA. 2010;107:15427–15230. doi: 10.1073/pnas.1007455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiener MC, White SH. Biophys. J. 1992;61:437–447. doi: 10.1016/S0006-3495(92)81848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber T, Rajamoorthi K, Kurze VF, Beyer K, Brown MF. J. Am. Chem. Soc. 2002;124:298–309. doi: 10.1021/ja011383j. [DOI] [PubMed] [Google Scholar]
- 29.Girshman J, Greathouse JV, Koeppe RE, II, Andersen OS. Biophys. J. 1997;73:1310–1319. doi: 10.1016/S0006-3495(97)78164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small DM, Cabral DJ, Cistola DP, Parks JS, Hamilton JA. Hepatology. 1984;4:77S–79S. doi: 10.1002/hep.1840040814. [DOI] [PubMed] [Google Scholar]
- 31.Froud RJ, East JM, Rooney EK, Lee AG. Biochemistry. 1986;25:7535–7544. doi: 10.1021/bi00371a042. [DOI] [PubMed] [Google Scholar]
- 32.Peitzsch RM, McLaughlin S. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- 33.Seddon JM. Biochim. Biophys. Acta. 1990;1031:1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 34.Lundbæk JA, Maer AM, Andersen OS. Biochemistry. 1997;36:5695–5701. doi: 10.1021/bi9619841. [DOI] [PubMed] [Google Scholar]
- 35.Zamyatnin AA. Annu. Rev. Biophys. Bioeng. 1984;13:145–165. doi: 10.1146/annurev.bb.13.060184.001045. [DOI] [PubMed] [Google Scholar]
- 36.Bruckdorfer KR, Cramp FC, Goodall AH, Verrinder M, Lucy JA. J. Cell Sci. 1974;15:185–199. doi: 10.1242/jcs.15.1.185. [DOI] [PubMed] [Google Scholar]
- 37.Chernomordik LV, Kozlov MM. Nature Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisner A, Heggen M, Tillman K, Mourzina Y, Offenhäusser Mater. Res. Soc. Symp. 2010;1206 1206-M16-29. [Google Scholar]
- 39.Ingólfsson HI, Sanford RL, Kapoor R, Andersen OS. J. Vis. Exp. 2010:e2131. doi: 10.3791/2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller SL, Gruner SM, Gawrisch K. Biochim. Biophys. Acta. 1996;1278:241–246. doi: 10.1016/0005-2736(95)00229-4. [DOI] [PubMed] [Google Scholar]
- 41.Vitkova V, Meleard P, Pott T, Bivas I. Eur. Biophys. J. 2006;35:281–286. doi: 10.1007/s00249-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 42.Ingólfsson HI, Koeppe RE, II, Andersen OS. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin S, Harary H. Biochemistry. 1976;15:1941–1948. doi: 10.1021/bi00654a023. [DOI] [PubMed] [Google Scholar]
- 44.Seeman P. Pharmacol. Rev. 1972;24:583–655. [PubMed] [Google Scholar]
- 45.Hwang TC, Koeppe RE, II, Andersen OS. Biochemistry. 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- 46.Lundbæk JA, Birn P, Tape SE, Toombes GE, Søgaard R, Koeppe RE, II, Gruner SM, Hansen AJ, Andersen OS. Mol. Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 47.Artigas P, Al'aref SJ, Hobart EA, Diaz LF, Sakaguchi M, Straw S, Andersen OS. Mol. Pharmacol. 2006;70:2015–2026. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- 48.Ingólfsson HI, Koeppe RE, II, Andersen OS. FEBS Lett. 2011;585:3101–3105. doi: 10.1016/j.febslet.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim T, Lee KI, Morris P, Pastor RW, Andersen OS, Im W. Biophys. J. 2012;102:1551–1560. doi: 10.1016/j.bpj.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Providence LL, Andersen OS, Greathouse DV, Koeppe RE, II, Bittman R. Biochemistry. 1995;34:16404–16411. doi: 10.1021/bi00050a022. [DOI] [PubMed] [Google Scholar]
- 51.Duwe HP, Kaes J, Sackmann E. J. Phys. France. 1990;51:945–962. [Google Scholar]
- 52.Zhou Y, Raphael RM. Biophys. J. 2005;89:1789–1801. doi: 10.1529/biophysj.104.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ly HV, Longo ML. Biophys. J. 2004;87:1013–1033. doi: 10.1529/biophysj.103.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingólfsson HI, Andersen OS. Biophys. J. 2011;101:847–855. doi: 10.1016/j.bpj.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee AG. Prog. Lipid Res. 1991;30:323–348. doi: 10.1016/0163-7827(91)90002-m. [DOI] [PubMed] [Google Scholar]
- 56.Ramadurai S, Holt A, Schafer LV, Krasnikov VV, Rijkers DT, Marrink SJ, Killian JA, Poolman B. Biophys. J. 2010;99:1447–1454. doi: 10.1016/j.bpj.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen OS, Finkelstein A, Katz I, Cass A. J. Gen. Physiol. 1976;67:749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rokitskaya TI, Antonenko YN, Kotova EA. Biophys. J. 1997;73:850–854. doi: 10.1016/S0006-3495(97)78117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawyer DB, Koeppe RE, II, Andersen OS. Biochemistry. 1989;28:6571–6583. doi: 10.1021/bi00442a007. [DOI] [PubMed] [Google Scholar]
- 60.Lundbæk JA, Andersen OS. J. Gen. Physiol. 1994;104:645–673. doi: 10.1085/jgp.104.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen OS. In: Renal Function. Giebisch GH, Purcel EF, editors. New York: Ed. The Josiah Macy, Jr. Foundation; 1978. pp. 71–99. [Google Scholar]
- 62.Greisen P, Jr, Lum K, Ashrafuzzaman M, Greathouse DV, Andersen OS, Lundbæk JA. Proc. Natl. Acad. Sci. USA. 2011;108:12717–12722. doi: 10.1073/pnas.1103192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott JR, Needham D, Dilger JP, Haydon DA. Biochim. Biophys. Acta. 1983;735:95–103. doi: 10.1016/0005-2736(83)90264-x. [DOI] [PubMed] [Google Scholar]
- 64.Feller SE, Gawrisch K, MacKerell AD., Jr J. Am. Chem. Soc. 2002;124:318–326. doi: 10.1021/ja0118340. [DOI] [PubMed] [Google Scholar]
- 65.Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Biophys. J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richieri GV, Ogata RT, Kleinfeld AM. Mol. Cell. Biochem. 1999;192:87–94. [PubMed] [Google Scholar]
- 67.Mabrey S, Sturtevant JM. Biochim. Biophys. Acta. 1977;486:444–450. doi: 10.1016/0005-2760(77)90094-7. [DOI] [PubMed] [Google Scholar]
- 68.Drummond CJ, Grieser F, Healy TW. J. Chem. Soc., Faraday Trans. I. 1989;85:521–535. [Google Scholar]
- 69.Tomoaia-Cotisel M, Zsako J, Mocanu A, Lupea M, Chifu E. J. Coll. Interface Sci. 1987;117:464–476. [Google Scholar]
- 70.Morigaki K, Walde P. Curr. Opin. Coll. Interf. Sci. 2007;12:75–80. [Google Scholar]
- 71.Namani T, Ishikawa T, Morigaki K, Walde P. Colloids Surf. B Biointerfaces. 2007;54:118–123. doi: 10.1016/j.colsurfb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 72.Anel A, Richieri GV, Kleinfeld AM. Biochemistry. 1993;32:530–536. doi: 10.1021/bi00053a018. [DOI] [PubMed] [Google Scholar]
- 73.Andersen OS. J. Gen. Physiol. 2008;131:395–397. doi: 10.1085/jgp.200810012. [DOI] [PMC free article] [PubMed] [Google Scholar]