Abstract
Purpose of review
In thalassemia, ineffective erythropoiesis is characterized by apoptosis of the maturing nucleated erythroid cells. New studies also suggest that limited erythroid cell differentiation plays a role in the development of ineffective erythropoiesis. This would further exacerbate anemia and increase iron absorption.
Recent findings
During erythroid differentiation and maturation, it is critical that the components of hemoglobin are made in stoichiometric amounts. It is, therefore, conceivable that factors that modify this process intrinsically or extrinsically will also affect erythropoiesis. Several proteins have the potential to alter erythroid replication and differentiation in conditions of ineffective erythropoiesis. Elevated erythropoietin levels increase the number of erythroid precursors bearing a phosphorylated form of Jak2. This, in a pathological condition, may contribute to limited erythroid differentiation. Unbalanced synthesis of globins and heme modifies the activity of the heme-regulated inhibitor kinase, affecting proliferation and differentiation of the erythroid precursors. In addition, inefficient elimination of reactive oxygen species, which are increased under conditions of iron overload, may also hamper erythropoiesis.
Summary
Use of Jak2 inhibitors may limit the overproduction of immature erythroid cells in thalassemia, with the potential of reversing extramedullary hematopoiesis and preventing splenectomy. In addition, preventing iron overload and formation of reactive oxygen species may also be beneficial in limiting tissue damage and ineffective erythropoiesis.
Keywords: heme, hepcidin and Jak2, ineffective erythropoiesis, iron metabolism, thalassemia
Introduction
The pathophysiological features observed in thalassemia are likely triggered by increased but inefficacious erythropoiesis, underscoring its dominant role over other physiological processes, such as iron metabolism and bone homeostasis. Identification of new players that control erythropoiesis [1,2••-4••] will enable studies that may lead to new approaches for limiting ineffective erythropoiesis and excessive iron absorption.
Thalassemias: monogenic disorders with pleiotropic effects
The thalassemias are due to a large number of mutations causing abnormal globin gene expression and resulting in total absence or quantitative reduction of globin chain synthesis [5]. Alpha-thalassemia is usually due to deletions within the alpha-globin gene cluster, leading to loss of function of one or both alpha-globin genes in each locus [6]. If three out of four genes are mutated, the condition is called hemoglobin H (HbH) disease, resulting in a hemolytic anemia that can worsen with febrile illness or exposure to certain chemicals, drugs, or infectious agents. HbH disease is characterized by moderate-to-severe anemia, hepatosplenomegaly, and jaundice. If all four alpha-globin genes are deleted, the resulting condition is called alpha-thalassemia major, which is so severe that death often occurs in utero. In contrast to alpha-thalassemia, the majority of the molecular defects associated with beta-thalassemia are usually point mutations involving only one or a limited number of nucleotides. Mutations in the promoter and within the transcribed sequence result in a major defect of beta-globin gene expression either at the transcriptional or posttranscriptional levels [5]. Notably, more than 200 different mutations have been associated with the beta-thalassemia condition [5,7]. A list of these hemoglobin variants is available online at the database HbVar (http://globin.bx.psu.edu/hbvar) [7].
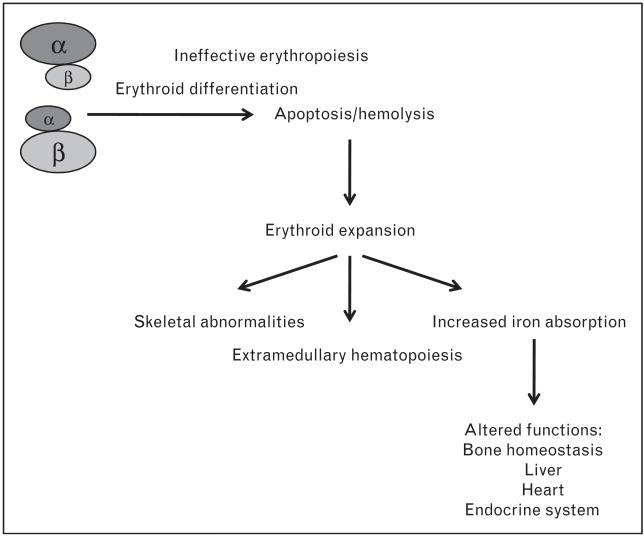
Ineffective erythropoiesis could develop under conditions in which erythroid progenitor precursors either fail to mature, die in the process of becoming erythrocytes, or develop into erythrocytes that are abnormal and die prematurely. Although the erythron is expanded in ineffective erythropoiesis, this results in only a limited number of erythrocytes being produced, far fewer than the same number of erythroid progenitor cells could generate under normal circumstances. The notion of ineffective erythropoiesis in thalassemia was first described using ferrokinetic parameters by Huff et al. [8] and further elaborated on by Finch and colleagues [9-12]. Ineffective erythropoiesis was further characterized showing that apoptosis of the erythroid precursors, due to chain imbalances, leads to medullary as well as intravascular hemolysis [13,14]. However, the apoptotic patterns in thalassemic patients as well as in mouse models of thalassemia point to additional mechanisms that may contribute to this process, as will be described later in this review.
Ineffective erythropoiesis leads to erythroid marrow expansion (Fig. 1). Extramedullary erythropoietic tissues, primarily in the thorax and the paraspinal regions, can lead to characteristic deformities of the skull and face, osteopenia, and demineralization of the bones, which are then prone to fractures. The excessive erythropoietic activity exacerbates the anemia by progressive splenomegaly. Splenic sequestration of red cells may develop, accelerating destruction of abnormal as well as transfused normal red blood cells (RBCs). Splenectomy may help decrease transfusion requirements for patients with splenomegaly. Unfortunately, after the spleen is removed, patients may be at a greater risk for stroke and infections [15•].
Figure 1.
Pleiotropic effects observed in thalassemia
Patients affected by the most severe forms of thalassemia require chronic blood transfusions to sustain life and chelation therapy to prevent iron overload. Those affected by beta-thalassemia intermedia do not require chronic blood transfusions but eventually develop elevated body iron loads also due to increased gastrointestinal iron absorption [16]. As loading continues, the capacity of transferrin, the main transport protein of iron, to bind and detoxify this essential metal may be exceeded. The resulting nontransferrin-bound iron (NTBI) fraction within plasma may promote the generation of reactive oxygen species (ROS), propagators of oxygen-related damage [17-19]. Iron overload is responsible for the most damaging effects of the thalassemias, making iron chelation a major focus of the management of these diseases. In addition, as iron accumulates in the organs, dysfunction of the liver, endocrine glands, and heart become the main factors in limiting the survival of patients with beta-thalassemia [20,21].
Iron absorption is increased in conditions of ineffective erythropoiesis
Iron absorption studies in patients affected by beta-thalassemia intermedia show that the rate of iron loading from the gastrointestinal tract is approximately three to four times greater than normal [16,22-24]. In nontransfused patients with severe thalassemia, abnormal dietary iron absorption results in an increased body iron burden between 2 and 5 g per year depending on the severity of erythroid expansion [25]. Analysis of nontransfused adult patients with HbH disease also indicates that iron overload is common in this disorder [26]. If regular transfusions are required, as in beta-thalassemia major patients, this doubles the rate of iron accumulation. In addition to the transfusion-related iron overload, increased iron absorption also plays a role in beta-thalassemia major, in which its importance is inversely related to Hb levels [27,28].
Erythropoiesis and iron metabolism are extremely intertwined in that alteration of one of the two may have a major impact on the second. In thalassemia, the mechanisms that control iron homeostasis are likely mediated by the relative levels of ineffective erythropoiesis, hypoxia, and iron overload [29]. Hepcidin, a circulating peptide hormone [30], responds to iron overload, limiting or preventing the activity of ferroportin, the iron export molecule, which is expressed in enterocytes and macrophages [31]. However, hepcidin expression can be greatly reduced in conditions of high erythroid demand, apoptosis, hypoxia, and if levels of erythropoietin (Epo) are increased [32,33••,34•,35••,36]. Therefore, though very low levels of hepcidin are extremely helpful in resolving acute or transient blood loss, its continued downregulation will result in iron overload due to the inadequacy of ineffective erythropoiesis in addressing anemia.
The situation in thalassemia is complicated by the relative level of ineffective erythropoiesis and the body iron content that increases over time due to elevated rates of iron absorption or repeated blood transfusions. In addition, blood transfusion can partially suppress ineffective erythropoiesis, limiting iron absorption.This was illustrated in studies with mouse models of beta-thalassemia and then using human thalassemic specimens. However, the mouse models need to be carefully compared to beta-thalassemia patients before any conclusion can be extended to humans [37•]. For instance, patients affected by thalassemia intermedia can exhibit lower Hb levels than the corresponding mouse counterpart. With these Hb levels, ineffective erythropoiesis may be more severe in patients than in mice, and this will likely result in lower hepcidin synthesis affecting iron distribution also. In beta-thalassemia major, mice are generally not transfused. Therefore, ineffective erythropoiesis is more severe, and synthesis of hepcidin is expected to be lower than that found in thalassemia major patients who are chronically transfused [38••,39••]. Keeping these differences in mind, the studies in mice were the first to indicate that hepcidin is downregulated in thalassemia [40-42,43••]. Mice affected by beta-thalassemia intermedia (th3/+ and th1/th1) had relatively low hepcidin expression levels, given their degree of iron overload. In these animals, the level of hepcidin increased with increasing levels of organ iron concentrations (Fig. 2), indicating that in this model hepcidin is still partially responsive to iron overload. In fact, analysis of 1-year-old th3/+ mice indicated that hepcidin expression was similar to or greater than that of control mice. However, in these older animals, upregulation of ferroportin in the duodenum was likely responsible for causing iron overload at that age [43••]. In contrast, the extreme level of ineffective erythropoiesis in mice affected by thalassemia major (th3/th3) [44] prevents hepcidin from sensing the iron burden and keeping its expression very low. Similarly to humans, blood transfusion in these animals increased hepcidin expression and partially suppressed ineffective erythropoiesis [43••,45•]. These studies were confirmed and further extended using human specimens in which urinary hepcidin levels were lower than would be predicted, given the iron burden, and transfusion led to its increase [39••,46]. Overall, these studies suggest that abnormal iron absorption could be prevented by administration of hepcidin (Gardenghi et al., in preparation). Such therapeutic approaches are worth investigating for their potential to reduce iron overload in thalassemias and other forms of anemia associated with ineffective erythropoiesis.
Figure 2.
Relationship between hepcidin and iron content in the liver and spleen in th3/+ mice over time
Ineffective erythropoiesis in thalassemia: a role for Jak2 and Jak2 inhibitors?
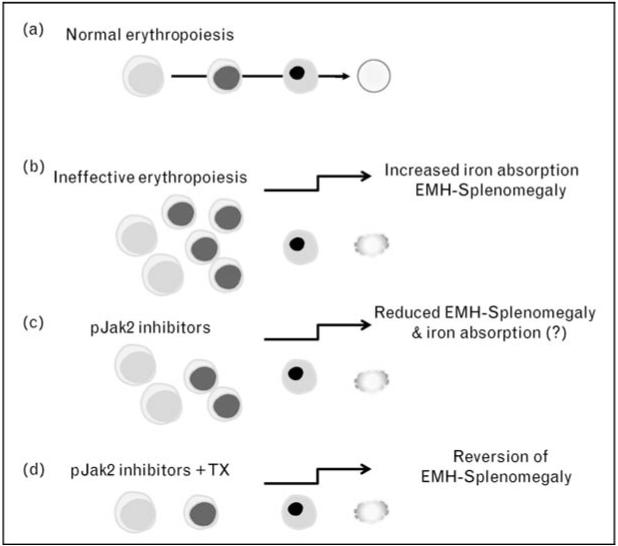
Decreased erythroid cell differentiation may contribute to ineffective erythropoiesis, exacerbating anemia [4••]. The slow progression of the disease in mice affected by thalassemia intermedia closely mimics that in transfusion-independent thalassemic patients who eventually develop splenomegaly. Although the enlarged spleen in these patients sequesters erythrocytes, reducing Hb levels, the exact cause of the splenomegaly and characterization of the various splenic populations is unknown. Our data, from both thalassemic mice and patient specimens, shed light on this phenomenon, suggesting that an increased number of proliferating erythroid progenitors accumulate in the spleen under conditions of ineffective erythropoiesis [4••]. In these studies, a greater than normal percentage of erythroid cells were in S-phase, exhibiting an erythroblast-like morphology [4••]. Thalassemic erythroid cells were associated with the expression of cell cycle promoting genes such as EpoR, Jak2, cyclin-A, Cdk2, Ki-67, and the antiapoptotic protein Bcl-XL. They also differentiated less than normal cells in vitro. Administration of a Jak2 inhibitor to thalassemic mice partially reversed ineffective erythropoiesis and decreased spleen size with limited effect on anemia [4••] (Fig. 3).
Figure 3.
Potential causes that limit erythroid differentiation in thalassemia
If this model is correct, splenomegaly might arise as the rate of differentiation is further reduced, leading to increased sequestration of erythrocytes progressively exacerbating the process. Transfusion-independent thalassemia intermedia patients, if affected by splenomegaly, often develop a need for blood transfusion therapy, and many patients eventually require splenectomy. It is appealing to speculate that thalassemia intermedia patients affected by splenomegaly could be treated temporarily with Jak2 inhibitors to reduce the spleen size and, in the presence of blood transfusion, to prevent further anemia. Moreover, our recent data on transfused and nontransfused th3/+ and th3/th3 mice suggest that even patients affected by beta-thalassemia major, who may develop splenomegaly and extramedullary hematopoiesis (EMH), might benefit from administration of Jak2 inhibitors (Melchiori et al., in preparation). In these settings, the use of Jak2 inhibitors would be expected to limit or reduce splenomegaly, thereby preventing or delaying the need for splenectomy and indirectly improving the management of anemia by reducing the rate of blood transfusions.
A further implication of this model is, as the level of erythroid differentiation decreases, the anemia would worsen thereby increasing serum Epo levels. Although Epo administration may be beneficial under some circumstances [47], when splenomegaly, iron overload, and ineffective erythropoiesis are already present, increasing Epo levels would further aggravate the ineffective erythropoiesis, raising the number of progenitor erythroid cells, which would then fail to differentiate [4••]. However, high Epo levels alone are unlikely to drive this mechanism. Although high Jak2 activity driven by Epo is necessary to increase cell proliferation, it is not likely to be sufficient to prevent cell differentiation. In fact, mutations associated with constitutive activation of Jak2 in erythropoiesis lead to polycythemia vera rather than ineffective erythropoiesis [48]. Therefore, based on this model, additional extreme physiological conditions, in addition to the activation of Jak2, need to occur to interfere with erythroid cell differentiation (Fig. 4).
Figure 4.
Potential correlation between Jak2 and ineffective erythropoiesis
Unbalanced synthesis of heme and globin chains: could they affect erythroid differentiation in thalassemia?
Absent or reduced erythroid differentiation in beta-thalassemia is a difficult concept to fathom. It seems paradoxical that when the body most needs erythrocytes, it would diminish their production. Several intrinsic and extrinsic mechanisms can be postulated to explain this phenomenon. Intrinsic mechanisms may play a major role in conditions of very low or absent beta-globin synthesis and would affect erythropoiesis as soon as hemoglobin production was impaired. For instance, cells with defective beta-globin synthesis may possess an intrinsic safety mechanism that limits the differentiation process in the absence of a stoichiometric quantity of alpha-globin and beta-globin chains. Under these conditions, and in the presence of high Epo levels, the cells may proliferate or, alternatively, slow down rather than differentiate or die. Alternatively, an excess of heme might provide a signal to prevent cell differentiation, which would otherwise lead to a level of alpha-globin aggregates and ROS too toxic for survival (Fig. 4).
Heme-regulated translation mediated by the heme-regulated inhibitorkinase (HRI) provides one major mech-anismthat ensures balanced synthesis ofglobins and heme. HRI phosphorylates the alpha-subunit of eukaryotic translational initiation factor 2 (eIF2alpha) in heme deficiency, thereby inhibiting protein synthesis globally. In beta-thalassemia, HRI is essential in determining RBC size, number, and Hb content, in reducing excess synthesis of globin chains and heme under suboptimal conditions, and finally in reducing the severity of the disease [1,2••-4••]. Therefore, still unknown downstream effectors of HRI may be activated in thalassemic cells and contribute to limiting erythroid cell differentiation.
Reactive oxygen species decrease the lifespan of red blood cells: could they also limit erythroid differentiation?
Expansion of the erythron, in combination with hypoxia, greatly increases iron absorption, leading to generation of ROS, which in turn may also affect erythropoiesis. There is substantial evidence indicating that some aspects of the disorder in beta-thalassemia are mediated by oxidative stress [49•]. In red blood cells derived from patients with beta-thalassemia, ROS levels were elevated, and the corresponding reduced glutathione (GSH) levels were lower than their normal counterparts [50]. Oxidative stress in thalassemic blood cells may explain clinical symptoms, such as the anemia due to the short survival of mature RBC in the circulation, and therefore treatment with antioxidants may have a potential clinical benefit [50].
In support of this notion, addition of antioxidants, such as the compound N-acetylcysteine (NAC) and its derivative AD4, reduced oxidative stress in blood cells derived from thalassemic patients. The effect of antioxidants significantly reduced the rate of hemolysis of thalassemic RBCs and their phagocytosis by macrophages [51•• ]. In addition, intraperitoneal injection of AD4 to th3/+ mice reduced the parameters of oxidative stress [51••].However, long-term studies need to be performed to evaluate whether antioxidants can prevent the worsening of ineffective erythropoiesis, splenomegaly, and anemia as seen in these animals over time.
Another piece of evidence that ROS may affect erythropoiesis comes from studying the Forkhead Box O (FoxO) family of transcription factors, which were shown to play an essential role in the regulation of oxidative stress in hematopoietic stem and erythroid cells (reviewed in [52•]. In particular, these proteins belong to the Forkhead family of winged helix transcription factors, of which the most representative is the mammalian homolog DAF-16 in Caenorhabditis elegans [52•]. Activation of DAF-16 results in a significant increase in C. elegans lifespan, partly through its mediation of defense against oxidative stress [52•].
Studies in a mouse model null for FoxO3 indicated that this protein is essential in red cell survival [3••]. Absence of FoxO3 was associated with reduction in erythrocyte lifespan as well as an enhanced mitotic arrest in intermediate erythroid progenitor cells, resulting in a decreased rate of erythroid maturation. FoxO3-null erythrocytes also showed decreased expression of ROS-scavenging enzymes and evidence of oxidative damage. In fact, mice deficient in FoxO3 die rapidly when exposed to oxidative stress induced by phenylhydrazine. Interestingly, the mitotic arrest as well as the shortened lifespan of the FoxO3-null erythrocytes improved following treatment of the mice with the reducing agent NAC. This raises the interesting possibility that ROS levels regulate not only RBC survival, but also the maturation process of the progenitor erythroid cells. Activation of FoxO3 on one hand could directly protect the erythroid cells from ROS-related damages, whereas on the other hand it would slow down their maturation. This would decrease hemoglobin synthesis and, indirectly, the formation of additional ROS molecules (Fig. 4).
Conclusion
Recent studies in thalassemia suggest the possibility that intrinsic and extrinsic mechanisms play some role inlimiting erythroid differentiation, thereby worsening anemia. These mechanisms might contribute differentially to ineffective erythropoiesis in each patient depending on the level of beta-globin synthesis and other extrinsic factors such as iron overload. Recently, the synthesis of transferrin receptor 1 (TfR-1) [53••] and the activity of the transcriptional factor C/EBPalpha, which is required for hepcidin expression, have been associated to the Epo/EpoR/Jak2/Stat5 pathway [34•]. In addition, it has been shown that phosphorylation and recycling of Fpn1 requires Jak2 activity [54••]. Therefore, Jak2 might represent one of the major links at the interface between erythropoiesis and iron metabolism. Use of Jak2 inhibitors, antioxidant, and analogs of the protein hepcidin may be of value in limiting ineffective erythropoiesis and abnormal iron absorption. Future studies will address whether these scenarios and potential therapeutic options are sound. Further study in mouse models of beta-thalassemia and other hemoglobinopathies characterized by ineffective erythropoiesis followed by rigorous studies in patients will address these questions.
Acknowledgements
This work was sponsored by grants from NIH-NIDDK R21DK065169, the Carlo and Micol Schejola Foundation, the Roche Foundation for Anemia Research (RoFAR), the Cooley’s Anemia Foundation (CAF), and the Children’s Cancer and Blood Foundation. The author would like to thank B. Psaila and K.M. Young for reading the manuscript and for helpful discussions.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as: •of special interest ••of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 226-227).
- 1.Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest. 2005;115:1562–1570. doi: 10.1172/JCI24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. Heme-regulated translation mediated by the HRI provides one major mechanism that ensures balanced synthesis of globins and heme. Translational regulation by HRI is critical to reduce excess synthesis of globin proteins or heme in thalassemia and other hemoglobinopathies and thus reduces the severity of these diseases.
- 3••.Marinkovic D, Zhang X, Yalcin S, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–2144. doi: 10.1172/JCI31807. The FoxO3 member of the FoxO family of transcription factors is essential to prevent overaccumulation of ROS. In the absence of FoxO3, erythrocytes exhibited decreased expression of ROS-scavenging enzymes and had a ROS-mediated shortened lifespan and evidence of oxidative damage.
- 4••.Libani IV, Guy EC, Melchiori L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112:875–885. doi: 10.1182/blood-2007-12-126938. This study introduced the notion that decreased erythroid cell differentiation contributes to ineffective erythropoiesis, exacerbating anemia. To some extent, this is mediated by Jak2. This observation may have important consequences as the use of Jak2 inhibitors has the potential to offer an alternative to splenectomy and profoundly change the management of this disorder.
- 5.Steinberg MH, Forget BG, Higgs DR, et al. Disorders of hemoglobin: genetics, pathophysiology and clinical management. Cam- bridge University Press; Cambridge, UK: 2001. [Google Scholar]
- 6.Vichinsky EP. Changing patterns of thalassemia worldwide. Ann N Y Acad Sci. 2005;1054:18–24. doi: 10.1196/annals.1345.003. [DOI] [PubMed] [Google Scholar]
- 7.Giardine B, van Baal S, Kaimakis P, et al. HbVar database of human hemoglobin variants and thalassemia mutations: 2007 update. Hum Mutat. 2007;28:206. doi: 10.1002/humu.9479. [DOI] [PubMed] [Google Scholar]
- 8.Huff RL, Hennessy TG, Austin RE, et al. Plasma and red cell iron turnover in normal subjects and in patients having various hematopoietic disorders. J Clin Invest. 1950;29:1041–1052. doi: 10.1172/JCI102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finch CA, Sturgeon P. Erythrokinetics in Cooley’s anemia. Blood. 1957;12:64–73. [PubMed] [Google Scholar]
- 10.Finch CA, Deubelbeiss K, Cook JD, et al. Ferrokinetics in man. Medicine. 1970;49:17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Pootrakul P, Huebers HA, Finch CA, et al. Iron metabolism in thalassemia. Birth Defects Orig Artic Ser. 1988;23:3–8. [PubMed] [Google Scholar]
- 12.Cazzola M, Finch CA. Iron balance in thalassemia. Prog Clin Biol Res. 1989;309:93–100. [PubMed] [Google Scholar]
- 13.Centis F, Tabellini L, Lucarelli G, et al. The importance of erythroid expansion in determining the extent of apoptosis in erythroid precur-sors in patients with beta-thalassemia major. Blood. 2000;96:3624–3629. [PubMed] [Google Scholar]
- 14.Schrier SL. Pathophysiology of thalassemia. Curr Opin Hematol. 2002;9:123–126. doi: 10.1097/00062752-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 15•.Taher A, Mehio G, Isma’eel H, et al. Stroke in thalassemia: a dilemma. Am J Hematol. 2008;83:343. doi: 10.1002/ajh.21117. This article underlines recent observations that suggest a potential correlation between splenectomy and stroke in beta-thalassemia.
- 16.Pippard MJ, Callender ST, Warner GT, et al. Iron absorption and loading in beta-thalassaemia intermedia. Lancet. 1979;2:819–821. doi: 10.1016/s0140-6736(79)92175-5. [DOI] [PubMed] [Google Scholar]
- 17.Pippard MJ, Callender ST, Finch CA. Ferrioxamine excretion in iron-loaded man. Blood. 1982;60:288–294. [PubMed] [Google Scholar]
- 18.Pootrakul P, Breuer W, Sametband M, et al. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalasse- mia/HbE patients treated with an oral chelator. Blood. 2004;104:1504–1510. doi: 10.1182/blood-2004-02-0630. [DOI] [PubMed] [Google Scholar]
- 19.Rachmilewitz EA, Weizer-Stern O, Adamsky K, et al. Role of iron in inducing oxidative stress in thalassemia: can it be prevented by inhibition of absorption and by antioxidants? Ann N Y Acad Sci. 2005;1054:118–123. doi: 10.1196/annals.1345.014. [DOI] [PubMed] [Google Scholar]
- 20.Propper RD, Cooper B, Rufo RR, et al. Continuous subcutaenous adminis- tration of deferoxamine in patients with iron overload. N Engl J Med. 1977;297:418–423. doi: 10.1056/NEJM197708252970804. [DOI] [PubMed] [Google Scholar]
- 21.Olivieri NF, Weatherall DJ. Clinical aspects of beta-thalassemia. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of hemoglobin: genetics, pathophysiology and clinical management. Cambridge University Press; Cambridge, England: 2001. [Google Scholar]
- 22.Bannerman RM, Keusch G, Kreimer-Birnbaum M, et al. Thalassemia inter- media, with iron overload, cardiac failure, diabetes mellitus, hypopituitarism and porphyrinuria. Am J Med. 1967;42:476–486. doi: 10.1016/0002-9343(67)90276-8. [DOI] [PubMed] [Google Scholar]
- 23.Cossu P, Toccafondi C, Vardeu F, et al. Iron overload and desferrioxamine chelation therapy in beta-thalassemia intermedia. Eur J Pediatr. 1981;137:267–271. doi: 10.1007/BF00443255. [DOI] [PubMed] [Google Scholar]
- 24.Fiorelli G, Fargion S, Piperno A, et al. Iron metabolism in thalassemia intermedia. Haematologica. 1990;75:89–95. [PubMed] [Google Scholar]
- 25.Hershko C, Rachmilewitz EA. Mechanism of desferrioxamine-induced iron excretion in thalassaemia. Br J Haematol. 1979;42:125–132. doi: 10.1111/j.1365-2141.1979.tb03704.x. [DOI] [PubMed] [Google Scholar]
- 26.Hsu HC, Lin CK, Tsay SH, et al. Iron overload in Chinese patients with hemoglobin H disease. Am J Hematol. 1990;34:287–290. doi: 10.1002/ajh.2830340410. [DOI] [PubMed] [Google Scholar]
- 27.Erlandson ME, Walden B, Stern G, et al. Studies on congenital hemolytic syndromes. Part IV: gastrointestinal absorption of iron. Blood. 1962;19:359–378. [PubMed] [Google Scholar]
- 28.Cazzola M, Pootrakul P, Huebers HA, et al. Erythroid marrow function in anemic patients. Blood. 1987;69:296–301. [PubMed] [Google Scholar]
- 29.Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–1702. [PubMed] [Google Scholar]
- 30.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 32.Pak M, Lopez MA, Gabayan V, et al. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. This work shows that the von Hippel-Lindau/hypoxia-inducible transcription factor (VHL/HIF) pathway is an essential link between iron homeostasis and hepcidin regulation in vivo.
- 34•.Pinto JP, Ribeiro S, Pontes H, et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signalling and regulation of C/EBP{alpha} Blood. 2008;111:5727–5733. doi: 10.1182/blood-2007-08-106195. This study suggests a direct involvement of Epo in hepcidin regulation through the transcriptional factor C/EBPalpha.
- 35••.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. This study showed that a protein called GDF15 is secreted by thalassemic red cells suppressing hepcidin expression in the liver. This observation may have important consequences as GDF15 might be utilized as a scientific and therapeutic tool to investigate ineffective erythropoiesis and iron metabolism.
- 36.Tamary H, Shalev H, Perez-Avraham G, et al. Elevated growth differentiation factor 15 expression in patients with congenital dyserythropoietic anemia type I. Blood. 2008;112:5241–5244. doi: 10.1182/blood-2008-06-165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Rechavi G, Rivella S. Regulation of iron absorption in hemoglobinopathies. Curr Mol Med. 2008;8:646–662. doi: 10.2174/156652408786241401. This is a comprehensive review of mouse models of thalassemia and sickle cell anemia and how they have been utilized to study the relationship between erythropoiesis and iron metabolism.
- 38••.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. doi: 10.1002/pbc.20616. This study determined the hepcidin levels in patients with congenital chronic anemias, including thalassemic patients.
- 39••.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. This study determined the hepcidin levels in patients with thalassemic intermedia and major, showing a strong correlation between high erythropoietic drive and severe hepcidin deficiency.
- 40.Adamsky K, Weizer O, Amariglio N, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124:123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 41.Breda L, Gardenghi S, Guy E, et al. Exploring the role of hepcidin, an antimicrobial and iron regulatory peptide, in increased iron absorption in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:417–422. doi: 10.1196/annals.1345.069. [DOI] [PubMed] [Google Scholar]
- 42.De Franceschi L, Daraio F, Filippini A, et al. Liver expression of hepcidin and other iron genes in two mouse models of beta-thalassemia. Haematologica. 2006;91:1336–1342. [PubMed] [Google Scholar]
- 43••.Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in {beta}-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivella S, May C, Chadburn A, et al. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta -globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- 45•.Jenkins ZA, Hagar W, Bowlus CL, et al. Iron homeostasis during transfusional iron overload in beta-thalassemia and sickle cell disease: changes in iron regulatory protein, hepcidin, and ferritin expression. Pediatr Hematol Oncol. 2007;24:237–243. doi: 10.1080/08880010701360700. This study indicates that in patients affected by beta-thalassemia in which the erythropoietic drive was relieved by transfusion, the hepcidin levels are high, purely reflecting the response of this hormone to excess liver iron.
- 46.Kattamis A, Papassotiriou I, Palaiologou D, et al. The effects of erythropoietic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 47.Perrine SP. Fetal globin stimulant therapies in the beta-hemoglobinopathies: principles and current potential. Pediatr Ann. 2008;37:339–346. doi: 10.3928/00904481-20080501-10. [DOI] [PubMed] [Google Scholar]
- 48.Wang YL, Vandris K, Jones A, et al. JAK2 mutations are present in all cases of polycythemia vera. Leukemia. 2007;22:1289. doi: 10.1038/sj.leu.2405047. [DOI] [PubMed] [Google Scholar]
- 49•.Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8:609–619. doi: 10.2174/156652408786241384. A comprehensive review of the role of ROS in hemolytic anemias.
- 50.Amer J, Goldfarb A, Fibach E. Flow cytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur J Haematol. 2003;70:84–90. doi: 10.1034/j.1600-0609.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 51••.Amer J, Atlas D, Fibach E. N-acetylcysteine amide (AD4) attenuates oxidative stress in beta-thalassemia blood cells. Biochim Biophys Acta. 2008;1780:249–255. doi: 10.1016/j.bbagen.2007.11.009. This article describes that patients affected by sickle cell anemia and thalassemia exhibited 10-fold to 30-fold higher ROS production and 20–50% lower GSH content in RBC, platelets, and polymorphonuclear cells (PMN) compared with normal counterparts. These results suggest that these patients may benefit from antioxidant therapies, limiting oxidative damage to RBC, PMN, and platelets and potentially alleviating symptoms associated with their pathology.
- 52•.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. A thorough review of the role of FoxO family members in regulating hematopoietic cell homeostasis.
- 53••.Kerenyi MA, Grebien F, Gehart H, et al. Stat5 regulates cellular iron uptake of erythroid cells via IRP-2 and TfR-1. Blood. 2008;112:3878–3888. doi: 10.1182/blood-2008-02-138339. This study suggests a link between EpoR/Jak/Stat signaling and iron metabolism, showing that in mice that completely lack Stat5 activity the cell surface levels of TfR-1 on erythroid cells were decreased more than 2-fold. The authors showed that reduction could be attributed to reduced transcription of TfR-1 mRNA and iron regulatory protein 2 (IRP-2), the major translational regulator of TfR-1 mRNA stability in erythroid cells.
- 54•.De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. This study links Jak2 to ferroportin. Hepcidin binds to the iron exporter ferroportin, inducing its degradation and thus preventing iron entry into plasma. This paper indicates that Jak2 phosphorylates ferroportin, following binding of this protein to hepcidin. Phosphorylation of ferroportin then triggers its internalization and de- gradation.