Abstract
The stable conformations of GABAA-benzodiazepine receptor bivalent ligands were determined by low temperature NMR spectroscopy and confirmed by single crystal X-ray analysis. The stable conformations in solution correlated well with those in the solid state. The linear conformation was important for these dimers to access the binding site and exhibit potent in vitro affinity and was illustrated for α5 subtype selective ligands. Bivalent ligands with an oxygen-containing linker folded back upon themselves both in solution and the solid state. Dimers which are folded do not bind to Bz receptors.
Keywords: conformation, benzodiazepines, NMR, GABA
Introduction
The GABAA/BzR complex contains a chloride ion channel which comprises part of the major inhibitory neurotransmitter system in the CNS.1 This system regulates numerous neurological functions including convulsions, anxiety and sleep activity, as well as memory and learning processes.2-5 This membrane-bound heteropentameric protein polymer is composed principally of α, β and γ subunits. Recombinant receptors containing these subunits closely mimic the biological, electrophysiological and pharmacological properties of native GABAA receptors.5-7 Agents selective for specific BzR subtypes may permit one to separate out the pharmacological activities of these different isoforms.8-12 This is a goal of paramount importance in the search for new anxiolytic agents and new anticonvulsant compounds with decreased side effects.13-17
Currently, transforming monomers into a bivalent ligand is one of the successful strategies for developing potent ligands with enhanced selectivity.18-20 Bivalent ligands are defined as compounds which contain two pharmacophores joined through a connecting unit or linker. The general structure for bivalent ligands is described as P-X-P (P: pharmacophore; X: linker) (see Table I). The proper selection of a suitable linker X is crucial for potent receptor binding.
TABLE I.

| Compounds | Mono unit 1 |
Mono unit 2 |
Spanner | Stable Conformation |
Ki (αnβ3γ2) = nM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X-ray | NMR | α1 | α2 | α3 | α4 | α5 | α6 | ||||
| 1a | A | – | C2H5 | – | – | 28.4 | 21.4 | 25.8 | 5.3 | 0.49 | 2 8.8 |
| 2 | A | A | (CH2)3 | linear | linear | >1000 | >1000 | 858 | 1550 | 15 | >2000 |
| 3 | A | A | (CH2)5 | – | linear | 231 | 661 | 2666 | ND | 5.4 | 54.22 |
| 4 | C | C | (CH2)3 | – | linear | 1852 | 4703 | 8545 | ND | 100.5 | 5000 |
| 5 | A | A | ch2och2 | – | folded | 3795 | 2694 | 1864 | ND | 76.14 | ND |
| 6a | B | – | C2H5 | – | – | 287 | 45 | 96 | 1504 | 13.8 | 1000 |
| 7 | B | B | (CH2)2O(CH2)2 | folded | folded | 460 | 5000 | ND | ND | 5000 | 5000 |
| 8 | B | B | (CH2)3 | linear | linear | 236 | 7.4 | 272 | >5000 | 194.2 | >5000 |
monomer
Recent studies on the binding selectivity of the inverse agonist RY-80 (1) indicated preferential binding to α5 BzR/GABAA subtypes.21, 22 Therefore, the bivalent ligand Xli-093 (2) was developed by incorporating the pharmacophore of 1 with a three-carbon linker.22 This bivalent ligand exhibited selective affinity for the α5 subtype and behaved as a selective antagonist of the effects of diazepam in oocytes at this α5 subtype. Effects at the other three diazepam-sensitive sites were minimal. Encouraged by this, a series of new bivalent ligands were designed and synthesized (Table I).
It was hoped that these new dimers might exhibit enhanced selectivity and potency at the α5 BzR/GABAA subtypes. It was also expected that insertion of an oxygen atom into the linker might increase water solubility and hence enhance molecular hydrophilicity which should play an important role in the pharmacokinetic properties of the ligand.
The nature of the functional groups in a ligand plays an important role in receptor binding, of course, as well as the conformation in solution. The more information about the stable conformation(s) of molecules the better the understanding of the structure-activity relationships. It was essential from the beginning of the present study to determine the conformation of these bivalent ligands, which contained 3 to 5 atom linkers, since the steric requirements for affinity to the Bz receptor must be satisfied.23
In traditional medicinal chemistry, computer assisted molecular modeling programs and X-ray analysis contribute greatly in the search for stable conformations. However, some problems with these methods should not be neglected. Using computer modeling to determine the stable conformation of molecules containing many free rotating bonds, such as those contained in bivalent ligands, is difficult. Although X-ray crystallography is the ultimate arbiter of chemical structure, it has many limitations beyond the obvious need for crystals: it often does not reflect accurately the conformation in solution, nor is it informative regarding conformational equilibria. This information is crucial in drug design. However, NMR spectroscopy is a powerful technique in drug discovery and its role in conformational analysis cannot be surpassed by other spectroscopic methods.24
Herein is described a method utilizing low temperature NMR for the determination of the solution stable conformation of a series of GABAA-benzodiazepine bivalent ligands with different monomeric units and linkers. The conformations in solution were determined by NMR spectroscopy and compared with those in the crystal structure. The combination of low temperature NMR and X-ray analysis provided accurate structural information required for understanding structure-activity relationships and drug design. The influence of the molecular structure of the linker on the conformation is also discussed.
Results
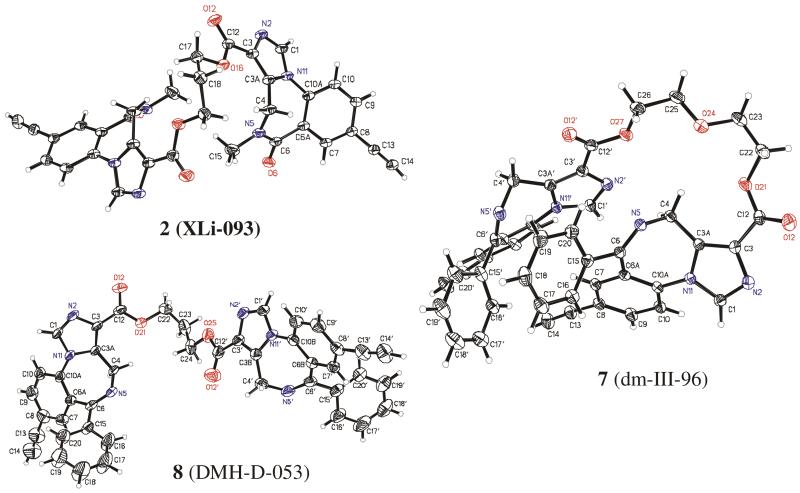
Recently it was shown the active dimer 2 existed in a linear conformation in the solid state while dimer 7 with a oxygen containing linker folded back upon itself, as illustrated in Figure I.25
Figure I. Crystal structure of 2 (left top), 7 (right) and 8 (left bottom).
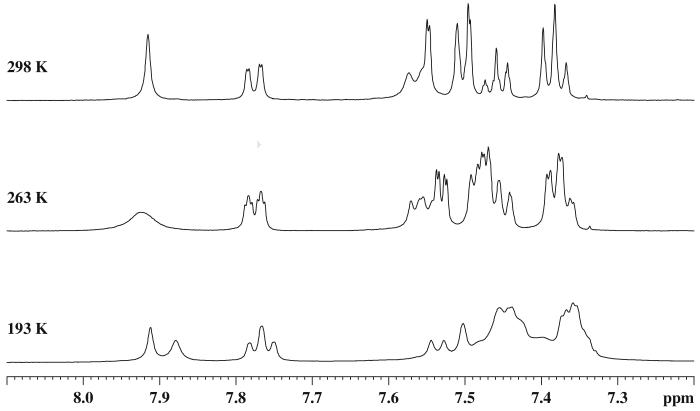
Since the bioactive conformation in solution may or may not parallel that in the crystal structure, the lowest energy solution structure must be established in order to correlate conformation with biological activity. Thus, NMR experiments at variable temperatures were performed and data were collected in different solvents. In methylene chloride or chloroform at room temperature, only a single set of signals was detected for both bivalent ligands 2 and 7. At low temperature, it was found that the linear dimer 2 exhibited only a small splitting of about 3 Hz for some of the aromatic protons in the 1HNMR spectra25, while two clearly separated sets of signals were observed for the folded dimer 7 (Figure II). For example, as seen in Figure II for 7, the signal of H1 (7.92 ppm at 298 K) was split into two peaks at δ7.91 and 7.88 ppm, respectively, at 193° K. Similar results were observed in the 13C spectrum where C1 (134.9 ppm at 298°K) split into two signals at 135.3 and 135.4 ppm at 198° K. The doubling of the signals is consistent with disruption of the symmetry between the two domains of the molecule as expected if 7 adopted a static folded structure similar to the crystalline state.
Figure II.
Aromatic region of 1HNMR spectra of 7 in CD2Cl 2 at variable temperatures.
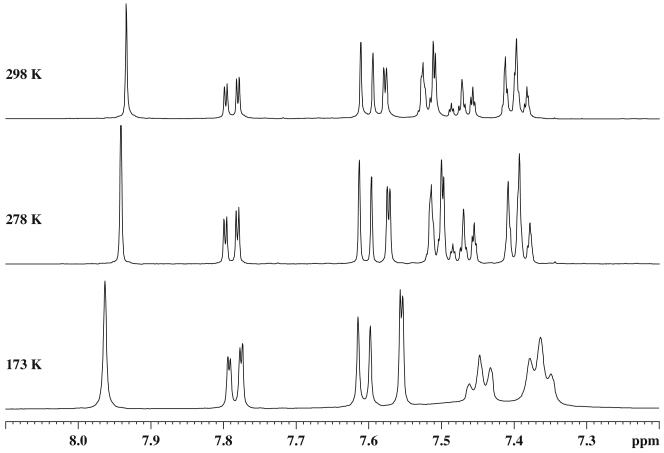
However, the possibility could not be ruled out that the split in the signals was caused by slowing a dynamic process within each domain, such as conformational interconversion of the seven-membered ring. In order to investigate this possibility, the NMR spectra of the monomer 6 were run at low temperature as well. At temperatures as low as 173 K only one set of signals was observed in both the 1H and 13C NMR spectra of 6 (Figure III).25 At lower temperatures, however, this was quite different from what was observed for dimer 7 at room temperature; the spectra of 6 and 7 were indistinguishable at 25°C. Moreover, some additional line broadening of some of the aromatic signals was observed at the lowest temperature in both the monomer 6 and in its dimer 7.
Figure III.
Aromatic region of 1HNMR spectra of 6 in CD2Cl 2 at different temperatures.
The analysis of these data indicated that the line broadening at the lowest temperature was due to one of the conformational processes mentioned above, whereas the doubling of the peaks in 7 was caused by the presence of two domains. Certainly an interdomain interaction existed between the two heterocyclic units of 7, but not in monomer 6. It was therefore concluded the internal mobility of the molecule decreased when the temperature was lowered which permitted observation of the two sets of signals of 7 on the NMR time scale. It was thus suggested that only when the molecule preferred the folded conformation in solution were two sets of signals observed. The preferred conformations of the molecules in CDCl3 and CD2Cl2 correlated quite well with those observed in the crystal structures (Table I).
The study was then expanded by varying the nature of the linker and monomer. Dimers 3 and 5 contain the same monomeric unit as 2, whereas 8 contains an all carbon linker. It was found that, 2 3, 4 and 8 exhibited only one set of NMR signals at low temperature, whereas the NMR signals of ligands 5 and 7 split into two sets at low temperature. Low temperature NMR studies were performed in CD2Cl2. It was concluded that 5 and 7 preferred a folded conformation, while 2, 3, 4 and 8 assumed a linear conformation. These conclusions are supported by a crystal structure obtained for bivalent ligand 8 which indicated 8 was present in a linear conformation in the solid state. These results are illustrated in Table I.
Since the goal was to design and synthesize bivalent ligands for biological applications in aqueous solution, the question arose: Do the conformations in CDCl3 or CD2Cl2 resemble those in aqueous solution? Attempts to run the NMR experiments in water failed since the ligands were not sufficiently soluble in D2O. However, the spectra of both the linear and folded dimer 2 and 7 could be carried out in MeOH-d4. The solvating properties of methanol, of course, more closely resemble those of water, and this more closely mimics aqueous physiological condition as well. The conformations of dimers in MeOH-d4 were consistent with conformations in hydrophobic solvents.
Discussion
From the results described above, it is clear that dimers which contain an oxygen atom in the linker tend to adopt a folded conformation. Analysis of these data indicated in the hydrophilic solvent, 7 also had a higher tendency to fold back upon itself than 2, as it did in the hydrophobic media (Figure IV and Figure V). In fact, the tendency of 7 to assume a folded structure appeared to be higher in methanol than in CD2Cl2 or CDCl3, as the free rotation of the molecule was limited (Figure VI). On the other hand, for dimer 2, which preferred a linear structure in the solid state and in lipophilic solvents, only one set of signals was observed at room temperature in MeOH-d4(Figure VI).
Figure IV.
Aromatic region of 1H NMR spectra of 2 in MeOH-d4 at different temperatures.
Figure V.
Aromatic region of proton spectra of 7 in MeOH-d4 at different temperatures.
Figure VI.
Partial HSQC spectrum of 7 in MeOH-d4 at 298 K.
It is interesting to note that in the preparation of the samples, dimer 2 was less soluble in MeOH-d4 than was 7. It has been established that ligands are easier to dissolve in a solvent when the ligands surface energy can be minimized. Bivalent ligand 7 was more polar than dimer 2 and has more tendency to fold back. Consequently, 7 was presumably, easier to dissolve in methanol because its surface energy was minimized. On the other hand, when ligand 2 was dissolved in a more polar solvent such as methanol, the surface energy may be forced to be minimized.
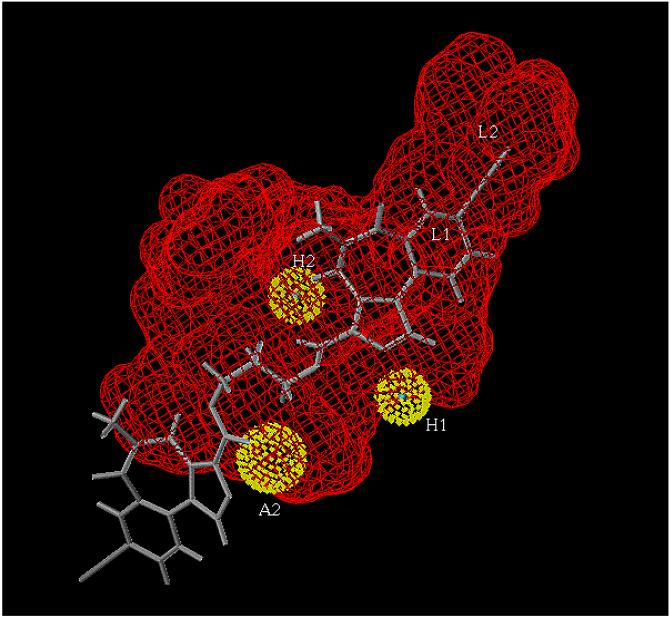
Since the conformation of molecules 2 and 7 in methanol agree with those in CD2Cl2 or CDCl3, the behavior of these ligands in CD2Cl2 or CDCl3 should reflect those in aqueous solution. The stable conformation of the compounds determined in CD2Cl2 or CDCl3 were correlated with the newly generated receptor binding data (Table I). In the pharmacophore/receptor model, the bivalent ligands in the linear conformation align well(Figure VII).
Figure VII.
Bivalent ligand 2 (Xli093) aligned in the included volume of the pharmacophore/receptor model for the 5 3 2 subtype.
Importantly, bivalent ligands 2, 3, 4 and 8 with carbon only linkers prefered the linear conformation as the stable conformation, independent of the number of linker atoms. In contrast, replacing the middle carbon of either linker (CH2)3 or (CH2)5 with an oxygen atom altered the stable conformation of the molecules 5 and 7 from linear to folded. The only difference between bivalent pairs 2 and 5 with linear and folded conformations as the stable ones, respectively, was the center atom. In compound 2 the middle atom was carbon, while in 5, oxygen was present. Consequently, it was decided to focus attention on the conformational difference between carbon and oxygen containing linkers.
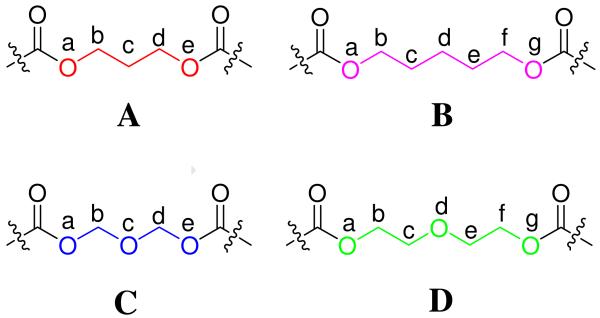
It was well-known26, 27 that the carbon chain in both small molecules and polymers favored the anti conformation which results in a linear arrangement of atoms. From examination of the linkers (Figure VIII), it was easily seen that linkers C and D can be regarded as oligomers of oxymethylene (OCH2)2 and oxyethylene (OCH2CH2)2.
Figure VIII.
The linkers of the bivalent ligands.
It was well documented that the preference for the gauche-gauche conformation28-30 of a simple open-chain acetal such as dimethoxymethane (CH3OCH2OCH3, 9) could be predicted on the basis of the anomeric effect and related stereoelectronic effects. In this conformation the polar C–O bonds are favorably oriented such that a lone pair orbital of the oxygen atom was almost antiperiplanar to the C–O bond (Figure IX, lone pair orbital and C–O bond in same color). This permits maximum overlap of the n orbital of the oxygen atom with the σ* orbital of the C–O bond. This was not possible in the anti-anti or gauche-trans conformations and the two rabbit-ear interactions28-32 (Figure IX) engendered by each pair of adjacent oxygen atoms in the anti-anti conformer are avoided.
Figure IX.
The conformations for dimethoxymethane.
Furthermore, in the related polymer of 9 with the two-bond repeating sequence, poly(oxymethylene) (POM), the gauche conformation was, in fact, markedly preferred over trans and the polymer existed in a helical (all gauche) conformation27, 33-35 rather than in the all-anti one.
Similarly, much effort has been spent on the investigation of the conformational characteristics of 1,2-dimethoxyethane (glyme, CH3OCH2CH2OCH3, 10) as a model molecule for understanding the conformations of poly(oxyethylene) (POE). It had long been established that POE chains have a large fraction of bonds in gauche conformations and assumed a helical conformation overall.26, 27, 33-35 It has been proposed that the oxygen gauche effect27, 36, 37 and 1, 5-C–H–O interaction38-40 within the molecule were responsible for the gauche-rich conformations.
Based on this pioneering work, the correlation was made that the conformation of the linkers in the bivalents 2–5 and 7–8 adopt anti (B), gauche-gauche (C), trans-gauche-trans (D) conformations, respectively, regardless of the monomeric units which comprise them. The arrangement in space (disregarding the direction) of every unit in the linkers is depicted in Figure X. The end-to-end distance of each unit (C and D) which adopted the gauche conformation was shorter than the one in the anti conformer. The more units in the linker, the shorter the gauche linker. Moreover, it was recognized that the C–O bond length (1.43Å is often appreciably shorter than the C–C bond (1.54 Å).41 Therefore, it was believed that the linkers with the oxygen atom in the middle favored the helical conformation and rendered the two monounits in each dimer sufficiently close to each other with suitable dihedral angles to facilitate the intramolecular lipophilic-lipophilic (aromatic-aromatic) interaction. This was regarded as one of the most important factors to stabilize the folded structure as the preferred conformation.42-46 For these same reasons, bivalent ligands with the linker B adopted the linear conformation.
Figure 10.
Newman projection for linkers B, C and D.
For the higher analog of POM and POE, namely, poly(trimethylene oxide) [–(CH2)3–O–]x (POM3), trans-gauche-gauche-trans was sightly preferred over all-trans and trans-trans-gauche-trans comformers in the crystalline state.27 The preference for the gauche state in this case was only 0.2 kcal/mol where as in the POM and POE examples was 1.5 and 0.4 kcal/mol, respectively.33 Since the energy difference between gauche and trans was low, the linker A had more flexibility than the other linkers (C and D) to rotate freely and less tendency to occur as gauche. This could lead to improper end-to-end distances or dihedral angles for the interaction between the aromatic monounits. Hence dimers connected with linker A could not be stabilized in the folded conformation even though the same aromatic monounits were contained in the molecules.
Bivalent ligands were evaluated in competition binding assays for specific GABAA membrane proteins using [3H] flunitrazepam as the radiolabel. This assay measures the ability of the ligand to displace flunitrazepam. Data is reported as Ki according to the Cheng-Prusoff equation.47 Biological data are presented in Table I for dimers synthesized using different spanners (or linkers). The linkers in 5 and 7 contained an oxygen atom. Compounds 2, 3, 4, and 8 contained all carbon linkers. Binding data indicate decreased affinity when bivalents contain an oxygen atom in the linker. Compounds 2 and 3 showed increased selectivity for the α5 subtype as compared to parent monomer 1. Compound 5 which was analogous to 2 with the exception of the oxygen atom present in the linker, bound with less affinity at the α5 subtype. Likewise bivalent 8 showed increased selectivity versus monomer 6. However, the bivalent 7 containing an oxygen atom in the linker did not bind. The data suggests that dimers which contain a single oxygen atom in the linker bind less decreased affinity to the Bz receptor. This is due to their propensity to adopt a folded conformation. A strategy to increase hydrophilicity and avoid a folded conformation is to extend the linker length and insert two opposing oxygen atoms. This research is currently underway.
Conclusion
In summary, comparison of the results of low temperature NMR studies to crystal structures has provided enough information to demonstrate that low temperature NMR can be used as a quick method to identify dimeric ligands with a tendency to fold back upon themselves, as compared with those preferring a linear conformation. A correlation with binding data shows that the suitability of a ligand in the α5 BzR/Gabaergic subtype is heavily influenced by its conformation in solution. Variable temperature NMR thus can be used as a tool for screening bivalent ligands for their in vivo suitability. It is also clear the presence of one oxygen atom in the linker was the principle cause for the dimer to fold back onto itself. Ligands which contain two offsetting oxygen atoms in the linker are now under study in our laboratory.
Experimental
Synthesis
Inverse agonist 1 (RY080) was synthesized via the reported procedure.3, 48 Hydrolysis of the ester function of 1 provided the acid 9 in excellent yield and this material was subjected to a standard CDI-mediated coupling reaction to furnish bivalent ligands 2-5 in 60% yield (Scheme I).22, 49
Scheme XI.
Synthesis of bivalent analogs of Xli-093.
The acid 11, obtained from the ester 10, which was available from the literature,3, 50 was stirred with CDI in DMF, followed by stirring with the required diol and DBU to provide bromide dimers 12 or 13, respectively. They were converted into the trimethylsilylacetylenyl 14 or 15, respectively under standard conditions (Pd-mediated, Heck-type coupling).51, 52 The bisacetylene 7 or 8 (individually) was easily obtained by treatment of the trimethylsilyl ligand 14 or 15 with fluoride anion, as shown in Scheme II.
Scheme XII.
Synthesis of bivalent analogues of DMH-D-053.
Materials and General Instumentation
Chemicals were purchased from Aldrich Chemical Co. or Tokyo Chemical Industries and were used without further purification except where otherwise noted. Anhydrous THF was distilled from sodium/benzophenone ketyl. TLC analyses were carried out on Merch Kieselgel 60 F254, and flash column chromatography was performed on silica gel 60b purchased from E. M. Laboratories. Melting points were taken on a Thomas-Hoover melting point apparatus or an Electrothermal Model IA8100 digital melting point apparatus and are reported uncorrected. NMR spectra were recorded on a Bruker 300 or 500 MHz multiple-probe spectrometer. Infrared spectra were recorded on a Nicolet DX FTIR BX V5.07 spectrometer or a Mattson Polaris IR-10400 instrument. Low-resolution mass spectral data (EI/CI) were obtained on a Hewlett-Packard 5985B GC-mass spectrometer, while high resolution mass spectral data were taken on a VG autospectrometer (Double Focusing High Resolution GC/Mass Spectrometer, UK). Microanalyses were performed on a CE Elantech EA1110 elemental analyzer.
Competition Binding Assays
Competition binding assays were performed in a total volume of 0.5 mL at 4°C for 1 hour using [3H] flunitrazepam as the radioligand. For these binding assays, 20-50 mg of membrane protein harvested with hypotonic buffer (50 mM Tris-acetate pH 7.4 at 4 degree) was incubated with the radiolabel as previously described.53 Nonspecific binding was defined as radioactivity bound in the presence of 100 μM diazepam and represented less than 20% of total binding. Membranes were harvested with a Brandel cell harvester followed by three ice-cold washes onto polyethyleneimine-pretreated (0.3%) Whatman GF/C filters. Filters were dried overnight and then soaked in Ecoscint A liquid scintillation cocktail (National Diagnostics; Atlanta, GA). Bound radioactivity was quantified by liquid scintillation counting. Membrane protein concentrations were determined using an assay kit from Bio-Rad (Hercules, CA) with bovine serum albumin as the standard.
Radioligand Binding Assays
(Dr Mckernan and Atack)49 In brief, the affinity of compounds for human recombinant GABA(A) receptors was measured by competition binding using 0.5 nM [3H]flunitrazepam. Transfected HEK Cells (beta2 gamma2 and desired alpha subtype) were harvested into phosphate-buffered saline, centrifuged at 3,000 g and stored at −70°C until required. On the day of the assay, pellets were thawed and re-suspended in sufficient volume of 50 mM Tris/acetate (pH 7.4 at 4°C) to give a total binding of approximately 1500-2000 dpm. Non-specific binding was defined in the presence of 100 mM (final concentration) diazepam. Test compounds were dissolved in DMSO at a concentration of 10 mM and diluted in assay buffer to give an appropriate concentration range in the assay, such that the final DMSO concentration in the assay was always less than 1%. Total assay volume was 0.5 mL and assays were carried out in 96-well plates and incubation time started by the addition of 0.1 mL of re-suspended cell membranes. Following incubation for 1 hour at 4°C, assays were terminated by filtration through GF/B filters, washed with 10 mL ice cold buffer, dried and then counted using a liquid scintillation counter. The percentage inhibition of [3H] flunitrazepam binding, the IC50 and the Ki values were calculated using the Activity Base Software Package (ID Business Solutions, Guildford , UK) according to the Cheng-Prusoff equation.47
1,3-Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) propyl diester 2
(XLi093) (Procedure A). To a solution of carbonyl diimidazole (230.3 mg, 0.57 mmol) in anhydrous DMF (5 mL) was added 8-ethynyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]-benzodiazepine-3-carboxylic acid 9 (200 mg, 0.71 mmol). The solution which resulted was stirred for 2 h at rt. Analysis by TLC (silica gel) indicated the absence of starting material. To the solution which resulted was then added 1,3-propanediol (27.1mg, 0.36 mmol) in dry DMF (0.5 mL) and also DBU (114.2 mg, 0.75 mmol) in dry DMF (0.10 mL) at rt. The mixture was stirred at rt for 4.5 h until analysis by TLC (silica gel) indicated the reaction was complete. The reaction mixture was then poured into ice water (30 mL) and extracted with CH2Cl2 (3 × 50 mL). The combined organic layer was washed with H2O (5 × 50 mL), brine and dried (Na2SO4). The solvent was removed under reduced pressure and the residue was purified by flash chromatography (silica gel, EtOAc/CH3OH, 4: 1) to provide 2 (157 mg) as a white solid in 73.4% yield. 2: mp >230° C (dec.); IR (NaCl) 3247, 1725, 1641, 1359, 1253, 1061 cm −1 ; H NMR (300 MHz, CDCl3) 2.37(m, 2H), 3.24(s,2H), 3.26 (s, 6H), 4.40(s, 2H), 4.57(t, 4H, J= 6.2Hz), 5.31(br, 2H), 7.41(d,, 2H, J=8.3 Hz) 7.72(d,d, 2H, J = 6.43Hz, and J = 1.86Hz), 7.89 (s,2H), 8.19(d, 2H, J = 1.76Hz); 13C NMR (75.5 MHz, CDCl3) 26.2, 34.4, 40.7, 60.2, 78.7, 79.7, 120.4, 121.6, 127.7, 130.1, 133.4, 134.1, 134.9, 161.3, 164.1; MS (FAB,NBA) m/e (relative intensity) 603(M+ +1, 100). This material was employed for the X-ray crystal structure. It was homogenous in two independent TLC systems [Rf =0.31 in EtOAc/CH3OH, 4 :1; Rf =0.32 in CH2Cl2/CH3OH, 9 : 1]. Anal. Calcd for C33H26N6O6 ·2/3 CH3OH: C, 64.81; H, 4.63; N, 13.47. Found: C, 64.56; H, 4.72; N, 13.76.
1,5-Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3carboxy) pentyl diester 3 (XLi210)
Ligand 3 was prepared by following the procedure A and acid 9 by replacing the1,3-propanediol with 1,5-pentanediol to provide 3 as a white solid in 89.2% yield. 3: mp 132-138°C ; IR (KBr) 3422, 3280, 2931, 1714, 1635, 1487, 1249, 1064 cm−1; 1H NMR (500 MHz, CDCl3) 1.90(m, 4H), 3.24(s,6H), 3.52 (s, 2H), 4.39(s, 8H), 5.29(s, 2H), 7.36(dd, 2H, J=8.1Hz, 16Hz), 7.70(m, 2H) 7.70(m, 2H), 7.86 (s,2H), 8.18(s, 2H); MS (FAB,NBA) m/e (relative intensity) 631(M++1, 13). Anal. Calcd for C35H30N6O6 ·5/3 H2O: C, 63.61; H, 4.83; N, 12.72. Found: C, 63.16; H, 4.72; N, 13.06.
1,3-Bis(8-ethyl-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) propyl diester 4 (XLi356)
1,3-Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]-benzodiaze-pine -3-carboxy) propyl diester 2 (500 mg, 0.83 mmol) was dissolved in EtOH (150 mL) after which Pd/C (176 mg) was added in solution at rt. The slurry was stirred for 5h under one atmosphere of H2(bench top, balloon of H2). The catalyst was removed by filtration and washed with EtOH. The EtOH was removed under reduced pressure to furnish a residue. This material was purified by flash chromatography (silica gel, EtOAc : EtOH/8 : 2) to provide 4 (504 mg, 99%) as white crystals: mp 125-133°C; IR (NaCl) 3407, 2964, 2358, 1725, 1640, 1499 cm−1; 1H NMR (CDCl3) δ1.29 (m, 6H), 2.39(m, 2H), 2.78 (dd, 4H, J=7.5 Hz, 15.1 Hz), 3.26 (s, 6H), 4.48 (br, 2H), 4.56 (t, 4H, J=6.1 Hz, 12.2 Hz), 5.16(br, 2H), 7.33 (d, 2H, J = 8.2Hz), 7.48 (d, 2H, J=1.8 Hz), 7.89 (t, 4H, J=3.2 Hz, 5.3 Hz), 8.15; MS(EI) m/e (relative intensity) 611(M++1, 100). Anal. Calcd for C33H34N6O6 •2H2O: C, 61.33; H, 5.92; N, 13.00. Found: C, 61.74; H, 5.91; N, 12.63.
Bis(8-acetyleno-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5a][1,4]benzodiazepine-3-carboxy) dimethyl glycol diester 5 (XLi374)
Ligand 5 was prepared by following the procedure A and acid 9, by replacing 1,3-propanediol with methylene glycol to afford dimer 5 (80% yield): mp >220 °C (dec.); IR (KBr) 3419, 3237, 2910, 1714, 1635, 1561, 1498 cm −1; 1H NMR (500 MHz, CD2Cl) d 3.18(s, 6H), 3.31(s,2H), 4.37 (br, 2H), 5.29(br, 2H), 5.74 (s, 4H), 7.40(d, 2H, J=8.1 Hz), 7.74(dd, 2H, J=1.6Hz and J=8.2Hz), 7.90 (s, 2H), 8.15 (s, 2H); MS(FAB,NBA) m/e (relative intensity) 605(M++1, 100). Anal. Calcd for C32H24N6O7 ·3/2 CH3COOC2H5: C, 61.99; H, 4.93; N, 11.41. Found: C, 61.36; H, 4.52; N, 11.96.
8-Bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylic acid 11
The ester 10 (2g) was dissolved in EtOH (50 mL) and aq sodium hydroxide (10 mL, 2N) was added to the solution. The mixture was heated to reflux for 0.5 hour. After the EtOH was removed under reduced pressure, the solution was allowed to cool. The pH value was adjusted to 4 by adding 10% aq HCl dropwise. The mixture was filtered and the solid was washed with water and ethyl ether. The solid was dried to provide 11(1.8g, 96.6%): mp >250 °C; IR (KBr) 3450 (b), 2844, 1707, 1615, 1493, 1166, 700 cm−1 1H NMR (300 MHz, DMSO-d6) 4.14 (d, 1H, J=12.6Hz), 5.79 (d, 1H, 12.6Hz), 7.41-7.54 (m, 6H), 7.88 (d, 1H, J=8.7Hz), 8.03 (dd, 1H, J=8.7Hz, J=2.1Hz), 8.47 (s, 1H); MS (EI) m/e (rel intensity) 381 (M+, 20), 383 (19).
1,3-Bis(8-bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) propyl diester 13 (DMH-D-070)
(Procedure B) The carboxylic acid 11 (2 g, 5.2 mmol) was dissolved in DMF (20mL), after which CDI (1.02 g, 6.3 mmol) was added at rt and the mixture was stirred for 2 h. Then 1,3-propanediol (0.19 mL, 2.6 mmol) and DBU (0.78 mL, 5.2 mmol) were added to the mixture and stirring continued overnight. The reaction solution was then cooled with an ice-water bath, after which water was added to precipitate a solid. This material was purified further by flash chromatography on silica gel (gradient elution, EtOAc:EtOH 20:1, 15:1, 10:1) to provide the bisbromide 13 (DMH-D-070) as a white solid (1.3 g, 61.9%): mp 187.5-189 °C; IR (KBr) 3112, 2968, 1708, 1610, 1559, 1491, 1269, 1160, 1123, 1073 cm−1 ; 1H NMR (300 MHz, CDCl3) 2.35 (m, 2H), 4.08 (d, 2H J=12.6Hz), 4.55 (m, 4H), 6.05 (d, 2H, J=12.6Hz) 7.37-7.53 (m, 12H), 7.6 (d, 2H, J=2.1Hz), 7.81 (dd, 2H, J=2.1Hz, 8.6 Hz), 7.93 (s, 2H); 13C NMR (75.5 MHz, CDCl3 28.2, 44.9, 61.4, 120.7, 124.2, 129.3, 129.6, 130.6, 134.1, 134.4, 134.7, 135.0, 138.9, 138.9, 162.6, 167.9; MS (FAB, NBA) m/e (rel intensity) 803 (M++1, 15); Anal. Calcd. For C39H28N6O4Br2: C, 58.23; H, 3.51; N, 10.45. Found: C, 57.92; H, 3.43; N, 10.29.
1,3-Bis(8-trimethylsilylacetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]-diazepine-3-carboxy) propyl diester 15(DMH-D-048)
(Procedure C). To a suspension of bisbromide 13 (1.005 g, 1.25 mmol) in acetonitrile (50 mL) and triethylamine (65 mL), was added bis(triphenylphosphine)-palladium (II) acetate (0.15 g, 0.2 mmol). The solution which resulted was degassed and trimethylsilylacetylene (0.7 mL, 5 mmol) was added after which it was degassed again (argon followed by vacuum). The mixture was heated to reflux and stirring maintained overnight. After removal of the solvent under reduced pressure, the residue was dissolved in CH2Cl2 and washed with water. 3-Mercaptopropyl functionalized silica gel (0.6g) was added into the organic layer and stirring continued for 1 hour. The silica gel/Pd complex was removed by filtration and the filtrate was concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (gradient elution, EtOAc:EtOH 20:1, 15:1, 10:1) to furnish the bistrimethylsilyl dimer 15 (DMH-D-048, 680 mg, 60.8%) as a white solid: mp 169-172 °C; IR (KBr) 3449, 2950, 1725, 1720, 1715, 1496, 1250, 1160, 1080, 847 cm−1; 1H NMR (300 MHz, CDCl3) 0.25 (s, 18H), 2.35 (m, 2H), 4.05 (d, 2H, J=12.6Hz), (m, 2H), 4.55 (m, 4H), 6.02 (d, 2H, J=12.6Hz), 7.37-7.55 (m, 14H), 7.75 (dd, 2H, J=1.8Hz, 8.4Hz), 7.94 (s, 2H); 13C NMR (75.5 MHz, CDCl3) −0.3, 28.3, 44.9, 61.4, 97.4, 102.3, 122.4, 122.6, 128.0, 128.3, 129.0, 129.4, 130.5, 134.1, 134.9, 135.1, 139.0, 139.2, 139.2, 162.6, 168.5; MS (FAB, NBA) m/e (rel intensity) 839 (M++1, 100); Anal. Calcd. For C49H46N6O4Si2: C, 70.14; H, 5.53; N, 10.02. Found: C, 69.97; H, 5.35; N, 9.77.
1,3-Bis(8-acetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy)propyl diester 8 (DMH-D-053)
(Procedure D) A solution of bistrimethylsilyl dimer 15 (330 mg, 0.4 mmol) in THF (70 mL) was stirred with tetrabutylammonium fluoride hydrate (250 mg, 0.96 mmol) at −78°C for 5 min. After this, H2O (35 mL) was added to the solution to quench the reaction and stirring continued at low temperature for one half hour. The solution was extracted with EtOAc (3×100 mL), and the organic layer was washed with water. After removal of the solvent under reduced pressure, ethyl ether was added to the residue to precipitate a solid. The mixture was filtered and the solid was washed with CH2Cl2-Et2O (ca 1:15) to provide the bisacetylenyl dimer 8 (DMH-D-053, 220 mg, 80%) as a yellow solid which crystallizes in CH2Cl2: mp 172-175 °C; IR (KBr) 3450, 3280, 2950, 1720, 1715, 1495, 1250, 1120, 1050 cm;−1; 1H NMR (300 MHz, CDCl3) 2.35 (m, 2H), 3.18 (s, 2H), 4.08 (d, 2H, J=12.3Hz), 4.56 (m, 4H), 6.04 (d, 2H, J=12.6Hz), 7.36-7.59 (m, 14H), 7.78 (dd, 2H, J=8.4Hz, 1.7Hz), 7.95 (s, 2H); 13C NMR (75.5 MHz, CDCl3) 28.8, 45.4, 61.9, 80.2, 81.3, 121.4, 122.7, 128.1, 128.3, 129.0, 129.3, 130.5, 134.2, 135.2, 135.3, 135.6, 138.9, 139.2, 162.6, 168.5; MS (FAB, NBA) m/e (rel intensity) 695 (M++1, 100). Anal. Calcd. For C43H30N6O4•¼CH2Cl2: C, 72.63; H, 4.30; N, 11.75. Found: C, 72.36; H, 4.27; N, 11.36.
Bis(8-bromo-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 12(DM-III-93)
Ligand 12 was prepared from acid 11, under the same conditions employed in procedure B, by replacing 1,3-propanediol with diethylene glycol to yield a yellow solid (93.7%) 12: mp 165-168°C; IR (KBr) 3060, 2956, 1725, 1610, 1558, 1491, 1267, 1161, 1123, 1074 cm−1; 1H NMR (300 MHz, CDCl3) 3.93 (t, 4H, J=4.8 Hz), 4.06 (d, 2H, J=12.6Hz), 4.54 (m, 4H), 6.05 (d, 2H, J=12.6Hz), 7.39-7.50 (m, 12H), 7.57 (d, 2H, J=2.7Hz), 7.80 (dd, 2H, J=2.1Hz, 8.4 Hz), 7.90 (s, 2H); 13C NMR (75.5 MHz, CDCl3) 44.9, 63.6, 69.0, 120.7, 124.2, 128.3, 129.0, 129.3, 129.6, 130.6, 134.1, 134.4, 134.6, 135.0, 138.9, 139.0, 162.5, 167.9; MS (FAB, NBA) m/e (rel intensity) 833 (M+ +1, 5). Anal. Calcd. For C40H30Br2N6O5•0.15CHCl3: C, 56.72; H, 3.57; N, 9.88. Found: C, 56.61; H, 3.55; N, 9.92.
Bis(8-trimethylsilylacetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 14 (DM-III-94)
Ligand 14 was prepared from dibromide 12, under the same conditions employed in Procedure C by replacing 1,3-propanediol with diethylene glycol to produce a yellow solid (49.5%) 14: mp 205-208°C; IR (KBr) 3433, 2960, 1730, 1700, 1612, 1493, 1255, 1169, 1120, 1071, 847 cm−1; 1H NMR (300 MHz, CDCl3)δ0.25 (s, 18H), 3.93 (t, 4H, J=5.4Hz), 4.04 (d, 2H, J=12.6Hz), 4.55 (m, 4H), 6.04 (d, 2H, J=12.6Hz), 7.37-7.53 (m, 14H), 7.74 (dd, 2H, J=1.2Hz, 8.4Hz), 7.91 (s, 2H); 13C NMR (75.5 MHz, CDCl3) −0.3, 45.0, 63.6, 69.0, 97.5, 102.4, 122.5, 122.7, 128.1, 128.3, 129.0, 129.4, 130.5, 134.2, 135.0, 135.1, 135.2, 139.1, 139.3, 162.7, 168.6; MS (FAB, NBA) m/e (rel intensity) 869 (M++1, 100). Anal. Calcd. For C50H48N6O5Si2•¼H2O: C, 68.81; H, 5.60; N, 9.62. Found: C, 68.88; H, 5.66; N, 9.51.
Bis(8-acetylenyl-6-phenyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxy) diethylene glycol diester 7 (dm-III-96)
Ligand 7 was prepared from diester 14, under the same conditions employed in procedure B, by replacing 1,3-propanediol with diethylene glycol to provide a yellow solid (81.6%) 7: mp 173−177°C; IR (KBr) 3432, 3280, 1720, 1715, 1496, 1254, 1175, 1120, 1074cm−1; 1H NMR (300 MHz, CDCl3) 3.12 (s, 2H), 3.93 (t, 4H, J=4.5Hz), 4.06 (d, 2H, J=12.6Hz), 4.55 (m, 4H), 6.05 (d, 2H, J=12.6Hz), 7.38-7.56 (m, 14H), 7.75 (dd, 2H, J=8.4Hz, 1.8Hz), 7.91 (s, 2H); 13C NMR (75.5 MHz, CDCl3) 45.0, 63.6, 69.0, 79.8, 81.3, 121.3, 122.7, 128.1, 128.3, 129.0, 129.3, 130.5, 134.2, 135.2, 135.3, 135.6, 139.0, 139.1, 162.6, 168.4; MS (FAB, NBA) m/e (rel intensity) 725 (M++1, 63). Anal. Calcd. For C44H32N6O5•¼EtOAc•3/2H2O: C, 69.89; H, 4.82; N, 10.87. Found: C, 70.12; H, 4.45; N, 10.58.
ACKNOWLEDGMENT
The authors thank NIMH (MH-46851), the Research Growth Initiative of the University of Wisconsin-Milwaukee, and NSF (instrumentation grant NSF-9512622) for support of this work. Moreover, the authors acknowledge NIDA and ONR for support of the X-ray crystallography.
X-RAY CRYSTALLOGRAPHIC DATA.
Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. 687205(DMH-D-053), 222395(Xli093), and 222396(DM-III-96). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or deposit@ccdc.cam.ac.uk).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES.
- 1.Squires RF, Braestrup C. Nature. 1977;266:732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]
- 2.Ninan PT, Insel TM, Cohen RM, Cook JM, Skolnick P, Paul SM. Sci. 1982;218:1332–1334. doi: 10.1126/science.6293059. [DOI] [PubMed] [Google Scholar]
- 3.Wong G, Skolnick P. Eur. J. Pharmacol.-Mol. Pharmacol. Section. 1992;225:63–68. doi: 10.1016/0922-4106(92)90040-3. [DOI] [PubMed] [Google Scholar]
- 4.Mendelson WB, Cain M, Cook JM, Paul S, Skolnick P. Science. 1983;219:414–416. doi: 10.1126/science.6294835. [DOI] [PubMed] [Google Scholar]
- 5.Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, Rossier J. Nature. 1986;321:864–866. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]
- 6.Sieghart W. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 7.Nayeem N, Green TP, Martin IL, Barnard EA. J. Neurochem. 1994;62:815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- 8.Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. Psychopharmacology. 1999;146:205–213. doi: 10.1007/s002130051108. [DOI] [PubMed] [Google Scholar]
- 9.Benson JA, Low K, Keist R, Mohler H, Rudolph U. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 11.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 12.Möhler H, Fritschy JM, Rudolph U. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 13.Liu RY, Zhang PW, McKernan RM, Wafford K, Cook JM. Med. Chem. Res. 1995;5:700–709. [Google Scholar]
- 14.Liu RY, Hu RJ, Zhang PW, Skolnick P, Cook JM. J. Med. Chem. 1996;39:1928–1934. doi: 10.1021/jm950887n. [DOI] [PubMed] [Google Scholar]
- 15.Yu S, Ma CR, He XH, McKernan R, Cook JM. Med. Chem. Res. 1999;9:71–88. [Google Scholar]
- 16.Bailey DJ, Tetzlaff JE, Cook JM, He XH, Helmstetter FJ. Neurobiol. Learn. Mem. 2002;78:1–10. doi: 10.1006/nlme.2001.4050. [DOI] [PubMed] [Google Scholar]
- 17.Platt DM, Rowlett JK, Spealman RD, Cook J, Ma CR. Psychopharmacology. 2002;164:151–159. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- 18.Erez M, Takemori AE, Portoghese PS. J. Med. Chem. 1982;25:847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 19.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. J. Med. Chem. 1985;28:1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 20.Portoghese PS, Larson DL, Sayre LM, Yim CB, Ronsisvalle G, Tam SW, Takemori AE. J. Med. Chem. 1986;29:1855–1861. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]
- 21.Roth B. unpublished results. 2006. [Google Scholar]
- 22.Li XY, Cao H, Zhang CC, Furtmueller R, Fuchs K, Huck S, Sieghart W, Deschamps J, Cook JM. J. Med. Chem. 2003;46:5567–5570. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, He XH, Ma CR, Liu RY, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. J. Med. Chem. 2000;43:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- 24.Burger's Medicinal Chemistry & Drug Discovery. 6 ed. John Wiley & Sons Inc.; 2003. [Google Scholar]
- 25.Han DM, Forsterling FH, Li XY, Deschamps JR, Cao H, Cook JM. Bioorg. Med. Chem. Lett. 2004;14:1465–1469. doi: 10.1016/j.bmcl.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Eliel EL, Wilen SH. Stereochemistry of Organic Compounds. Wiley-Interscience; 1994. [Google Scholar]
- 27.Flory PJ. Statistical Mechanics of Chain Molecules. Interscience Publishers; 1968. [Google Scholar]
- 28.Lemieux RU. Molecular Rearrangement. Interscience Publishers; 1964. [Google Scholar]
- 29.Eliel EL. J. Acc. Chem. Res. 1970;3:1–8. [Google Scholar]
- 30.Graczyk PP, Mikolajczyk M. Topics in Stereochemistry. John Wiley & Sons; 1994. [Google Scholar]
- 31.Hutchins RO, Kopp LD, Eliel EL. J. Am. Chem. Soc. 1968;90:7174–7175. [Google Scholar]
- 32.Eliel EL, Giza CA. J. Org. Chem. 1968;33:3754–3758. [Google Scholar]
- 33.Uchida T, Kurita Y, Kubo M. J. Polym. Sci. 1956;19:365–372. [Google Scholar]
- 34.Abe A, Mark JE. J. Am. Chem. Soc. 1976;98:6468–6476. [Google Scholar]
- 35.Ohsaku M. Macromolecules. 1978;11:970–976. [Google Scholar]
- 36.Wolfe S. Acc. Chem. Res. 1972;5:102–111. [Google Scholar]
- 37.Jaffe RJ, Smith GD, Yoon DY. Phys. Chem. 1993;97:12745–12751. [Google Scholar]
- 38.Bultinck P, Van Alsenoy C, Goeminne A. J. Phys. Chem. 2001;105:9203–9210. [Google Scholar]
- 39.Bultinck P, Goeminne A, Van de Vondel D. J. Mol. Struct. 1999;467:211. [Google Scholar]
- 40.Glendening ED, Feller D, Thompson MA. J. Am. Chem. Soc. 1994;116:10657–10669. [Google Scholar]
- 41.Glasstone S, Laidler KJ, Eyring H. The Theory of Rate Processes. McGraw-Hill; 1941. [Google Scholar]
- 42.Azumaya I, Uchida I, Kato T, Yokoyama A, Tanatani A, Takayanagi H, Yokozawa T. Agnew. Chem. Int. Ed. 2004;43:1360–1363. doi: 10.1002/anie.200352788. [DOI] [PubMed] [Google Scholar]
- 43.Rashkin MJ, Waters ML. J. Am. Chem. Soc. 2002;124:1860–1861. doi: 10.1021/ja016508z. [DOI] [PubMed] [Google Scholar]
- 44.Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K. J. Am. Chem. Soc. 2002;124:104–112. doi: 10.1021/ja0105212. [DOI] [PubMed] [Google Scholar]
- 45.Hobza P, Selzle HL, Schlag EW. J. Am. Chem. Soc. 1994;116:3500–3506. [Google Scholar]
- 46.Burley SK, Petsko GA. Sci. 1985;229:23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y, Prusoff WH. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 48.Fryer RI, Schmidt RA, Sternbach LH. J. Pharm. Sci. 1964;53:264–268. doi: 10.1002/jps.2600530303. [DOI] [PubMed] [Google Scholar]
- 49.Li X. Synthesis of Selective Ligands for GABAA/Benzodiazepine Receptors. University of Wisconsin-Milwaukee; Milwaukee: 2004. [Google Scholar]
- 50.Sternbach LH, Fryer RI, Metlesics W, Reeder E, Sach G, Saucy G, Stempel A. J. Org. Chem. 1962;27:3788–3796. [Google Scholar]
- 51.Austin WB, Bilow N, Kelleghan WJ, Lau KSY. J. Org. Chem. 1981;46:2280–2286. [Google Scholar]
- 52.Heck RF. Palladium Reagents in Organic Synthesis; Academic Press. Academic Press; 1985. [Google Scholar]
- 53.Choudhary MS, Craigo S, Roth BL. Mol. Pharmacol. 1992;42:627–633. [PubMed] [Google Scholar]