Abstract
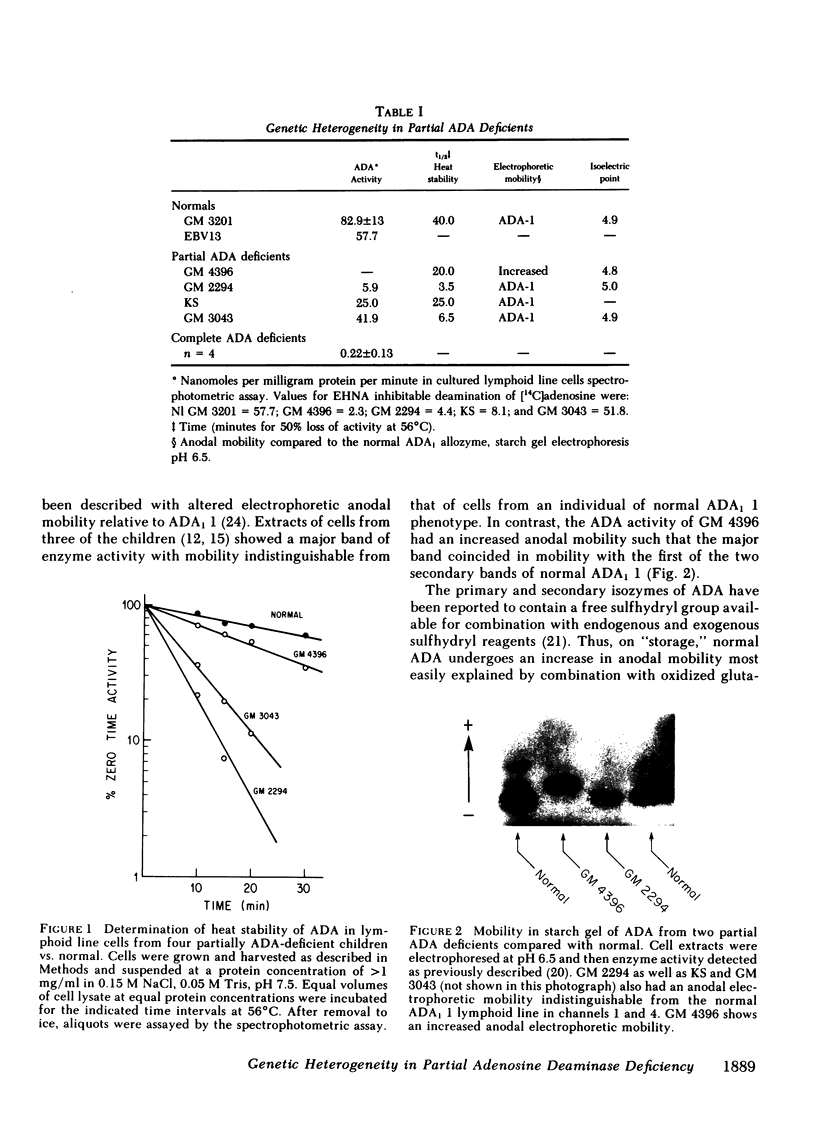
Inherited deficiency of the enzyme adenosine deaminase (ADA) results in a syndrome of severe combined immunodeficiency (SCID). Children with ADA- -SCID lack ADA in all cells and tissues. In contrast, a "partial" deficiency of ADA has been described in six immunologically normal children from four different "families." These children lack ADA in their erythrocytes but retain variable amounts of activity in their lymphoid cells. We have examined ADA activity in lymphoid line cells from four of these children, who are unrelated, for evidence of genetic heterogeneity. One child, who is Caucasian, has an enzyme with increased electrophoretic mobility, a diminished isoelectric point (pI 4.8 vs. Nl = 4.9) and very low activity (2.3 vs. Nl = 82.9 +/- 12.9 nmol/mg protein per min); as a second child has an enzyme with normal electrophoretic mobility but increased isoelectric point (pI = 5.0), markedly diminished heat stability at 56 degrees C (t1/2 = 4.2' vs. Nl = 40') and low activity (12.1); a third has an enzyme with only diminished heat stability (t1/2 = 6.5'), no detectable abnormality in charge and almost normal activity (41.9); while the fourth exhibits only diminished ADA activity (25.0) with no striking qualitative abnormalities. Thus, we have found evidence for three different mutations at the structural locus for ADA in three of these individuals, (a) an acidic, low activity heat stable mutation (b) a basic, somewhat higher activity, heat labile mutation, and (c) a relatively normal activity heat labile mutation. In the fourth, there is as yet no compelling evidence for a mutation at the structural locus for ADA and a mutation at a regulatory locus cannot be excluded.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borkowsky W., Gershon A. A., Shenkman L., Hirschhorn R. Adenosine deaminase deficiency without immunodeficiency: clinical and metabolic studies. Pediatr Res. 1980 Jul;14(7):885–889. doi: 10.1203/00006450-198007000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. Conversion of human erythrocyte-adenosine deaminase activity to different tissue-specific isozymes. Evidence for a common catalytic unit. J Clin Invest. 1975 Mar;55(3):661–667. doi: 10.1172/JCI107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Martiniuk F., Rosen F. S. Adenosine deaminase activity in normal tissues and tissues from a child with severe combined immunodeficiency and adenosine deaminase deficiency. Clin Immunol Immunopathol. 1978 Mar;9(3):287–292. doi: 10.1016/0090-1229(78)90100-9. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Ratech H., Rubinstein A., Papageorgiou P., Kesarwala H., Gelfand E., Roegner-Maniscalco V. Increased excretion of modified adenine nucleosides by children with adenosine deaminase deficiency. Pediatr Res. 1982 May;16(5):362–369. doi: 10.1203/00006450-198205000-00009. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner V., Jenkins T., Seaman C., Piomelli S., Borkowsky W. Erythrocyte adenosine deaminase deficiency without immunodeficiency. Evidence for an unstable mutant enzyme. J Clin Invest. 1979 Oct;64(4):1130–1139. doi: 10.1172/JCI109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson D. A., Cook P. J., Harris H. Further data on the adenosine deaminase (ADA) polymprphism and a report of a new phenotype. Ann Hum Genet. 1969 May;32(4):361–367. doi: 10.1111/j.1469-1809.1969.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Harris H. The investigation of reactive sulphydryls in enzymes and their variants by starch gel electrophoresis. Studies on red cell adenosine deaminase. Ann Hum Genet. 1969 Jul;33(1):81–87. doi: 10.1111/j.1469-1809.1969.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Lane A. B., Nurse G. T., Hopkinson D. A. Red cell adenosine deaminase (ADA) polymorphism in Southern Africa, with special reference to ADA deficiency among the !Kung. Ann Hum Genet. 1979 May;42(4):425–433. doi: 10.1111/j.1469-1809.1979.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Rabson A. R., Nurse G. T., Lane A. B. Deficiency of adenosine deaminase not associated with severe combined immunodeficiency. J Pediatr. 1976 Nov;89(5):732–736. doi: 10.1016/s0022-3476(76)80792-5. [DOI] [PubMed] [Google Scholar]
- Jenkins T. Red-blood-cell adenosine deaminase deficiency in a "healthy" Kung individual. Lancet. 1973 Sep 29;2(7831):736–736. doi: 10.1016/s0140-6736(73)92568-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martiniuk F., Hirschhorn R. Human neutral alpha-glucosidase C: genetic polymorphism including a "null" allele. Am J Hum Genet. 1980 Jul;32(4):497–507. [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Chen S. H., Scott C. R. Use of the integrated steady state rate equation to investigate product inhibition of human red cell adenosine deaminase and its relevance to immune dysfunction. J Biol Chem. 1978 Jan 25;253(2):323–325. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N., Tartaglia A. P., Gilsanz F., Sparkes R. S. Control of red blood cell adenine nucleotide metabolism studies of adenosine deaminase. Prog Clin Biol Res. 1978;21:319–338. [PubMed] [Google Scholar]
- Polmar S. H., Stern R. C., Schwartz A. L., Wetzler E. M., Chase P. A., Hirschhorn R. Enzyme replacement therapy for adenosine deaminase deficiency and severe combined immunodeficiency. N Engl J Med. 1976 Dec 9;295(24):1337–1343. doi: 10.1056/NEJM197612092952402. [DOI] [PubMed] [Google Scholar]
- Siciliano M. J., Bordelon M. R., Kohler P. O. Expression of human adenosine deaminase after fusion of adenosine deaminase-deficient cells with mouse fibroblasts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):936–940. doi: 10.1073/pnas.75.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield J. A., Creagan R. P., Nichols E. A., Ruddle F. H. Assignment of a gene for adenosine deaminase to human chromosome 20. Hum Hered. 1974;24(1):1–11. doi: 10.1159/000152631. [DOI] [PubMed] [Google Scholar]
- Valentine W. N., Paglia D. E., Tartaglia A. P., Gilsanz F. Hereditary hemolytic anemia with increased red cell adenosine deaminase (45- to 70-fold) and decreased adenosine triphosphate. Science. 1977 Feb 25;195(4280):783–785. doi: 10.1126/science.836588. [DOI] [PubMed] [Google Scholar]