Abstract
Objective
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a newly recognized anti-neuronal antibody-mediated inflammatory brain disease causing severe psychiatric and neurological deficits in previously healthy children. The aim of this study was to report characteristic clinical features and outcomes of children diagnosed with anti-NMDAR encephalitis.
Methods
Consecutive children presenting with newly acquired psychiatric and/or neurologic deficits consistent with anti-NMDAR encephalitis and evidence of CNS inflammation were screened over a 12-month period. Children were included in the study if they had confirmatory evidence of anti-NMDAR antibodies in the serum and/or cerebrospinal fluid (CSF). Details of clinical presentation and results of investigations were reported. Type and duration of treatment and outcomes at last follow-up were documented.
Results
Seven children were screened and three children with anti-NMDAR encephalitis were identified. All patients presented with neurological or psychiatric (‘neuropsychiatric’) abnormalities, seizures, speech disorder, sleep disturbance, and fluctuating level of consciousness. The two older patients also had more prominent psychiatric features, while the younger child had significant autonomic instability and prominent involuntary movement disorder. None had an underlying tumor. Immunosuppressive therapies resulted in near or complete recovery; however, two of the patients had early relapse requiring re-treatment.
Conclusion
Anti-NMDAR encephalitis is an important cause of neuropsychiatric deficits in children that must be included in the differential diagnosis of CNS vasculitis and other inflammatory brain diseases. Early diagnosis and treatment are essential for neurologic recovery.
Inflammatory diseases of the central nervous system (CNS) in children present a diagnostic challenge to clinicians. The wide differential diagnosis includes infectious and post-infectious processes, systemic inflammatory conditions such as systemic lupus erythematosus, and primary CNS vasculitis. Recently, newly recognized anti-neuronal antibody-mediated inflammatory disorders such as anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis have become an important diagnostic consideration in children presenting with severe newly acquired neuropsychiatric symptoms(1).
Anti-NMDAR encephalitis was initially described as a paraneoplastic process in women with ovarian teratoma (2). In contrast`, in the pediatric population it is most commonly diagnosed in the absence of tumors (3).Children may present with psychosis, seizures, movement disorder, decreased level of consciousness, and/or life-threatening autonomic instability (4). Rapid clinical deterioration may occur. Elevated serum inflammatory markers and cerebrospinal fluid (CSF) pleocytosis are commonly found. Brain imaging is often normal or non-diagnostic (5–6). The diagnosis relies on testing for anti-NMDAR antibodies. Rheumatologists’ awareness of anti- NMDAR encephalitis is limited; no reports have been published in the Rheumatology literature so far. However, early recognition is crucial, since it is treatable and early treatment is found to be tightly linked to prognosis (4)
The aim of this study is to report the presenting clinical features, investigation results, treatment, and outcomes of consecutive children diagnosed with anti-NMDAR encephalitis, a novel inflammatory brain disease in children.
METHODS
Patients
A single center prospective cohort study of children ages <18 years diagnosed with an anti- NMDAR encephalitis between July 1, 2009 and June 30, 2010 was conducted. Children were screened if they 1) had evidence of a newly acquired neuropsychiatric deficit compatible with anti-NMDAR encephalitis such as psychosis, seizures, movement disorder, and/or autonomic dysfunction and, 2) additional supportive evidence of inflammation in the blood, CSF or neuroimaging. Children were included in the study if they had confirmatory evidence of anti-NMDAR antibodies in the serum and/or cerebrospinal fluid (CSF).The study excluded children with primary CNS vasculitis and those with systemic underlying conditions such as rheumatic diseases or infections. Approval from the Research Ethics Board was obtained (REB No #1000019862).
Clinical data
Demographic information, preceding systemic and neurologic symptoms, past medical history, history of current illness, and features of detailed neurologic, psychiatric and rheumatologic examinations were recorded. Neurologic status was determined by in depth standardized assessment.
Laboratory data
Erythrocyte sedimentation rate (ESR); C-reactive protein (CRP), complete blood count (CBC), including the white blood cell (WBC) differential count; levels of C3 and C4 complement, and von Willebrand factor antigen (vWF Ag) were recorded. CSF was analyzed for cell count, protein level, oligoclonal bands, and opening pressure. Autoantibody testing included antinuclear antibody, rheumatoid factor, anti-double-stranded DNA, anti-Ro, anti-La, anti-SM, anti-RNP, anti-neutrophil cytoplasmic antibodies, and anti-cardiolipin antibodies. Viral and bacterial cultures, serology, and viral polymerase chain reaction were performed in both peripheral blood and CSF, according to the standardized institutional encephalitis workup (7).
Anti-NMDAR antibodies
Serum and CSF were tested for anti-NMDAR antibodies in the Laboratory of Neuro-Oncology and Paraneoplastic Disorders at the University of Pennsylvania (Director: Dr. Josep Dalmau) as previously reported (2, 6).
Neuroimaging and additional testing
All patients underwent standardized magnetic resonance (MR) imaging (MRI) including T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI) and post-gadolinium sequences, at diagnosis and subsequently when indicated. MR angiography (MRA), MR venogram (MRV), and conventional angiography were also performed as indicated. Patients tested for anti-NMDAR antibodies were screened for malignancies by abdominal/pelvic and/or testicular ultrasound. All patients underwent electroencephalograms (EEG). Brain biopsies were performed when indicated. Method and review of biopsies followed the previously reported institutional protocol(8).
Treatment and outcome
The use of immunosuppressive medication including intravenous immunoglobulin (IVIG), IV methylprednisolone, oral corticosteroids and IV rituximab was documented. Dose and duration were recorded. Adverse events were noted. Treatment was given at the discretion of the treating rheumatologist and/or neurologist. Neurological status at last follow up and relapses during the observation period were determined.
RESULTS
Patients
A total of seven children presented with clinical features compatible with anti-NMDAR encephalitis including psychiatric manifestations, seizures and/or movement disorders during the study interval. Three children (25%) had positive anti-NMDAR antibodies and were diagnosed with anti-NMDAR encephalitis. These included one male and two females, with a median age of 13 years (range 3–16 years). The remaining four children were subsequently diagnosed with 1) post-infectious inflammatory brain disease (2); 2) channelopathy (1); and 3) other inflammatory brain diseases (1).
Demographics, preceding symptoms, clinical features at presentation and results of investigations in the three children with anti-NMDAR encephalitis are summarized in Table 1. Treatment and outcomes are provided in Table 2.
Table 1.
Preceding symptoms, clinical features and lab characteristics at diagnosis, and associated test results of three children at diagnosis of anti-NMDA receptor encephalitis
| Patient 1 3 year old female |
Patient 2 16 year old male |
Patient 3 13 year old female |
||
|---|---|---|---|---|
| Preceding Symptoms | ||||
| Systemic | Fever×2 days | Fever, vomiting, | None | |
| diarrhea×2 days | ||||
| Neuropsychiatric | Ataxia | Agitation, confusion, | Right facial palsy, | |
| emotional lability | dysarthria | |||
| Clinical Findings at Diagnosis | ||||
| Neurologic | ||||
| Seizures | Yes | Yes | Yes | |
| Movement | Choreiform | None | None | |
| disorder | movements, orofacial | |||
| dyskinesia | ||||
| Other neurologic | Absence of speech, | Slowed speech, sleep | Dysarthria, aphasia, | |
| signs | sleep disturbance, | disturbance, | confusion, sleep | |
| fluctuating level of | purposeless | disturbance, | ||
| consciousness (LOC) | movements, | fluctuating LOC | ||
| fluctuating LOC | ||||
| Psychiatric | Agitation, bizarre | Delusions, echolalia, | Hallucinations, | |
| behaviour | echopraxia, violent | delusions, agitation | ||
| behaviour, agitation | ||||
| Autonomic signs | Fever, tachycardia | Hypertension | Fever | |
| Laboratory Tests | ||||
| ESR, mm/hour (0–10) | 97 | 1 | 112 | |
| CRP, mg/liter (0–8) | 3.7 | <0.6 | 29.6 | |
| WBC,×109/liter | 7.6 | 13.4 | 9.5 | |
| (normal 4–10) | ||||
| CSF Analysis | ||||
| WBC, ×106/liter | 13 | 25 | 80 | |
| (normal 0–3) | ||||
| Protein, gm/liter | 0.19 | 0.52 | 0.11 | |
| (normal 0.15–0.4) | ||||
| Opening pressure, | 26 | 20.5 | <17 | |
| cm H2O (normal<17) | ||||
| Oligoclonal bands | Present | Absent | Absent | |
| Neuroimaging | ||||
| Brain MRI | Normal | Swelling and | High signal focus | |
| high signal of right | in right frontal lobe. | |||
| hippocampus. | Diffuse dural | |||
| Subtle diffusion | enhancement. | |||
| restriction. | ||||
| Brain MRA | Normal | Normal | Normal | |
| Brain MRV | Normal | Normal | Normal | |
| Other | ||||
| Investigations | ||||
| EEG | Diffuse slowing. | Slowing over right | Diffuse slowing with | |
| No epileptic activity. | frontal-temporal | right temporal | ||
| region. | predominance. | |||
| Electrical seizure. | No epileptic activity. | |||
| Brain biopsy | Not done | Mild lymphocytic | Not done | |
| meningeal infiltrate; | ||||
| no vasculitis | ||||
| Malignancy | ||||
| Presence of tumor | No | No | No | |
ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, WBC = white blood cell count, MRI = magnetic resonance imaging, MRA = magnetic resonance angiography, MRV = magnetic resonance venography, EEG = electroencephalogram
Table 2.
Treatment and outcome of patients with anti-NMDA receptor encephalitis
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Initial therapy | |||
| IVIG | Yes | Yes | Yes |
| IV methyl- | Yes | Yes | No |
| prednisolone pulse | |||
| Oral prednisone | Yes | No | No |
| IV Rituximab | Yes | No | No |
| Duration of therapy | 7 months | 7 months | 3 months |
| Course | |||
| Relapse | No | yes | yes |
| Time to relapse | - | 13 months | 3 months |
| Re-treatment | - | IVIG×6 months | Rituximab |
| plus IVIG×6 months | |||
| Outcomes | |||
| Time at last follow-up | 12 months | 14 months | 4 months |
| Neurological deficits | None | Seizures | Seizures |
| at last follow-up | Speech difficulties | ||
| Memory impairment |
IV = intravenous, IVIG = intravenous immunoglobulin
Case reports
Patient 1
A 3-year-old previously healthy girl presented with a 2-week history of progressive ataxia along with pain and weakness of her left leg. She had had a transient low-grade fever 2 weeks prior to the onset of neurologic symptoms. Neurologic exam demonstrated a circumductive, high-stepping gait and mild weakness of the left leg. She subsequently developed choreiform movements involving the left lower extremity. Initial work-up, including CBC, ESR, toxicology screen, and MRI of brain and spine, was normal.
She returned 10 days later with increasing choreiform movements of the left leg and involvement of her right foot and left arm. In addition, she had developed sleep disruption, confusion and slowed speech. She was started on carbamazepine and her movements improved. Lumbar puncture was performed demonstrating an elevated CSF WBC (13×106/L), with 91% lymphocytes. CSF protein, lactate, amino acids, and infectious workup were normal.
Over the next two weeks, she developed continuous choreiform movements of all extremities, as well as dyskinesia of her orolingual and extra-ocular muscles. She had severe agitation, complete loss of speech, and minimal responsiveness. Subsequently, she had recurrent seizures, oxygen requirement, and autonomic changes including fever and tachycardia. She was treated with phenobarbital for seizure control, and trihexyphenidyl for abnormal movements. Repeat blood work revealed an elevated ESR (97 mm/h) and a normal CRP. She received empiric treatment with acyclovir and antibiotics, although repeated infectious and metabolic work-ups remained negative. All autoantibodies were negative. Repeat lumbar puncture demonstrated an elevated opening pressure (26 cm H2O), mild pleocytosis and positive oligoclonal banding. Repeat brain MRI/MRA/MRV remained normal. EEG demonstrated diffuse slow background activity and no epileptiform discharges. For suspected inflammatory brain disease, the child was treated with intravenous immunoglobulin (IVIG) (2 g/kg) and a 5-day course of pulse methylprednisolone (20 mg/kg/dose), followed by oral prednisone (2 mg/kg, weaned over 6 months).
CSF and serum were both strongly positive for anti-NMDAR antibodies. No associated malignancy was found on abdominal imaging. Due to severe symptoms, she received additional therapy with rituximab (500 mg/m2 × 2 doses, 14 days apart). She required gastrostomy tube insertion for feeding purposes and received intensive neurocognitive rehabilitation therapy. At the 12-month follow-up visit, she had made a complete neurological recovery.
Patient 2
A 16-year-old young male presented with generalized tonic-clonic seizures following a 2-day history of low-grade fever, vomiting and diarrhea. He had been a cannabis user for 2 years. Over the prior three weeks he was agitated, emotionally labile, confused, and had slowed speech. Neurologic examination revealed brisk reflexes and cloni bilaterally. Mental status exam revealed flat affect, delusional and disorganized thoughts, and poor eye contact. He then developed echolalia, echopraxia, catatonic posturing and purposeless movements, as well as several episodes of agitation and violent behavior.
Laboratory testing demonstrated slightly elevated WBC, normal ESR, CRP and autoantibody profile. Toxicology screen, including testing for Cannabis, was negative. Initial lumbar puncture showed CSF WBC 25×106/L (94% lymphocytes) and elevated CSF protein (0.52 gm/L). He was started on acyclovir and antibiotics, however infectious and metabolic investigations were negative. Video EEG showed complex partial seizures and a slowing pattern over the right frontal-temporal region. Initial MRI brain and conventional cerebral angiogram were normal. A non-lesional brain biopsy showed occasional lymphocytes in the meninges and no evidence for vasculitis. He was treated with pulse methylprednisolone (1000 mg/dose), followed by IV methylprednisolone (60 mg/day) for five days for presumed inflammatory brain disease. In addition he required multiple medications to control his agitation and behavioural abnormalities, valproic acid for seizure control, and amlodipine for transient hypertension. He slowly became less agitated without violent episodes and responded to commands.
After three weeks of admission, brain MRI was repeated revealing high signal and swelling of the right hippocampus, in keeping with limbic encephalitis. The repeat lumbar puncture showed high opening pressure with normal CSF studies. Serum and CSF tested positive for anti- NMDAR antibodies. No associated malignancy was found.
Given the confirmed diagnosis of anti-NMDAR encephalitis, the patient was commenced on IVIG (70 g/dose). He also received intensive rehabilitation, occupational therapy and physiotherapy for 2 months. At follow-up 11 months after diagnosis he was calm with no agitation or confusion, but had mild memory impairment and slow speech. At last follow-up at 13 months he presented with recurrence of seizures and began a second course of IVIG.
Patient 3
A previously healthy 13-year-old female presented with a right facial palsy and dysarthria. She was treated with a short course of prednisone for presumed Bell’s palsy. One week later she developed agitation, sleep disturbance and seizures. On assessment she had a right upper motor neuron facial palsy without other neurological deficits. CBC was normal, and lumbar puncture demonstrated CSF pleocytosis (WBC 80×106/L, 94% lymphocytes). MRI brain was normal, and EEG revealed background slowing over the right temporal region. She received phenytoin for seizure control and acyclovir for possible viral encephalitis.
During her admission, she exhibited expressive and receptive dysphasia, confusion, auditory hallucinations, and fluctuating level of consciousness. Further investigations demonstrated raised ESR and CRP. Autoantibodies, toxicology screen and infectious work-up were negative. An MRI/MRA/MRV was repeated at three weeks after symptom onset and showed a nonspecific focus of high FLAIR signal in the right frontal lobe, and diffuse dural, but no leptomeningeal enhancement. A repeat lumbar puncture demonstrated normal opening pressure, CSF WBC of 11×106/L, and CSF protein 0.11 g/L (0.15–0.40 g/L). During her admission she developed febrile neutropenia and transaminitis, thought to be secondary to medication effects. Anti-NMDAR antibodies were detected in the CSF. There was no evidence of malignancy.
The patient was treated with IVIG (70 g/dose), and subsequently demonstrated gradual improvement in cognitive function, psychiatric and neurologic symptoms. At 6 weeks she had mild dysarthria and aphasia. At 3 months, she presented with recurrence of seizures and received IV rituximab and re-treatment with IVIG.
DISCUSSION
Anti-NMDAR encephalitis is a novel anti-neuronal antibody-mediated disease in the spectrum of childhood inflammatory brain diseases. Children with anti-NMDAR encephalitis presented with characteristic progressive neuropsychiatric symptoms and non-specific evidence of CNS inflammation. Targeted testing for antibodies against NMDAR NR1/NR2 heteromers confirmed the diagnosis. Disease recognition and appropriate management led to significant recovery.
Our patients presented with a flu-like prodrome followed by psychiatric features and neurologic abnormalities including seizures and movement disorder, consistent with other studies (3, 5, 9–12). The course of anti-NMDAR encephalitis has been proposed to represent a severity continuum starting with bizarre behaviour and psychotic symptoms and subsequent progression to seizures, movement abnormalities, decreased consciousness and autonomic dysfunction(4). A significant proportion of patients require intensive care unit (ICU) admission and intubation(3, 6). Increasing clinical disease severity is thought to correlate with gradually decreasing availability of NMDAR function. Increasing titers of anti-NMDAR antibodies crosslink NMDARs, resulting in internalization and alteration of synaptic function. This reduction in surface NMDARs is reversible after antibody removal (13). Patient age may be a confounding factor, as younger children are often unable to express hallucinations or paranoid thoughts. Previous case studies have reported abnormal movements as the initial presenting feature in young children with anti-NMDAR encephalitis (8, 10–12). In contrast, case series of adolescents and young adults commonly report initial psychiatric symptoms (6, 9, 14).
All three patients had evidence of CNS inflammation. Correspondingly, nearly all cases reported in the literature describe CSF pleocytosis, with or without elevated CSF protein and/or oligoclonal bands (3, 6, 12). Measures of systemic inflammation appear to be less consistent. MRI findings in anti-NMDAR encephalitis are variable: Some patients show radiological evidence of limbic involvement, as did one of our patients, although the clinical picture usually corresponds to a diffuse encephalopathy rather than to a focal limbic dysfunction(6) Nonspecific FLAIR signal abnormalities can be found. Most importantly, the majority of patients have normal neuroimaging (3, 12).
There is no established treatment protocol for anti-NMDAR encephalitis. Treatment of our patients was administered in a step-wise fashion. All patients received a six-month course of IVIG. Two children were treated with steroid therapy. Additional rituximab, an anti-CD20 monoclonal antibody, was given to two children, in one case at diagnosis and the other at the time of early recurrence of symptoms. In the literature, good responses have been described with combination therapy of steroid and IVIG, and more severely affected patients have been treated with rituximab, cyclophosphamide or plasmapheresis (2–3, 6). Of interest, there have been adult cases documenting clinical recovery without specific treatment (9, 14).
An estimated 75% of anti-NMDAR encephalitis patients make a substantial or full recovery (3, 12). All three of our patients significantly improved over time. However, early relapses were documented in two children. The reported time to clinical recovery is variable, ranging from 2 weeks to 24 months (3, 6, 12). Patients frequently require intensive rehabilitation following their acute illness. Large clinical series indicate that patients without tumors, as is the case in many children, often require second line immunotherapies such as rituximab or cyclophosphamide, due to limited response to corticosteroids, IVIg or plasma exchange(15). Patients without tumors also have more relapses than those with tumors (15).
Characteristic neuropsychiatric features should alert clinicians to consider the diagnosis of anti-NMDAR encephalitis. A high index of suspicion is required when assessing children presenting with a combination of psychosis, seizures, movement disorder and/or autonomic instability. Testing for anti-NMDAR antibodies in children with CNS inflammation should be initiated prior to more invasive strategies such as brain biopsy. Early recognition and treatment may reverse the deficits and prevent permanent brain injury.
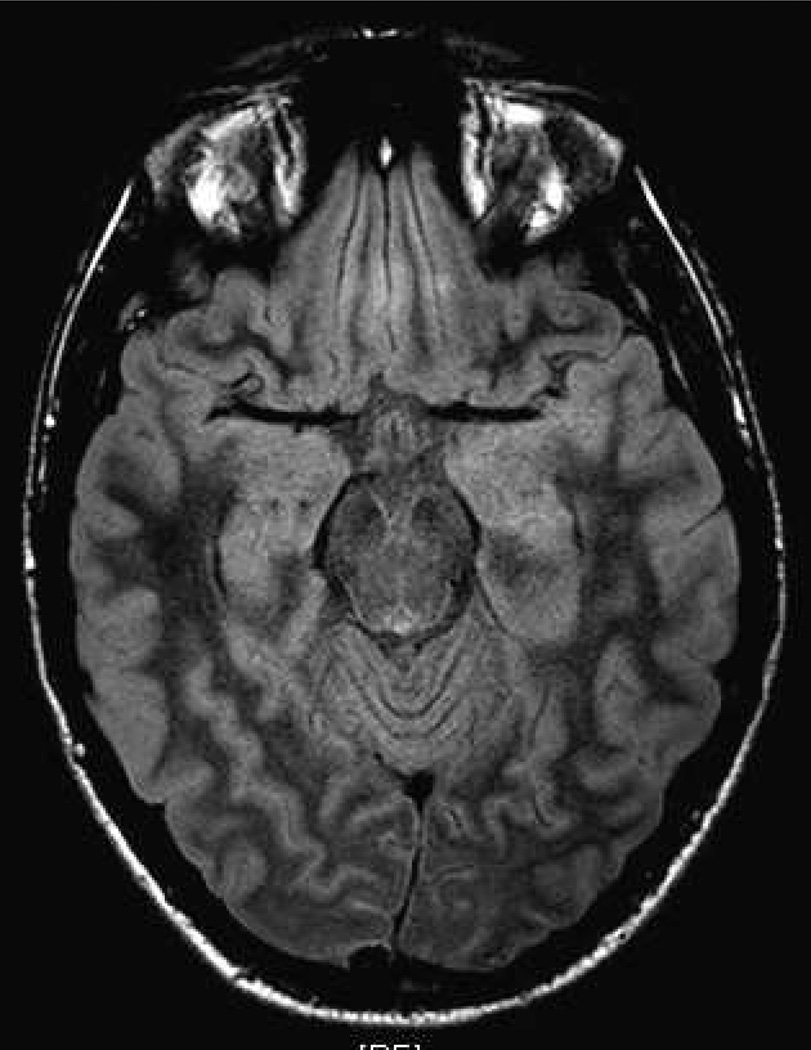
Figure 1. Subtle hippocampus swelling on FLAIR magnetic resonance imaging (MRI) sequences in a 16-year old male with anti-NMDAR encephalitis.
MRI Fluid-Attenuated Inversion Recovery (FLAIR) sequences study conducted at 6 weeks after onset of symptoms revealed subtle right hippocampus lesion in keeping with limbic encephalitis.
Footnotes
Disclosure of funding: None
References
- 1.Rosenfeld MR, Dalmau J. Update on paraneoplastic and autoimmune disorders of the central nervous system. Semin Neurol. 2010 Jul;30(3):320–331. doi: 10.1055/s-0030-1255223. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007 Jan;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009 Jul;66(1):11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: A severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2010 Oct 14; doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Sansing LH, Tuzun E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007 May;3(5):291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008 Dec;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, Jamieson F, et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin Infect Dis. 1998 Feb;26(2):398–409. doi: 10.1086/516301. [DOI] [PubMed] [Google Scholar]
- 8.Elbers J, Halliday W, Hawkins C, Hutchinson C, Benseler SM. Brain biopsy in children with primary small-vessel central nervous system vasculitis. Ann Neurol. 2010 Nov;68(5):602–610. doi: 10.1002/ana.22075. [DOI] [PubMed] [Google Scholar]
- 9.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008 Feb 12;70(7):504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biancheri R, Pessagno A, Baglietto MG, Irani SR, Rossi A, Giribaldi G, et al. Anti-N-methyl-D-aspartate-receptor encephalitis in a four-year-old girl. J Pediatr. 2010 Feb;156(2):332–334. doi: 10.1016/j.jpeds.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 11.Lebas A, Husson B, Didelot A, Honnorat J, Tardieu M. Expanding spectrum of encephalitis with NMDA receptor antibodies in young children. J Child Neurol. 2010 Jun;25(6):742–745. doi: 10.1177/0883073809343319. [DOI] [PubMed] [Google Scholar]
- 12.Poloni C, Korff CM, Ricotti V, King MD, Perez ER, Mayor-Dubois C, et al. Severe childhood encephalopathy with dyskinesia and prolonged cognitive disturbances: evidence for anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2010 May;52(5):e78–e82. doi: 10.1111/j.1469-8749.2009.03542.x. [DOI] [PubMed] [Google Scholar]
- 13.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010 Apr 28;30(17):5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niehusmann P, Dalmau J, Rudlowski C, Vincent A, Elger CE, Rossi JE, et al. Diagnostic value of N-methyl-D-aspartate receptor antibodies in women with new-onset epilepsy. Arch Neurol. 2009 Apr;66(4):458–464. doi: 10.1001/archneurol.2009.5. [DOI] [PubMed] [Google Scholar]
- 15.Dalmau J, Lancaster E, Marinez-Hernandez E, Rosenfeld M, Balice-Gordon R. Experience and Investigations on anti-NMDA receptor encephalitis. Lancet Neurol. 2010 doi: 10.1016/S1474-4422(10)70253-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]