Abstract
Large-scale molecular assemblies, or signaling clusters, at the cell membrane are emerging as important regulators of cell signaling. Here, we review new findings and describe shared characteristics common to signaling clusters from a diverse set of cellular systems. The well-known T cell receptor cluster serves as our paradigmatic model. Specifically, each cluster initiates recruitment of hundreds of molecules to the membrane, interacts with the actin cytoskeleton, and contains a significant fraction of the entire signaling process. Probed by recent advancements in patterning and microscopy techniques, the signaling clusters display functional outcomes that are not readily predictable from the individual components.
Introduction
Living cells interpret and respond to numerous signals from their environment. Receptors in the cell membrane are generally the initial point of interaction for incoming signals. At the most basic level these receptors provide specificity, for example by binding a unique chemical ligand. This information is transduced across the membrane to a cascade of chemical signaling reactions that perform logical operations and, ultimately, make decisions. In recent years, the role of spatial arrangement and assembly of signaling molecules into organized structures on the membrane is emerging as a significant component of signal regulation [1••,2,5,6,7•,8–13]. Advancements in the application of optical, spectroscopic, and materials patterning techniques to questions in cell biology have provided new angles of illumination on membrane signaling processes. Of particular interest is the discovery of submicronscale receptor signaling clusters in several cell systems [1••,2,5,6,7•,8–13]. These clusters can contain hundreds of molecules, are often associated with the cytoskeleton, and appear to encompass much more of the entire signaling cascade than originally thought to occur on the membrane surface. Most importantly, the physical assembly of the signaling system gives rise to emergent functional properties that can transcend the simple approximations of cooperativity, such as Hill coefficients and allosteric effects [14–16]. In this review, we present a study that compiles recurrent themes in membrane signaling that may link disparate systems based on large-scale spatial assembly of signaling molecules. Although current knowledge of many specific details in these systems is still incomplete, the common motifs presented here provide new insight and may help to predict yet undiscovered properties of these and related systems.
T cell receptor microclusters as a paradigm
Whereas small-scale receptor oligomerization has been discussed within the context of membrane signaling for decades, prominent examples of large-scale spatial patterns, such as the immunological synapse (IS) [8–11] have emerged more recently. The IS comprises an intercellular junction between immune cells and their target cells [8–11]. Upon binding, various receptor-ligand pairs become sorted into distinct spatial patterns that extend to microns in size across the interface. Among the various spatial components that can be identified within the IS, it has become clear that the T cell receptor (TCR) microclusters, along with clusters of other downstream signaling molecules, are the active signaling units—sometimes referred to as signalosomes [15] (Figure 1). There have been significant advances in our understanding of TCR microclusters over the past few years, and these new discoveries may reflect a more general theme in biology.
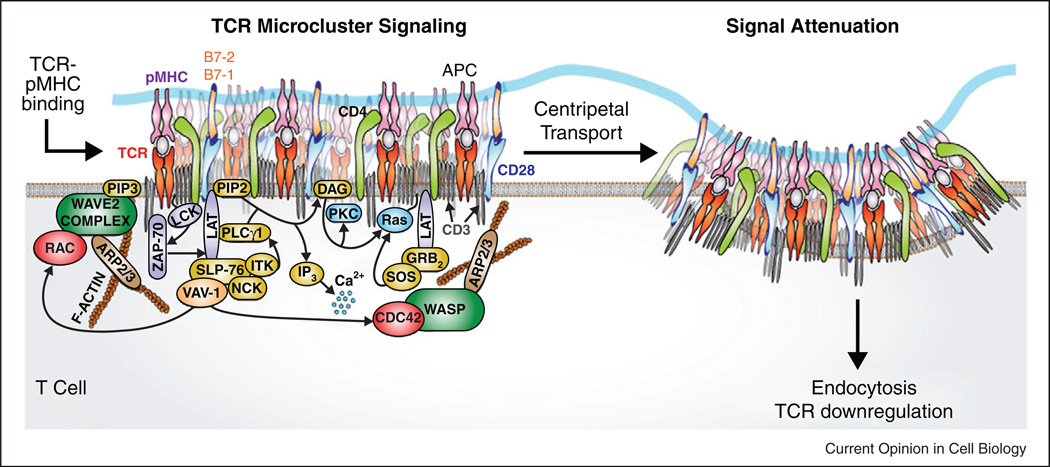
Figure 1. A signaling cluster.
Schematic of a T cell receptor microcluster, also called the TCR signalosome, which is densely packed with a diverse set of signaling molecules. Activation of actin assembly initiates centripetal transport. Further details can be found in the text and in references [1••,2–4].
Upon first engagement with antigen peptide-bound major histocompatibility complexes (pMHC), TCR molecules congregate into large clusters containing a hundred or more individual receptors. Here, other key molecules, such as lck [17,18], Lat [19], SLP76 [20,21] and actin [22,23] are heavily recruited [1••,2,3,13,24]. Much effort to understand TCR microclusters has focused on documenting their assembly and content (for in depth reviews see [1••,3,4]). Some very recent work probes the functionality of the signaling cluster by direct manipulation. Supported membranes have long been used as surrogate antigen presenting cell (APC) surfaces for T cell activation. By patterning structures onto the underlying substrate, it is possible to guide and restrict the assembly of signaling clusters in living T cells. This technique, which we refer to as a spatial mutation [25,26] has been used to demonstrate that T cell triggering thresholds are determined based on the number of agonist-bound ligand in a single microcluster, and not by the total number encountered by the cell (Figure 2). Thus, TCR microclusters appear to function with a high degree of internal cooperativity but little to no cooperativity between clusters [27•].
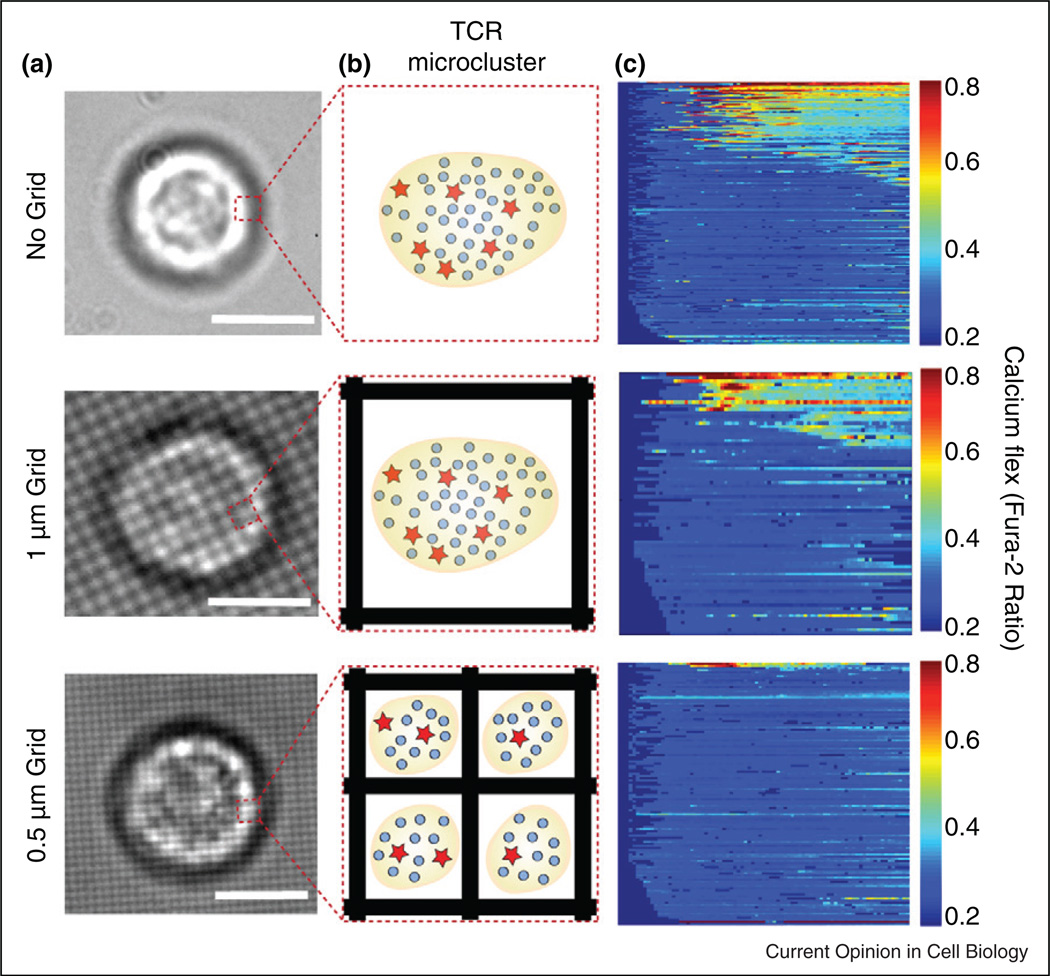
Figure 2. Titration of signaling clusters.
(a) Brightfield images of cells off and on the indicated grid pattern size (scale bar = 5 mm). (b) Schematic of TCR microcluster within indicated area in (a) with pMHC bound to activating agonists (stars) and non-activating null (circles) peptides. (c) Corresponding heat maps that display calcium flux for a population of cells on and off the grids, with each cell shown as a horizontal line and >100 cells per heat map.Adapted from reference [27•].
Interactions of signaling microclusters with the actin cytoskeleton
A common feature of signaling clusters is association with the actin cytoskeleton. Actin inhibition reveals that transport of TCR microclusters at the periphery is actin-dependent [22–24]. Further insights have been obtained from live cell imaging of the transport process as microclusters traverse maze-like configurations of mobility barriers [28]. Upon encountering a barrier during centripetal transport, the TCR microcluster trajectory is deflected along the barrier until it can continue its course through an opening. No elastic recoil of the cluster towards its original trajectory was observed. Furthermore, the speed of the deflected cluster scales with the cosine of its deflection angle to that of the flow, which is consistent with a dissipative or frictional coupling mechanism. In other studies with Jurkat cells, TCR and actin have been measured to move with different velocities [29•] and actin flow has been observed to slow but not stop over regions of trapped TCR [30]. Thus dynamic associations between TCR and actin allow force transmission without direct coupling. Similar actin coupling has also been observed for cadherin [31,32]. Cadherin clusters at adherens junctions regulate actin growth through the adaptor protein alpha-actinin [31–33]. Recently emerging imaging methods [16], such as image time autocorrelation analysis [34] (Figure 3), provide alternative ways to monitor and quantify interactions between the cytoskeleton and cell surface signaling clusters. For many of these studies, the use of patterned substrates to block or control TCR cluster movement is key.
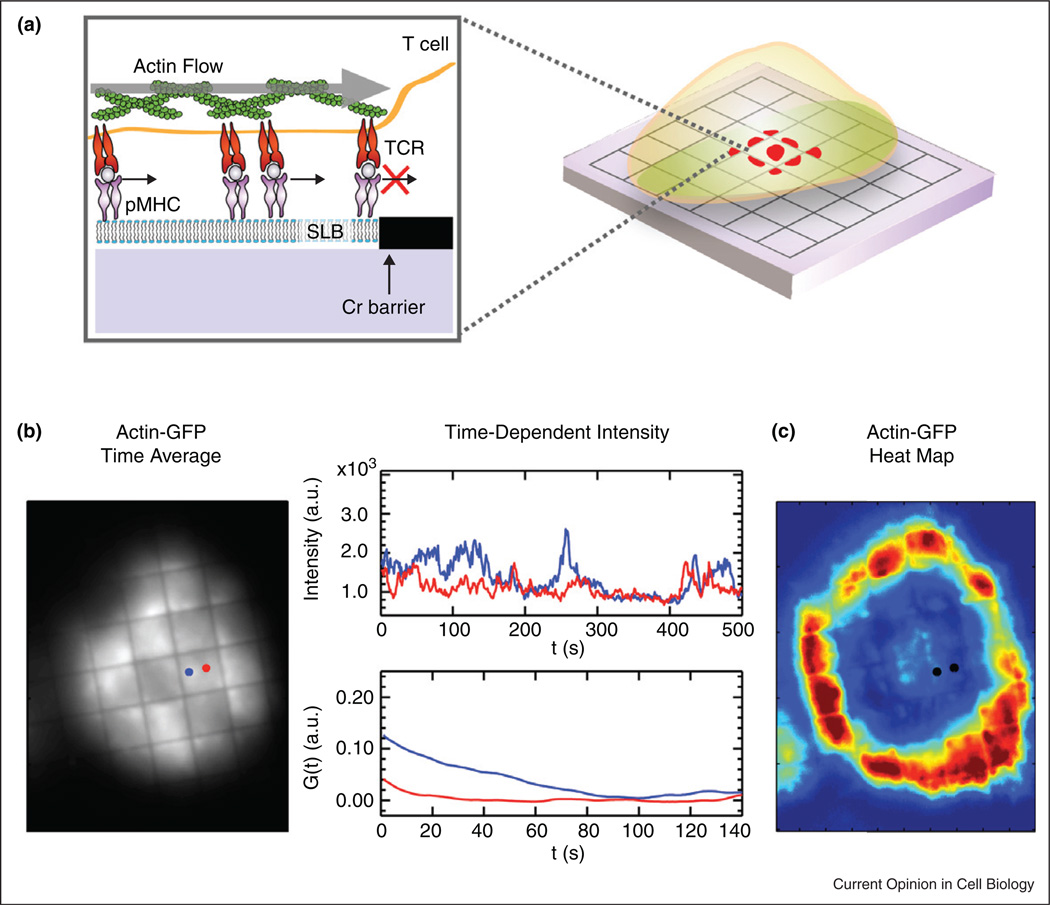
Figure 3. Measuring TCR–actin interactions.
(a) Schematic of a T cell on a substrate patterned with diffusion barriers. (b) Left, Time average of a stack of fluorescent actin-GFP. Right, Trace and plots illustrate temporal fluctuations along with time correlation, G(τ). (c) Pixel by pixel image of autocorrelation times reveals regions of high actin volatility and stability.
The association of actin with TCR microclusters may do more than drive lateral translocation. Two recent studies, using different but equally ingenious methods, have measured the on-off kinetics of the TCR–pMHC interactions in living cells. Both report these interactions to be more dynamic than previously thought, with much faster koff rates than was measured in vitro [35••,36••]. It may have been natural to assume that actin would stabilize TCR–pMHC at the molecular level, but Huppa et al. [36••] found that actin polymerization somehow contributes to the more dynamic TCR–ligand interaction. Consequently, actin appears to exert direct influence over the ligand-binding properties of TCR, providing a level of active control that the T cell can, in principle, modulate. This observation exemplifies how the emergent properties of signaling microclusters are not easily predictable from the primary inputs.
Triggering actin assembly
Several reviews discuss in great detail all the molecules thought to be involved in TCR–actin interactions [13,37–41]. One general theme is that molecules upstream of the Arp 2/3 complex become activated through a series of phosporylation-dependent reactions initiated at TCR microclusters [41]. Activation of the Arp 2/3 complex ultimately promotes actin branching and lamellipodia formation [42]. This event brings together signaling molecules, which may already be pre-clustered, to the inner leaflet of the membrane and the cytosolic side of the clusters. Recently, Lebensohn and Kirschner showed that Rac and cdc42, which activate Arp2/3, needed to be membrane-associated in the presence of the lipid PIP3 to fully activate Arp2/3 [43••]. More importantly, the WAVE complex must be activated to initiate Arp2/3 nucleation, which is the rate-limiting step for actin polymerization. This necessitates the simultaneous interaction of the WAVE complex with both Rac-GTP and acidic phospholipids at the membrane, such as PIP3. In accordance with this, some believe that negatively charged lipids, which are products of the TCR signaling cascade, may also promote actin nucleation [43••].
Impact of protein clustering
Crosslinking antibodies are commonly employed as alternative methods to trigger cell surface receptors. For example, antibodies to CD3, which is closely associated with TCR, are widely used to trigger TCR. Immobilized Anti-CD3 antibody on glass can induce Jurkat T cell activation, bypassing the need for TCR ligand binding [44,45]. Anti-CD3 not only crosslinks TCR into dimers, but also ultimately amplifies the TCR cluster assembly process (possibly in unnatural ways). MHC oligomers have also been widely used to activate T cells in solution; the monomer is inactive [46]. Crosslinking of CD28, a T cell costimulatory molecule, with a superagonist antibody without concomitant TCR or CD3 ligation produces similar results [47]. Hünig and Dennehy propose that interaction of the superagonist with bivalent CD28 can lead to supramolecular structures that favorably position receptors and signaling molecules to mediate signal transduction [47].
The effect of clustering on receptor signaling can also be observed when higher order complexes of downstream signaling molecules is induced. For example, the nucleotide switch that activates cdc42 can be bypassed through artificial dimerization of the GTPase with WASP [48]. Moreover, increased activation and affinity for Arp 2/3 have been observed for VCA domain dimers and N-WASP molecules that are incorporated into assemblies [49,50]. Padrick et al. [51] systematically tested three agents—EspFu, SH2 dimers, and PIP2 containing vesicles—that can cluster WASP proteins. They found that both allosteric activation and oligomerization are necessary for dramatically increasing actin assembly [51].
Protein cluster coupling to actin may be examined using antibody crosslinking experiments. A laser tweezers study measured drag forces on GPI-linked proteins as a function of antibody-crosslinking and revealed evidence of cluster size-based protein coupling to actin [52]. Clustering cell surface receptors can also lead to long-range spatial sorting in the context of actin-driven flows. In the T cell IS, differential clustering of the primary adhesion molecule lymphocyte function associated antigen-1 (LFA-1) leads to quantitative changes in its distribution within the synaptic junction [53•]. Bivalent or tetravalent antibody crosslinking of LFA-1, or its ligand, caused the protein to be sorted progressively closer to the lateral center of the circular junction [29•]. Local changes in receptor clustering are thus translated into global changes in receptor organization over the cell surface.
Interestingly, the idea of flow-assisted sorting has previously been observed for the effective clearance of antibody from the surface of motile trypanosome parasites [54]. Engstler et al. [54] showed that parasite swimming induced hydrodynamic flow, which led to the size-based sorting of antibodies to the posterior of the cell. Larger antibodies (IgM) are transported more rapidly than the smaller antibodies (IgG) to the flagellar pocket for endocytosis and degradation. In the case of the IS, the cell is static and does not undergo extensive hydrodynamic flow. Instead, the actin centripetal flow transports molecules and the cluster size determines the coupling strength and, ultimately, extent of radial transport [53•].
The growing use of antibody-based drugs in the clinical setting underscores the need to better understand receptor clustering and its broader effects on signal transduction processes. For example, an anti-CD20 antibody for cancer therapy induces lysosomal release of the reactive oxygen species for cell killing in an actin-dependent manner [55]. A potential link for this effect is the actin-associated protein Vav, which can control superoxide production [56]. Additionally, Benson et al. [57] observed increased killing of cancer cells by natural killer (NK) cells in multiple myeloma using an antibody against PD-1. Actin also appears to be affected, as the migration and cell morphology of the NK cells are changed with the antibody addition. The effect of these antibodies on actin is not well understood, and we speculate that such secondary effects may result from underlying, actin-involved receptor assembly processes.
Signaling clusters in other systems
Juxtacrine signaling in other cell systems also display features similar to those found in the T cell immunological synapse. One recent example is the EphA2-EphrinA1 system, which regulates cell adhesion, motility, and angiogenesis [58]. EphA2 binding to EphrinA1 leads to the formation of clusters that undergo actin-driven transport at the cell membrane [7•]. Spatial mutation studies, in which EphA2-EphrinA1 cluster transport was blocked, reveal discrete changes in the recruitment of the metalloprotease ADAM10 [7•]. This suggests the possible existence of mechanoregulation in this system as an emergent property of the signaling cluster [59].
In another example, the receptor tyrosine kinase MEK forms oligomers that preferentially sort to actin-dependent dorsal ruffles, where they undergo increased internalization [60]. Similar behavior has also been reported for activated epidermal growth factor receptor (EGFR) [61]. This same study further demonstrates that endocytosis may be independent of the traditional pathways, which are clathrin-pit or caveolae dependent [61]. Actin enrichment near the membrane has also been observed during trans-Notch-Delta binding [62]. To regulate signaling, cis-Delta-Notch complexes are quickly degraded [63], and it would be interesting to see if their internalization were actin-dependent.
Lipid rafts
Clusters of membrane signaling proteins can hardly be discussed without invoking the concept of membrane lipid rafts [64]. Despite immense experimental effort, the nature of lipid rafts and the balance of contributions from lipids or proteins in their formation remain controversial. What is clear is that signaling clusters of the types highlighted here are highly specific with respect to their molecular content, spatial positioning, and function. Lipids and cholesterol may contribute to their assembly and stabilization, but the proteins define the specificity and ultimate functionality.
Concluding remarks
Signaling clusters from a number of different biological systems share a surprisingly large number of physical features, such as actin recruitment and large-scale transport. Currently, much of biology is driven by to the goal of understanding how specific functions arise from underlying molecular mechanisms. In the case of signaling clusters, there is an opportunity to uncover common physical mechanisms that are employed in a diverse range of biological functions.
Acknowledgements
The authors would like to thank the many undergraduates, graduate students, postdoctoral fellows, and scientists who contributed to this research. Financial support is acknowledged from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 and Award U54 CA143836 from the National Cancer Institute (NCI).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1. Choudhuri K, Dustin ML. Signaling microdomains in T cells. FEBS Lett. 2010;584:4823–4831. doi: 10.1016/j.febslet.2010.10.015.. This is a comprehensive review on recent discoveries in T cell signaling.
- 2.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Seminario MC, Bunnell SC. Signal initiation in T-cell receptor microclusters. Immunol Rev. 2008;221:90–106. doi: 10.1111/j.1600-065X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 4.Huse M. The T-cell-receptor signaling network. J Cell Sci. 2009;122:1269–1273. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 5.Maddock JR, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 6.He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 7. Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729.. Using mobility barriers, the authors demonstrate that EphA2 signaling clusters can mediate mechanoregulation.
- 8.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 9.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 10.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 11.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomason PA, Wolanin PM, Stock JB. Signal transduction: receptor clusters as information processing arrays. Curr Biol. 2002;12:R399–R401. doi: 10.1016/s0960-9822(02)00885-0. [DOI] [PubMed] [Google Scholar]
- 13.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Kanagawa O, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 15.Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol. 2002;14:299–305. doi: 10.1016/s0952-7915(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 16.Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol. 2010;11:342–352. doi: 10.1038/nrm2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 18.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purbhoo MA, Liu H, Oddos S, Owen DM, Neil MA, Pageon SV, French PM, Rudd CE, Davis DM. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal. 2010;3:ra36. doi: 10.1126/scisignal.2000645. [DOI] [PubMed] [Google Scholar]
- 21.Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, Balbo A, Yamaguchi H, Houtman JC, Appella E, Schuck P, et al. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. EMBO J. 2010;29:2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci USA. 1998;95:6302–6307. doi: 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 24.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 26.DeMond AL, Groves JT. Interrogating the T cell synapse with patterned surfaces and photoactivated proteins. Curr Opin Immunol. 2007;19:722–727. doi: 10.1016/j.coi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Manz BN, Jackson BL, Petit RS, Dustin ML, Groves JT. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. PNAS. 2011;108:9089–9094. doi: 10.1073/pnas.1018771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105.. The authors examine how protein clusters in the immunological synapse interact with the actin cytoskeleton.
- 30.Yu CH, Wu HJ, Kaizuka Y, Vale RD, Groves JT. Altered actin centripetal retrograde flow in physically restricted immunological synapses. PLoS ONE. 2010;5:e11878. doi: 10.1371/journal.pone.0011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Smoligovets A, Smith AW, Wu H-J, Petit RS, Groves JT. Unpublished results. [Google Scholar]
- 35. Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944.. Mechanical assays are used to constrain the TCR–pMHC interactions to 2D and measure the kinetic rates. Kinetic rates that are faster than previous measurements in solution were observed and these results correlate well with T cell responses.
- 36. Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, Schutz GJ, Davis MM. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746.. The authors employ single molecule microscopy and fluorescence resonance energy transfer measurements and find that TCR–actin dynamics destabilize the interaction of TCR with its pMHC ligand.
- 37.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 38.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 39.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 40.Sechi AS, Wehland J. Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol. 2004;25:257–265. doi: 10.1016/j.it.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 42.Lai FP, Szczodrak M, Block J, Faix J, Breitsprecher D, Mannherz HG, Stradal TE, Dunn GA, Small JV, Rottner K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008;27:982–992. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. 2009;36:512–524. doi: 10.1016/j.molcel.2009.10.024.. This paper shows that contrary to previous findings, the WAVE2 complex is inactive in its native state and its activation requires simultaneous recruitment of various components to the membrane.
- 44.Meuer SC, Hodgdon JC, Hussey RE, Protentis JP, Schlossman SF, Reinherz EL. Antigen-like effects of monoclonal-antibodies directed at receptors on human T-cell clones. J Exp Med. 1983;158:988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nau GJ, Moldwin RL, Lancki DW, Kim DK, Fitch FW. Inhibition of IL-2 driven proliferation of murine lymphocyte-T clones by supraoptimal levels of immobilized anti-T-cell receptor monoclonal-antibody. J Immunol. 1987;139:114–122. [PubMed] [Google Scholar]
- 46.Casares S, Zong CS, Radu DL, Miller A, Bona CA, Brumeanu TD. Antigen-specific signaling by a soluble, dimeric peptide/major histocompatibility complex class II/Fc chimera leading to T helper cell type 2 differentiation. J Exp Med. 1999;190:543–553. doi: 10.1084/jem.190.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunig T, Dennehy K. CD28 superagonists: mode of action and therapeutic potential. Immunol Lett. 2005;100:21–28. doi: 10.1016/j.imlet.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Leung DW, Otomo C, Chory J, Rosen MK. Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc Natl Acad Sci USA. 2008;105:12797–12802. doi: 10.1073/pnas.0801232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho HYH, Rohatgi R, Lebensohn AM, Ma L, Li JX, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell. 2004;118:203–216. doi: 10.1016/j.cell.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 50.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci USA. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, Umetani J, Brautigam CA, Leong JM, et al. Hierarchical regulation of WASP/WAVE proteins. Mol Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki K, Sheetz MP. Binding of cross-linked glycosylphosphatidylinositol-anchored proteins to discrete actin-associated sites and cholesterol-dependent domains. Biophys J. 2001;81:2181–2189. doi: 10.1016/S0006-3495(01)75866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci USA. 2009;106:12729–12734. doi: 10.1073/pnas.0902621106.. External manipulation of protein clustering is shown to regulate spatial localization.
- 54.Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 55.Cragg MS, Alduaij W, Klein C, Umana P, Glennie MJ, Illidge TM. Novel lysosomal-dependent cell death following homotypic adhesion occurs within cell aggregates response. Blood. 2010;116:3373–3374. [Google Scholar]
- 56.Utomo A, Cullere X, Glogauer M, Swat W, Mayadas TN. Vav proteins in neutrophils are required for Fc gamma R-mediated signaling to Rac GTPases and nicotinamide adenine dinucleotide phosphate oxidase component p40(phox) J Immunol. 2006;177:6388–6397. doi: 10.4049/jimmunol.177.9.6388. [DOI] [PubMed] [Google Scholar]
- 57.Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang JY, Yu JH, Smith MK, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci Signal. 2008;1 doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- 59.Paszek M, Weaver V. Enforcing order on signaling. Science. 2010;327:1335–1336. doi: 10.1126/science.1187865. [DOI] [PubMed] [Google Scholar]
- 60.Abella JV, Parachoniak CA, Sangwan V, Park M. Dorsal ruffle microdomains potentiate Met receptor tyrosine kinase signaling and down-regulation. J Biol Chem. 2010;285:24956–24967. doi: 10.1074/jbc.M110.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–3610. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 62.Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- 63.Sprinzak D, Lakhanpal A, Lebon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]