Summary
The differential dysfunction of chromatic and achromatic visual pathways in early Parkinson’s disease (PD) was evaluated by means of visual-evoked potentials (VEPs) recorded in 12 patients (mean age 60.1 ± 8.3 years; range 46 to 74 years) in the early stages of PD and not yet undergoing treatment with L-dopa, and in 12 age-matched controls. Visual stimuli were full-field (14 deg) equiluminant red-green (R-G), blue-yellow (B-Y), and black-white (B-W) sinusoidal gratings of two cycles per degree, presented in onset (300 milliseconds) – offset (700 milliseconds) mode, at two contrast (K) levels (90% and 25%). The VEP mean latencies were significantly more delayed in PD patients than in controls for chromatic than for luminance stimuli, in particular for B-Y stimuli of low contrast (K90%: B-W =6.6 milliseconds, R-G =3.34 milliseconds, B-Y =15.48 milliseconds; K25%: B-W =7.8 milliseconds, R-G =14.8 milliseconds, B-Y =28.9). Latencies of chromatic VEPs were more variable that achromatic VEP latencies in both normal subjects and PD patients. Therefore, the frequency of latency abnormalities (within 30%) was not significantly different for the three visual stimuli. Our results show that, in addition to achromatic VEPs, chromatic VEPs are impaired in early PD patients not yet undergoing L-dopa therapy, indicating an acquired color deficiency in these patients. The greater delay for the B-Y VEPs suggests a higher vulnerability of visual blue-cone pathway in the early stages of the disease. However, the overall sensitivity of chromatic VEPs in detecting early visual impairment in PD is comparable with that of achromatic VEPs.
Keywords: Chromatic contrast, VEPs, Parkinson’s disease, Parvocellular, Koniocellular, Magnocellular system
Impairment of achromatic vision in Parkinson’s disease (PD) has been demonstrated using both psychophysical and electrophysiologic methods (Bodis–Wollner et al., 1987; Tartaglione et al., 1984). Abnormalities of color vision have been reported using clinical color vision tests such as the Farnsworth-Munsell 100 hue test and computerized techniques to measure color threshold and color contrast sensitivity (Birch et al., 1998; Buttner et al., 1992, 1995; Haug et al., 1995; Price et al., 1992) as well as chromatic VEPs (Barbato et al., 1994; Buttner et al., 1996). Little is known about the differential involvement of chromatic (red-green [R-G] and blue-yellow [B-Y]) visual subsystems. To answer this question, it is necessary to use stimuli with pure chromatic contrast (equiluminant) able to isolate the activity of retinocortical generators with R-G and B-Y chromatic opponency (Dacey and Lee, 1994; Engel et al., 1997; Lennie et al., 1990; Merigan and Maunsell, 1993). The properties of chromatic VEPs to equiluminant stimuli are strikingly different from those of achromatic VEPs (Crognale, 2002; Porciatti and Fanti, 1999; Porciatti and Sartucci, 1999), and are differently altered in disease (Buttner et al., 1996; Crognale et al., 1993; Porciatti et al., 1997; Porciatti and Sartucci, 1996; Schneck et al., 1997; Spinelli et al., 1996), suggesting specific vulnerability of visual pathway subpopulations.
Recently, normative data have been provided for VEPs to equiluminant R-G and B-Y gratings presented in onset-offset mode (Porciatti and Sartucci, 1999). Both R-G and B-Y VEPs display a major negative component at stimulus onset, whose latency dramatically increases with decreasing contrast compared with luminance ones. Moreover, B-Y VEPs have longer latencies and higher contrast threshold than R-G VEPs (Porciatti and Sartucci, 1999).
In a previous work, we showed that the chromatic pattern electroretinogram (PERG) is impaired in the early stages of PD, indicating a retinal substrate for the known chromatic deficit in this disease (Sartucci et al., 2003). The aim of the present study was to compare VEPs to R-G and B-Y equiluminant stimuli with those to achromatic black-white (B-W) in patients with PD not yet treated with levodopa. Preliminary results of this study have been previously reported in abstract form (Sartucci et al., 1999).
MATERIALS AND METHODS
Patients
Twelve new PD patients recruited at the Institute of Neurology of the Department of Neuroscience, University of Pisa, during the last 2 years were enrolled in the study. Six patients were females and six were males; their age ranged from 46 to 74 years (mean 60.1 ± 8.3 years). All had idiopathic PD according to the criteria of United Kingdom Brain Bank (Fahn et al., 1987; Gibb and Lees, 1988) and later revised by Gelb et al. [Gelb, 1999 #267] and did not receive L-dopa treatment. Computed tomography scan and MRI with contrast medium examination and acute challenge test with levodopa were performed in each patient at the moment of clinical examination, immediately before the inclusion in the study. Blink-reflex habituation (Matsumoto et al., 1992) was also checked. Disease severity was scored according to the Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al., 1987) and Hohen-Yahr scale (HY) (Hoehn and Yahr, 1967). Hoehn-Yahr grading was 1.6 ± 0.3 (range 1 to 2.5); UPDRS scoring subitem II was 8.6 ± 1.0, and subitem III was 14.5 ± 3.8. All were treated only with amantadine alone and/or together with selegiline. Exclusion criteria were the presence of other concomitant internal or neurologic disorders and history of psychiatric and cognitive disorders. We also excluded subjects with visual acuity at or below 0.8 Snellen fraction, known ophthalmologic disease, and abnormal color discrimination (Ishihara Test) after careful ophthalmologic examination, which included the assessment of visual acuity, pupil diameter and shape, fundus oculi, intrinsic and extrinsic ocular motility, and Goldman kinetic perimetry or Humphrey static perimetry (program 30-2). The main clinical and demographic features of the PD cases enrolled are summarized in Table 1.
TABLE 1.
Summary of Essential Demographic Data of the Patients and Control Observers, and Main Clinical Features of the Patients
| PD Patients (n =12) | Controls (n =12) | |
|---|---|---|
| Age, y (mean SD) | 60.1 ± 8.3 | 46.8 ± 12.9 |
| Gender (M/F) | 6/6 | 6/6 |
| Disease’s duration, mo (mean SD) | 26.4 ±7.5 | — |
| HY scale | 1.2 ±0.2 | — |
| UPDRS subitem II (mean SD) | 7.3 ±2.3 | — |
| UPDRS subitem III (mean SD) | 12.4 ±3.4 | — |
Twelve healthy subjects (six males and six females aged 27 to 66 years, mean age 46.8 ± 12.9 years) with normal visual acuity and color vision served as controls. All subjects had no or small refractive errors, which were corrected to normal vision when needed for the viewing distance of visual stimuli. They had normal color vision (Ishihara) and did not claim previous ocular or systemic disease. Pupillary and slit lamp examination findings were normal, as were intraocular pressure and optic disks. The experimental protocol was previously approved by the local ethics committee. All experiments fulfilled the tenets of the Declaration of Helsinki and an informed consent was obtained from each patient after the aims of the study and the procedure were fully explained.
Visual Stimuli
Visual stimuli were equiluminant horizontal sinusoidal gratings of two cycles per degree modulated either in luminance (B-W) or chromaticity (R-G and B-Y). R-G chromatic gratings were obtained by superimposing (out of phase by 180 degrees) red-black to green-black luminance gratings, and blue-yellow chromatic grating were obtained by superimposing (also out of phase by 180 degrees) blue-black to yellow-black luminance gratings. Red-black, green-black, blue-black, and yellow-black luminance gratings had the same Michelson contrast (90% or 25%), which was used to define the contrast of the chromatic grating. Gratings were generated by a VSG/2 graphic card (Cambridge Research, UK) and displayed on a color monitor (Barco CCID 7751, Kortrijk, Belgium), at a frame rate of 120 Hz, 512 lines per frame, and 14 bits per color per pixel, suitably linearized by gamma correction (Minolta Chromameter CS100, Japan). Custom software was used for generating stimuli, for establishing the psychophysical equiluminant point, and for recording/analyzing responses (Porciatti and Sartucci, 1999).
In all subjects, the equiluminant point was established for each eye by evaluating psychophysically the minimum visibility of R-G and B-Y gratings alternating sinusoidally at 15 and 10 Hz, respectively. Subjects adjusted the relative luminance of two opponent colors, red to total luminance: R/(R+G) =r and blue to total luminance: B/[B+(R+G)] =b, to null or minimize perception of flicker (Mullen, 1985; see Porciatti and Sartucci, 1999 for further details). It is important to note that changing the relative luminance of gratings composing the chromatic-contrast stimulus has no effect on the Michelson contrast of both original and composed gratings (Porciatti and Sartucci, 1999). Psychophysical color ratios yielding equiluminance were used to set chromaticity values of visual stimuli for VEPs. It has been previously shown that the psychophysical and electrophysiologic equiluminant points coincide (Fiorentini et al., 1996; Porciatti and Sartucci, 1999). In all subjects, for both R-G and B-Y patterns the equiluminant point was found at r and b values close to 0.5 (Vλ equiluminant point: see Fiorentini et al., 1996). This confirmed that color discrimination was normal in patients. The visible screen was 26 cm wide and 24 high, subtending 14 ×16 degrees when viewed from 100 cm (either psychophysical or electrophysiologic experiments). Mean luminance was 17 cd (candella) m−2, producing a retinal illuminance of 330 Troland when viewed through natural pupils (diameter 5 mm in all subjects).
Electrophysiologic Techniques
Equiluminant R-G and B-Y stimuli of two contrast levels, 90% and 25% respectively, were presented for 300 milliseconds (onset) and removed (contrast set to zero) for 700 milliseconds (offset). VEPs were recorded using Ag/AgCl superficial cup electrodes 9 mm in diameter, placed 2 cm above the inion (active) and at the right mastoid (reference); the vertex was grounded. Electrode resistance was kept below 5 KΩ, interelectrode one less than 500 Ω. VEPs signal were amplified (50,000 folds), band-pass filtered between 0.3 and 100 Hz (6-dB octave−1), digitized at 1,024 Hz with 12-bit resolution, and averaged online by a PC (Olidata, Cesena Italy), using custom software written in Labview language (Version 5.0, National Instruments, Austin, TX, 1998). Sweeps containing signals higher than 4 V, corresponding to final display of 80 mV were automatically rejected to minimize EEG contamination by eye blinking, ocular movement, or other environmental instrumental-biologic activities. Responses were evaluated separately for partial averages (10-sum or 20-sum packets) of the total average (at least 100 sums) to assess consistency (Porciatti and Sartucci, 1999) and two or three traces for each eye were superimposed to ensure reproducibility. Normative data for the present set of stimuli and recording conditions have been previously published (Porciatti and Sartucci, 1999).
Statistics
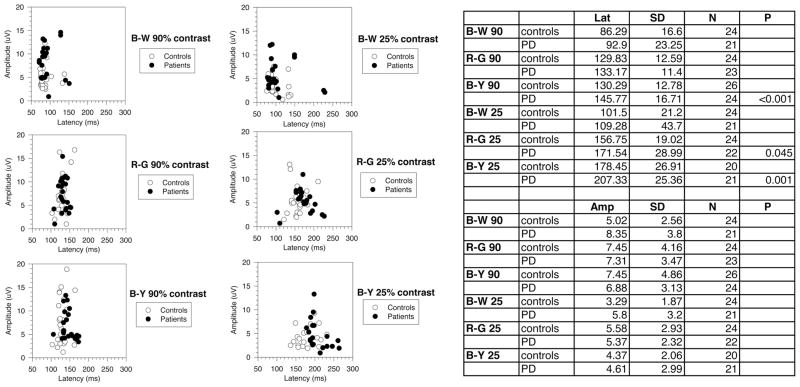
The VEP latency data of individual eyes of subjects (controls, n =24 eyes; PD patients, n =23 eyes; 1 patient was monocular) were analyzed. Some patients had nonrecordable responses for some stimulus conditions; these data were not included in the statistical design, in the evaluation of average response latency and amplitude (Table 2). However, all data were considered in measuring the rate of abnormal responses (Table 3). Data from patients exceeding 2.0 SD of the control mean for VEP latency were considered abnormal.
TABLE 2.
Mean Latency and Amplitude Values (± 1 SD) in Control Observers or Patients
| Latency | SD | n | P | ||
|---|---|---|---|---|---|
| B-W 90 | Controls | 86.29 | 16.6 | 24 | |
| Patients | 92.9 | 23.25 | 21 | ||
| R-G 90 | Controls | 129.83 | 12.59 | 24 | |
| Patients | 133.17 | 11.4 | 23 | ||
| B-Y 90 | Controls | 130.29 | 12.78 | 26 | |
| Patients | 145.77 | 16.71 | 24 | <0.001 | |
| B-W 25 | Controls | 101.5 | 21.2 | 24 | |
| Patients | 109.28 | 43.7 | 21 | ||
| R-G 25 | Controls | 156.75 | 19.02 | 24 | |
| Patients | 171.54 | 28.99 | 22 | 0.045 | |
| B-Y 25 | Controls | 178.45 | 26.91 | 20 | |
| Patients | 207.33 | 25.36 | 21 | 0.001 | |
| Amplitude | SD | n | P | ||
| B-W 90 | Controls | 5.02 | 2.56 | 24 | |
| Patients | 8.35 | 3.8 | 21 | ||
| R-G 90 | Controls | 7.45 | 4.16 | 24 | |
| Patients | 7.31 | 3.47 | 23 | ||
| B-Y 90 | Controls | 7.45 | 4.86 | 26 | |
| Patients | 6.88 | 3.13 | 24 | ||
| B-W 25 | Controls | 3.29 | 1.87 | 24 | |
| Patients | 5.8 | 3.2 | 21 | ||
| R-G 25 | Controls | 5.58 | 2.93 | 24 | |
| Patients | 5.37 | 2.32 | 22 | ||
| B-Y 25 | Controls | 4.37 | 2.06 | 20 | |
| Patients | 4.61 | 2.99 | 21 |
The VEP mean latencies were more delayed for chromatic than for luminance stimuli, especially for B-Y of low K levels. Significant differences compared with controls were observed for both R-G and B-Y of 25% K and for B-Y of 90% K. Latencies were longer for B-Y, as compared with R-G and B-W, at both K. Mean amplitude was not significantly different between controls and PD patients for all stimulus conditions.
TABLE 3.
Rate of Abnormal Responses (%) in Patients for R-G, B-Y, and LUM Checks of 90% and 25% Contrast Stimuli
| Red-Green K 90% | Red-Green K 25% | Blue-Yellow K 90% | Blue-Yellow K 25% | Black-White K 90% | Black-White K 25% |
|---|---|---|---|---|---|
| 0% | 21.7% | 30.4% | 30.4%% | 26.1% | 26.1% |
| n =0/23 | n =5/23 | n =7/23 | N =7/23 | n =6/23 | n =2/23 |
| — | 1 abs | 1 abs | 2 abs | 2 abs | 2 abs |
abs, VEP not elicitable; n, eyes tested.
RESULTS
Examples of onset-offset VEPs to R-G and B-Y equiluminant gratings of 90% and 25% contrast are reported in Fig. 1. For both kind of stimuli and contrast levels, the main response component is a negative-positive complex (N1-P1) at stimulus onset, which has been taken as representative of the chromatic VEP response. Amplitude (N1-P1) and latency (N1) of chromatic VEPs are strongly contrast-dependent (Porciatti and Sartucci, 1999; Rabin et al., 1994), as shown by the remarkable increase in latency and decrease in amplitude with decreasing contrast.
FIGURE 1.
Representative examples of VEP waveform to onset (300 milliseconds) – offset (700 milliseconds) horizontal sinusoidal gratings (spatial frequency two cycles per degree, field size 14 ×16 degrees) with different levels of pure chromatic contrast (RG: red-green; BY: blue-yellow; BW: black-white); two traces are superimposed for each stimulating pattern to ensure reliability. The upper line show responses to 90% contrast and the lower line to 25% contrast for all the three types of stimuli. Note that chromatic responses are represented mainly by a negative-positive complex (N1-P1;) whose amplitude and latency change substantially with contrast. Vertical lines indicate upper normal limits using 2 SD. Positivity is up.
The VEP data are summarized in Fig. 2 as amplitude versus latency scatterplots for responses measured in individual eyes, either in PD patients or in control observers. Visual inspection of Fig. 2 indicates that VEP latencies of PD patients overlapped with those of normal subjects. This overlap, however, was comparatively smaller for B-Y stimuli of 25% contrast.
FIGURE 2.
Amplitude versus latency scatterplots of chromatic VEPs, measured in individual eyes of PD patients. Filled circles indicate PD patients; empty circles indicate controls. It can be seen that scatter increases by reducing stimulus contrast, and that the data overlap considerably.
Abnormalities in VEP Latency and Amplitude
Average latency (±SD) in control subjects and patients is shown in Table 2. In general, VEP latencies of PD patients were delayed as compared with those of controls. The delay tended to be larger for chromatic than for luminance stimuli, in particular for B-Y stimuli of 25% contrast. Differences between PD and controls were significant for B-Y stimuli of 90% and 25% contrast (P <0.001) and borderline significant for R-G stimuli of 25% contrast (P =0.045). Mean amplitudes were not significantly different between control and PD patients for the different stimuli.
Table 3 summarizes the rate of latency abnormalities in PD patients. Rates of abnormal VEPs were as follows: for K90%, R-G 0% (n =0/23); B-Y 30.4% (n. =7/23; 1 eye with absent VEP), B-W 26.1% (n =6/23, 2 eyes with absent VEP); for K25%: R-G 21.7% (n =5/23; 1 eye with absent VEP); B-Y 30.4% (n =7/23; 2 eyes with absent VEP), B-W 26.1% (n =6/23; 2 eyes with absent VEP). Due to the large variability of chromatic versus achromatic VEPs, the rate of abnormalities is substantially comparable for all types of stimuli.
DISCUSSION
The aim of the present study was to establish whether the anatomically and physiologically distinct R-G and B-Y subdivisions of the color pathway are differently altered in early PD. In addition, we compared the sensitivities (rate of abnormalities) of equiluminant R-G, B-Y, and B-W onset VEPs to determine which condition has the highest potential to detect early visual impairment in PD.
Our results show that the average latency of equiluminant B-Y VEPs is significantly delayed in patients with early PD as compared with controls. The amount of delay is larger than that of both R-G and B-W VEPs. This indicates higher vulnerability of the B-Y chromatic pathway, in agreement with previous psychophysical investigations in PD (Birch et al., 1998; Haug et al., 1995) and the current belief that the blue-cone pathway is particularly vulnerable in many retinal and postretinal diseases. (Adams, 1982). A high vulnerability of the blue-cone pathway is generally explained by its limited retinal redundancy (Buttner et al., 1995; Crognale, 2002). Our results are in broad agreement with those of Buttner et al. (1996), who recorded chromatic equiluminant VEPs in PD patients and found comparable alterations for R-G and B-Y stimuli. We also found that, despite longer delays of chromatic VEPs as compared with achromatic VEPs, the rate of latency abnormalities is not significantly different. This finding may be understood in terms of larger variability of chromatic, as compared with achromatic, VEPs.
It is interesting that in patients with early PD, the chromatic PERG in response to equiluminant B-Y stimuli is comparatively more delayed than the PERG to either R-G or achromatic stimuli (Sartucci et al., 2003). This indicates that the alterations of the B-Y pathway in PD have a substantial retinal component.
In conclusion, the combined assessment of chromatic and achromatic VEPs to selected stimuli provides a means for a more comprehensive evaluation of the visual pathway and a better understanding of the pathophysiology of visual involvement in PD, suggesting that the blue-cone pathway is early and predominantly affected.
Acknowledgments
Supported by NIH/NEI R01 EY14957, NIH center grant P-30- EY014801, an unrestricted grant to the University of Miami from Research to Prevent Blindness and MIUR-PRIN 2003.
The authors thank Prof. U. Bonuccelli and Dr. C. Lucetti for permission to examine patients in their care, Prof. David C. Burr for generously providing the stimulus software facilities, and Mr. C. Orsini for his invaluable technical assistance.
References
- Adams AJ. Chromatic and luminosity processing in retinal disease. Am J Optom Physiol Opt. 1982;59:954–960. doi: 10.1097/00006324-198212000-00004. [DOI] [PubMed] [Google Scholar]
- Barbato L, Rinalduzzi S, Laurenti M, et al. Color VEPs in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1994;92:169–172. doi: 10.1016/0168-5597(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Birch J, Kolle RU, Kunkel M, et al. Acquired colour deficiency in patients with Parkinson’s disease. Vision Res. 1998;38:3421–3426. doi: 10.1016/s0042-6989(97)00398-2. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, et al. Visual dysfunction in Parkinson’s disease. Loss in spatiotemporal contrast sensitivity. Brain. 1987;110:1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Muller T, et al. Chromatic and achromatic visual evoked potentials in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1996;100:443–447. [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Muller T, et al. Distorted color discrimination in ‘de novo’ parkinsonian patients. Neurology. 1995;45:386–387. doi: 10.1212/wnl.45.2.386. [DOI] [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Patzold T, et al. Disorders of colour perception in Parkinson’s disease. J Neurol. 1992;239:239. [Google Scholar]
- Crognale MA. Development, maturation, and aging of chromatic visual pathways: VEP results. J Vision. 2002;2:438–450. doi: 10.1167/2.6.2. [DOI] [PubMed] [Google Scholar]
- Crognale MA, Switkes E, Rabin J, et al. Application of the spatiochromatic visual evoked potential to detection of congenital and acquired color-vision deficiencies. J Opt Soc Am A. 1993;10:1818–1825. doi: 10.1364/josaa.10.001818. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Engel S, Zhang X, Wandell B. Colour tuning in human visual cortex measured with functional magnetic resonance imaging. Nature. 1997;388:68–71. doi: 10.1038/40398. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. the members of the UPDRS Development Commitee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. New York: Macmillan; 1987. pp. 153–163. [Google Scholar]
- Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: unspecific decline of the responses to luminance and colour. Vision Res. 1996;36:3557–3566. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug BA, Kolle RU, Trenwalder C, et al. Predominant affection of the blue cone pathway in Parkinson’s disease. Brain. 1995:118. doi: 10.1093/brain/118.3.771. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Noro H, Kaneshige Y, et al. A correlation study between blink reflex habituation and clinical state in patients with Parkinson’s disease. J Neurol Sci. 1992;107:155–159. doi: 10.1016/0022-510x(92)90283-q. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Ann Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mullen KT. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J Physiol (Lond) 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Di Bartolo E, Nardi N, Fiorentini A. Responses to chromatic and luminance contrast in glaucoma: a psychophysical and electrophysiological study. Vision Res. 1997;37:1975–1987. doi: 10.1016/s0042-6989(97)00018-7. [DOI] [PubMed] [Google Scholar]
- Porciatti V, Fanti J. The PERG to red-green and blue-yellow chromatic contrast. Invest Ophthalmol Vis Sci. 1999;40:s13. [Google Scholar]
- Porciatti V, Sartucci F. Retinal and cortical evoked responses to chromatic contrast stimuli. Specific losses in both eyes of patients with multiple sclerosis and unilateral optic neuritis. Brain. 1996;119:723–740. doi: 10.1093/brain/119.3.723. [DOI] [PubMed] [Google Scholar]
- Porciatti V, Sartucci F. Normative data for onset VEPs to chromatic contrast. Clin Neurophysiol. 1999;110:772–781. doi: 10.1016/s1388-2457(99)00007-3. [DOI] [PubMed] [Google Scholar]
- Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology. 1992;42:887–890. doi: 10.1212/wnl.42.4.887. [DOI] [PubMed] [Google Scholar]
- Rabin J, Switkes E, Crognale M, et al. Visual evoked potentials in three-dimensional color space: correlates of spatiochromatic processing. Vision Res. 1994;34:2657–2671. doi: 10.1016/0042-6989(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Sartucci F, Lucetti C, Bonuccelli U, et al. VEPs to onset of equiluminant red-green and blue-yellow gratings in Parkinson’s disease. Invest Ophthalmol Vis Sci. 1999;40:S822. [Google Scholar]
- Sartucci F, Orlandi G, Lucetti C, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson’s disease. J Clin Neurophysiol. 2003;20:375–381. doi: 10.1097/00004691-200309000-00010. [DOI] [PubMed] [Google Scholar]
- Schneck ME, Fortune B, Switkes E, et al. Acute effects of blood glucose on chromatic visually evoked potentials in persons with diabetes and in normal persons. Invest Ophthalmol Vis Sci. 1997;38:800–810. [PubMed] [Google Scholar]
- Spinelli D, Angelelli P, De Luca M, Burr DC. VEP in neglect patients have longer latencies for luminance but not for chromatic patterns. Neuroreport. 1996;7:815–819. doi: 10.1097/00001756-199602290-00032. [DOI] [PubMed] [Google Scholar]
- Tartaglione A, Pizio N, Bino G, et al. VEP changes in Parkinson’s disease are stimulus-dependent. J Neurol Neurosurg Psychiatry. 1984;47:305–307. doi: 10.1136/jnnp.47.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]