Abstract
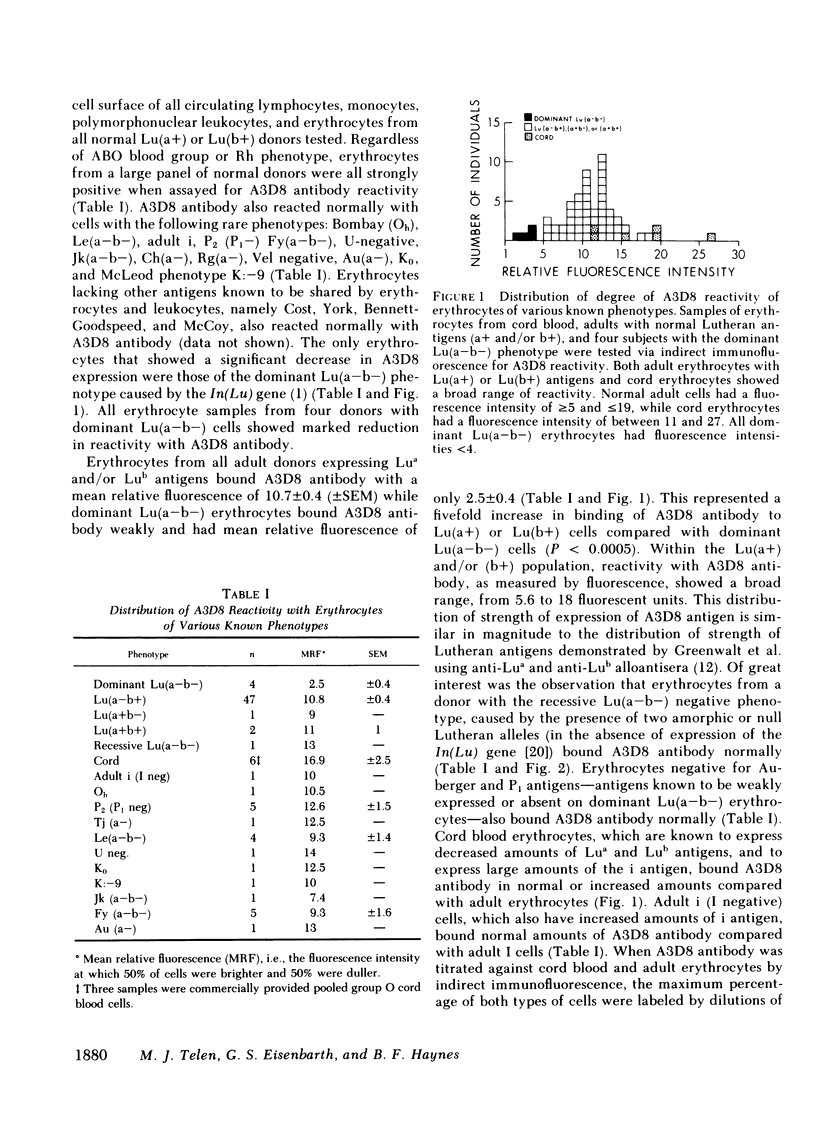
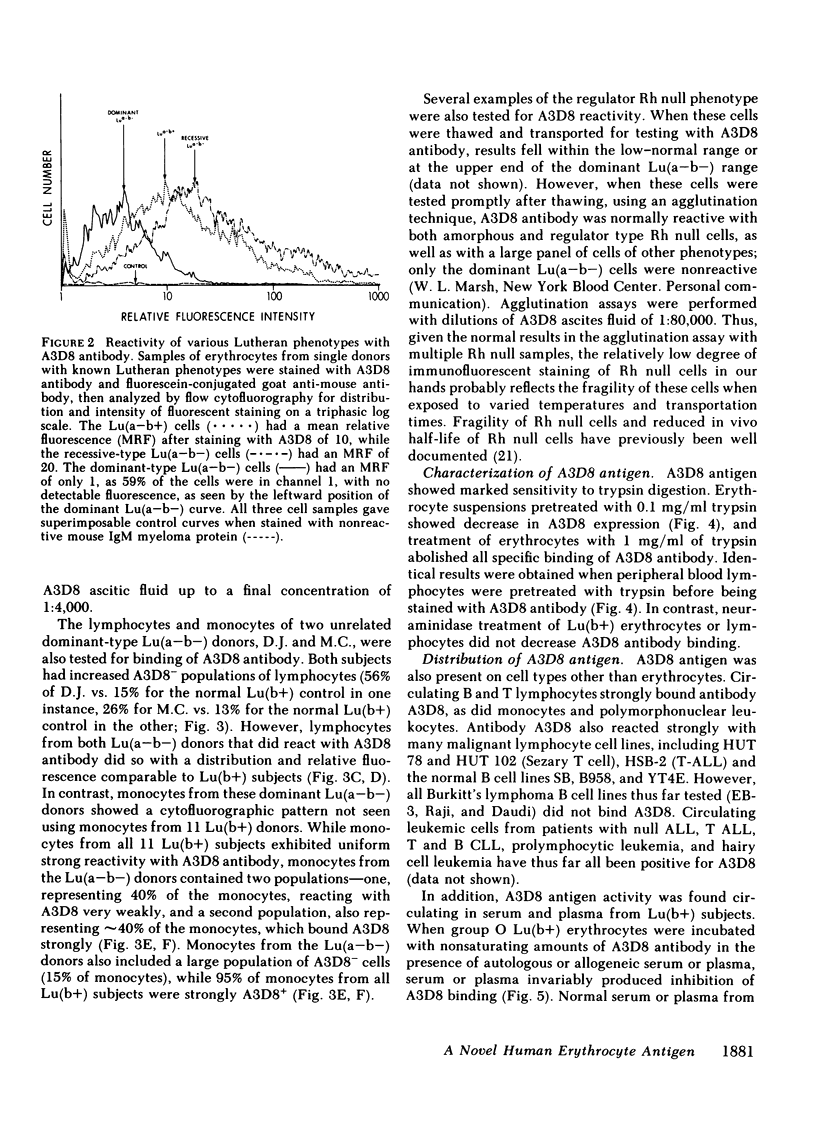
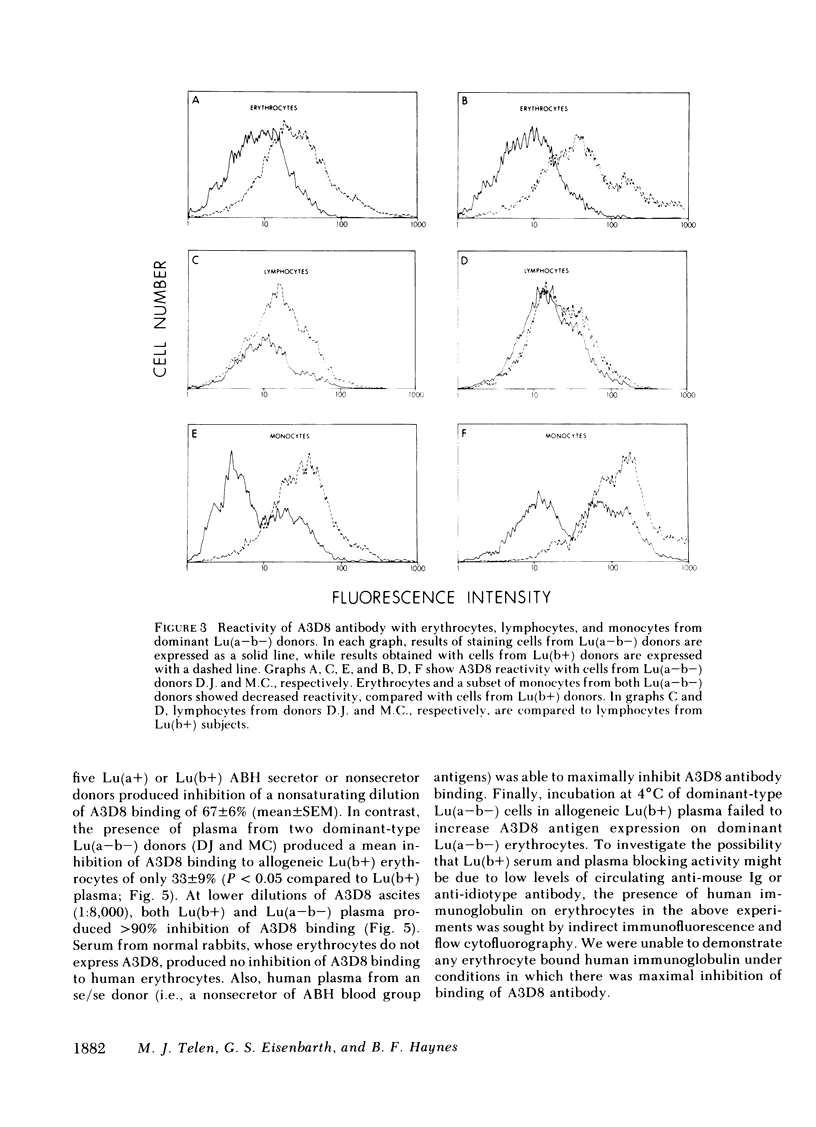
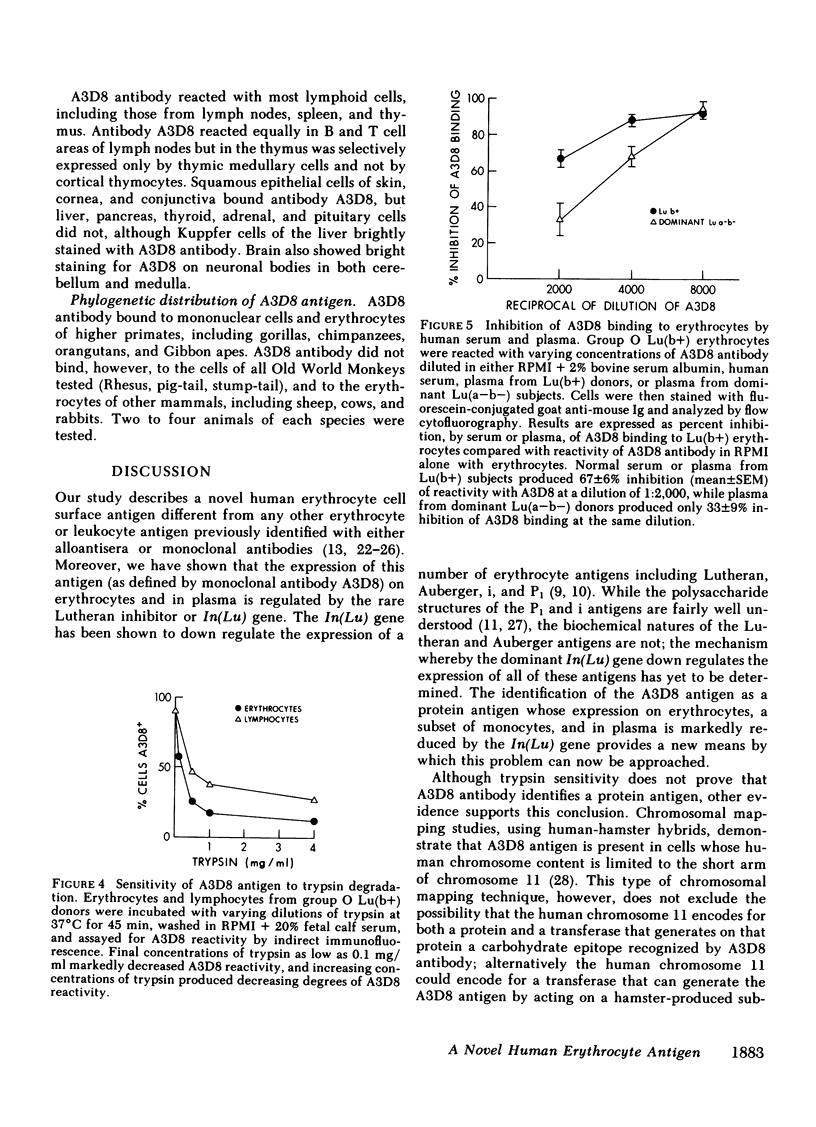
Our study describes a novel human erythrocyte protein antigen, the expression of which is regulated by the rare Lutheran inhibitor In(Lu) gene. We have produced a monoclonal antibody (A3D8) that bound strongly to erythrocytes from subjects with Lutheran phenotypes Lu(a+b+), Lu(a+b-), and Lu(a-b+) but bound negligibly to erythrocytes from subjects with the dominant form of Lu(a-b-) phenotype, reflecting inheritance of the In(Lu) gene. Importantly, erythrocytes from an individual with the recessive form of Lu(a-b-) phenotype (i.e., absence of the In(Lu) gene and absence of genes encoding for Lutheran antigens) showed reactivity with A3D8 antibody comparable to that seen with Lu(a+) or Lu(b+) erythrocytes. A3D8 antigen activity was also found on all leukocytes and in serum and plasma; this activity also appeared to be regulated by the In(Lu) gene in serum, plasma, and on a subset of leukocytes. Thus, we have identified a human erythrocyte protein whose expression is modified by the In(Lu) gene. This knowledge that such an antigen exists on erythrocytes and in normal plasma should allow further studies into the molecular genetics of the In(Lu) gene and into the functional and structural significance of the A3D8 antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anger B. R., Lloyd K. O., Oettgen H. F., Old L. J. Mouse monoclonal IgM antibody against human lung cancer line SK-LC-3 with specificity for H(O) blood group antigen. Hybridoma. 1982;1(2):139–147. doi: 10.1089/hyb.1.1982.1.139. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bove J. R., Allen F. H., Jr, Chiewsilp P., Marsh W. L., Cleghorn T. E. Anti-Lu4: a new antibody related to the Lutheran blood group system. Vox Sang. 1971 Oct;21(4):302–310. doi: 10.1111/j.1423-0410.1971.tb04785.x. [DOI] [PubMed] [Google Scholar]
- Brown F., Simpson S., Cornwall S., Moore B. P., Oyen R., Marsh W. L. The recessive Lu(a-b-) phenotype. A family study. Vox Sang. 1974 Mar;26(3):259–264. doi: 10.1111/j.1423-0410.1974.tb02694.x. [DOI] [PubMed] [Google Scholar]
- Contreras M., Tippett P. The Lu(a-b-) syndrome and an apparent upset of P1 inheritance. Vox Sang. 1974;27(4):369–371. doi: 10.1111/j.1423-0410.1974.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Crawford M. N., Tippett P., Sanger R. Antigens Aua, i and P1 of cells of the dominant type of Lu(a-b-). Vox Sang. 1974 Mar;26(3):283–287. doi: 10.1111/j.1423-0410.1974.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Silva J. S., McCOY J. L., Leonard C. M., Cannon G. B., Herberman R. B. Functional activities of rosette separated human peripheral blood leukocytes. J Immunol. 1975 Nov;115(5):1449–1455. [PubMed] [Google Scholar]
- Francke U., Foellmer B. E., Haynes B. F. Chromosome mapping of human cell surface molecules: monoclonal anti-human lymphocyte antibodies 4F2, A3D8, and A1G3 define antigens controlled by different regions of chromosome 11. Somatic Cell Genet. 1983 May;9(3):333–344. doi: 10.1007/BF01539142. [DOI] [PubMed] [Google Scholar]
- GURNER B. W., COOMBS R. R. Examination of human leucocytes for the ABO, MN, Rh, Tja, Lutheran and Lewis systems of antigens by means of mixed erythrocyte-leucocyte agglutination. Vox Sang. 1958 Jan;3(1):13–22. doi: 10.1111/j.1423-0410.1958.tb03554.x. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Tippett P. A human quantitative polymorphism related to Xg blood groups. Nature. 1981 Jan 29;289(5796):404–405. doi: 10.1038/289404a0. [DOI] [PubMed] [Google Scholar]
- Greenwalt T. J., Sasaki T. T., Steane E. A. The Lutheran blood groups: a progress report with observations on the development of the antigens and characteristics of the antibodies. Transfusion. 1967 May-Jun;7(3):189–200. doi: 10.1111/j.1537-2995.1967.tb05509.x. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Haynes B. F., Hensley L. L., Jegasothy B. V. Phenotypic characterization of skin-infiltrating T cells in cutaneous T-cell lymphoma: comparison with benign cutaneous T-cell infiltrates. Blood. 1982 Aug;60(2):463–473. [PubMed] [Google Scholar]
- Haynes B. F., Mann D. L., Hemler M. E., Schroer J. A., Shelhamer J. H., Eisenbarth G. S., Strominger J. L., Thomas C. A., Mostowski H. S., Fauci A. S. Characterization of a monoclonal antibody that defines an immunoregulatory T cell subset for immunoglobulin synthesis in humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2914–2918. doi: 10.1073/pnas.77.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holburn A. M., Masters C. A. The radioimmunoassay of serum and salivary blood group A and Lea glycoproteins. Br J Haematol. 1974 Oct;28(2):157–167. doi: 10.1111/j.1365-2141.1974.tb06650.x. [DOI] [PubMed] [Google Scholar]
- Judd W. J., Marsh W. L., Oyen R., Nichols M. E., Allen F. H., Jr, Contreras M., Stroup M. Anti-Lu14: a Lutheran antibody defining the product of an allele at the Lu8 blood group locus. Vox Sang. 1977;32(4):214–219. doi: 10.1111/j.1423-0410.1977.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Bai Y., Lomas C., Green C., Tippett P. Two monoclonal antibodies detecting high frequency antigens absent from red cells of the dominant type of Lu(a-b-) Lu:-3. J Immunogenet. 1982 Oct;9(5):353–357. doi: 10.1111/j.1744-313x.1982.tb00993.x. [DOI] [PubMed] [Google Scholar]
- MacIlroy M., McCreary J., Stroup M. Anti-Lu8, an antibody recognizing another Lutheran-related antigen. Vox Sang. 1972;23(5):455–457. doi: 10.1111/j.1423-0410.1972.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Marsh W. L. Anti-Lu5, anti-Lu6, and anti-Lu7. Three antibodies defining high frequency antigens related to the Lutheran blood group system. Transfusion. 1972 Jan-Feb;12(1):27–34. [PubMed] [Google Scholar]
- Molthan L., Crawford M. N., Marsh W. L., Allen F. H., Jr Lu9, another new antigen of the Lutheran blood-group system. Vox Sang. 1973 May;24(5):468–471. doi: 10.1111/j.1423-0410.1973.tb03487.x. [DOI] [PubMed] [Google Scholar]
- Taliano V., Guévin R. M., Tippett P. The genetics of a dominant inhibitor of the Lutheran antigens. Vox Sang. 1973 Jan;24(1):42–47. doi: 10.1111/j.1423-0410.1973.tb03855.x. [DOI] [PubMed] [Google Scholar]
- Tippett P. A case of suppressed Lu a and Lu b antigens. Vox Sang. 1971 Apr;20(4):378–380. doi: 10.1111/j.1423-0410.1971.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Voak D., Sacks S., Alderson T., Takei F., Lennox E., Jarvis J., Milstein C., Darnborough J. Monoclonal anti-A from a hybrid-myeloma: evaluating as a blood grouping reagent. Vox Sang. 1980 Sep;39(3):134–140. doi: 10.1111/j.1423-0410.1980.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Wiener A. S., Moor-Jankowski J., Gordon E. B., Davis J. The blood factors I and i in primates including man, and in lower species. Am J Phys Anthropol. 1965 Dec;23(4):389–396. doi: 10.1002/ajpa.1330230412. [DOI] [PubMed] [Google Scholar]



