Abstract
G protein-coupled receptors (GPCRs) are seven-transmembrane proteins that transmit diverse extracellular signals across a membrane. Herpesvirus genomes encode multiple GPCRs implicated in viral pathogenesis. Kaposi sarcoma-associated herpesvirus GPCR (kGPCR) activates proliferative pathways and, when expressed in endothelium in mice, sufficiently induces angiogenic tumor resembling human Kaposi’s sarcoma. IKKε, an IκB kinase (IKK)-related kinase, is implicated in inflammation-driven tumorigenesis. We report here that IKKε is critically required for kGPCR tumorigenesis and links kGPCR to NF-κB activation. Using kGPCR-induced tumor models, we found that IKKε expression was drastically up-regulated in Kaposi sarcoma-like lesions and that loss of IKKε abolished tumor formation. Moreover, kGPCR interacted with and activated IKKε. Activated IKKε promoted NF-κB subunit RelA (also known as p65) phosphorylation, which correlated with NF-κB activation and inflammatory cytokine expression. The robust expression of IKKε and phosphorylated RelA was observed in human Kaposi sarcoma. Finally, a kinase-defective mutant of IKKε effectively abrogated NF-κB activation and tumorigenesis induced by kGPCR. Collectively, our findings uncover a critical IKKε in promoting NF-κB activation and tumorigenesis induced by a viral GPCR.
Keywords: sarcomagenesis, inflammation associated
Kaposi sarcoma-associated herpesvirus (KSHV) is the etiological agent of Kaposi sarcoma (KS), primary effusion lymphoma, and multicentric Castleman disease (1–4). KS is the most common neoplasm in AIDS patients (5, 6). The KSHV genome encodes multiple molecules that trigger cell proliferation and induce tumor formation, recapitulating some key pathological features of KSHV-associated malignancies. The viral G protein-coupled receptor (referred to as kGPCR), when expressed in mice, is sufficient to drive the development of angio-proliferative tumors resembling human KS (7). Paradoxically, kGPCR is expressed in the lytic phase of KSHV-infected cells (8) and its expression is invariably observed in minor populations of KS tumor cells (7, 9), supporting the postulate that lytic replicating cells and latently-infected cells synergize to facilitate the sarcomagenesis of KS (7).
GPCRs are seven-transmembrane proteins that transmit physiological stimuli across membranes via coupling to small G proteins (10, 11). kGPCR is a functional homolog of the human interleukin 8 receptor (IL-8R) and a bona fide signaling molecule independent of association with its cognate ligand (12). Many of the classic proliferative signaling cascades are activated by kGPCR and are necessary for kGPCR tumorigenesis (13). Although the critical roles of the PI3K–AKT pathway in kGPCR tumorigenesis are emerging (14, 15), the significance of other signaling pathways in kGPCR oncogenesis and mechanisms of signal transduction thereof remain poorly defined (e.g., NF-κB activation) (16). Despite the fact that NF-κB activation is well studied for its regulation in diverse physiological conditions, it remains unclear how kGPCR activates the NF-κB pathway in particular.
The canonical NF-κB pathway is regulated by phosphorylation and subsequent proteolysis of the inhibitors of NF-κB (IκBs) (17). Upon the degradation of IκBs, the NF-κB dimer is unleashed from inhibition, translocates into the nucleus, and up-regulates gene expression (18, 19). The NF-κB family comprises five members: RelA (also known as p65), RelB, c-Rel, NF-κB1 (p105, a precursor of p50), and NF-κB2 (p100, a precursor of p52) (20). In contrast to the first three Rel proteins, NF-κB1 and NF-κB2 are transcriptionally inactive owing to the lack of the carboxyl terminal transactivation domain (21, 22). Thus, NF-κB activation and downstream gene expression are highly dependent on the three Rel proteins, among which RelA is the most abundant and extensively studied NF-κB subunit. Central to fundamental cellular processes, NF-κB activation is a recurring theme frequently found in a plethora of human cancers (23).
The IKK-related kinases, namely TANK-binding kinase 1 (TBK1) and IKKε (also known as IKKi), were originally discovered for their key roles in activating IFN regulatory factors during viral infection (24–26). Subsequently, IKKε was reported to function chiefly through phosphorylating and activating STATs upon IFN engagement (27). Notably, emerging studies implicate these IKK-related kinases in tumorigenic processes (28, 29), although the underpinning molecular mechanisms remain largely unknown. We report here that IKKε is up-regulated by the proinflammatory kGPCR and necessary for the angiogenic sarcomagenesis of kGPCR. Mechanistically, IKKε links a low-level, persistent NF-κB activation to the transmembrane kGPCR, via phosphorylating RelA. Our findings uncover IKKε in intimately linking two signaling nodes of pleiotropic functions, transmembrane GPCR and NF-κB transcription factors.
Results
IKKε Is Crucially Involved in kGPCR-Induced Tumorigenesis.
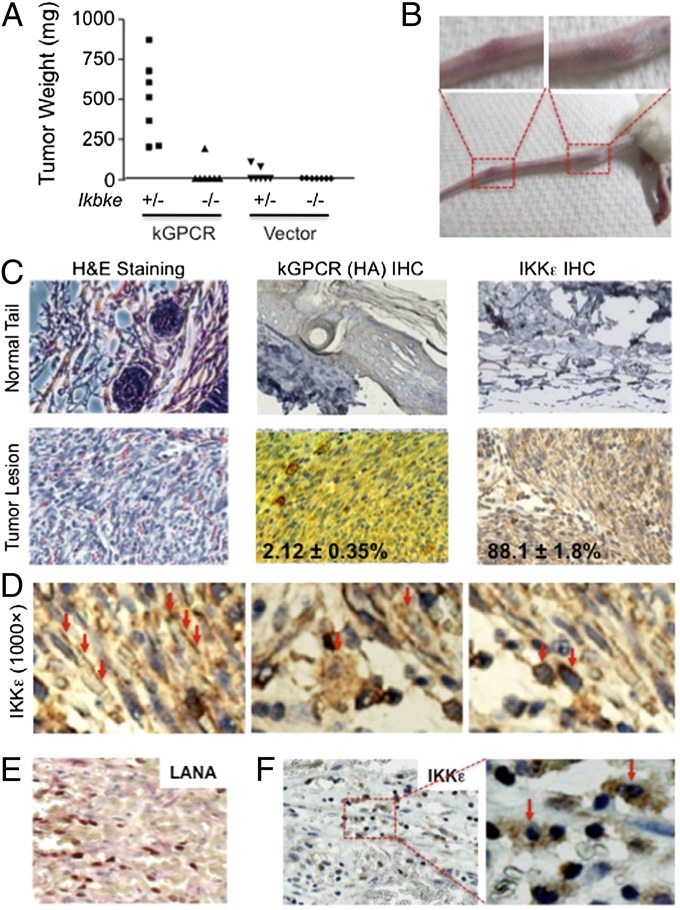
The IKKε kinase is highly inducible by inflammatory cytokines (26). kGPCR-induced tumors have inflammatory characteristics (30, 31). To probe the roles of IKKε in kGPCR tumorigenesis, we examined kGPCR-induced tumor formation in nude mice. Remarkably, loss of IKKε abolished tumor formation induced by kGPCR (Fig. 1A), indicating that IKKε is essential for kGPCR tumorigenesis. In the absence of kGPCR, we observed no tumor formation in nude mice regardless of the expression of IKKε in mouse embryonic fibroblasts (MEFs). Next, we examined the expression of IKKε in KSHV-associated lesions. To do this, we first turned to the TIE2-tva transgenic mouse model (mouse expressing avian leukosis virus receptor TVA under the control of vascular endothelial cell-specific TIE2 promoter) that exquisitely identified kGPCR as a primary oncogene of KSHV in the endothelium. The TIE2-tva mouse, when infected with avian leukosis virus (ALV)-derived retroviral vector RCAS [replication-competent avian sarcoma-leukosis virus long terminal repeat (LTR) with a splice acceptor] carrying kGPCR, developed angiogenic lesions remarkably resembling human KS lesions, providing an efficient system to recapitulate KSHV-associated angiogenesis (7). Among 20 TIE2-tva mice infected with ALV-derived retroviruses expressing kGPCR, 50% (10 mice) developed KS-like lesions manifested primarily on the skin of the tail, and occasionally on that of the paws and facial area (Fig. 1B and Fig. S1 A and B).
Fig. 1.
IKKε is critically involved in kGPCR-induced tumorigenesis. (A) Ikbke+/− and Ikbke−/− MEFs infected with control lentivirus (Vector) or lentivirus expressing kGPCR were mixed with SVEC cells and injected into nude mice s.c. Tumor weight was determined when mice were killed. (B–F) TIE2-tva mice were injected with RCAS-kGPCR and tumors on the tail were photographed (B). (C) H&E staining and immunohistochemical staining of the fixed tumor sections. Numbers indicate the percentage of kGPCR+ and IKKε+ cells. (D) Three staining patterns (indicated by red arrows) of IKKε in tumor tissues in C. Human KS tumors were stained for latent nuclear antigen LANA (E) and IKKε (F).
H&E staining showed that the tumor lesions were composed of densely packed, spindle-shaped cells and were extensively infiltrated with slits of erythrocytes, replicating some key characteristics of human Kaposi sarcoma (Fig. 1C). Consistent with previous reports, fewer than 5% of tumor cells expressed kGPCR (Fig. 1C), supporting the notion that kGPCR promotes endothelium angiogenesis via a paracrine mechanism. When tumor lesions were stained with anti-IKKε antibody, most tumor cells were highly positive for IKKε, which was rarely observed in those of control mouse tails (Fig. 1C and Fig. S1C). At a closer scrutiny, three staining patterns of IKKε intracellular distribution were clearly observed. Within most spindle cells, the main constituent of KS-like lesions, IKKε, was enriched within the vicinity of the plasma membrane and a small portion was located in the perinuclear region (Fig. 1D). In cells reminiscent of macrophage/stromal cell shapes, IKKε was largely cytoplasmic and displayed as punctae that were loosely connected to and surrounding the nucleus. Interestingly, a number of cells also contained punctate staining of IKKε inside the nucleus. Finally, IKKε was restricted to the perinuclear region in a subset of cells whose shape cannot be differentiated, largely owing to the lack of IKKε staining in cytoplasm and/or plasma membrane (Fig. 1D). The latter two were found predominantly in the space between slits of densely packed spindle cells and likely represent infiltrated immune cells (Fig. S1C).
To assess the involvement of IKKε in KSHV-associated malignancies, we further examined IKKε expression in human KS tumors. In KS tumor tissue that was marked by the latent nuclear antigen (LANA), a signature molecule of KSHV latently infected cells (Fig. 1E), IKKε was high in a significant subset of tumor cells, displaying both cytoplasmic and nuclear staining (Fig. 1F and Fig. S1D). Notably, all IKKε-positive cells showed nuclear staining, and about half of them had cytoplasmic IKKε staining. In cells in which IKKε was predominantly nuclear, the nuclei were dark blue owing to the overlay of blue (hematoxylin) and brown colors (IKKε). These cells may represent the infiltrated immune cells similar to those observed in mouse KS-like lesions. These results show that IKKε is highly expressed in tumor cells of human KS and mouse KS-like lesions.
kGPCR Interacts with and Up-Regulates IKKε.
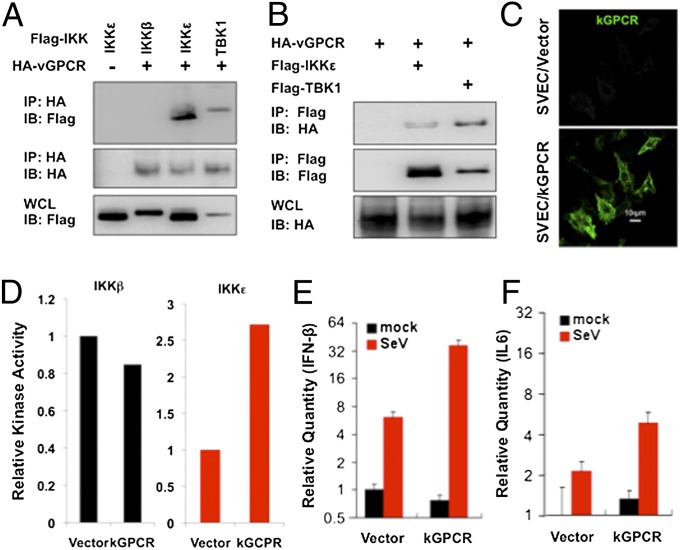
The robust IKKε expression in human KS tumors and mouse KS-like lesions prompted us to examine IKKε regulation by kGPCR. We first examined whether kGPCR physically associates with IKKε by coimmunoprecipitation. We found that kGPCR interactions with IKKε and TBK1, but not IKKβ, were readily detected in transfected 293T cells, and immunoprecipitated IKKε and TBK1 also interact with kGPCR (Fig. 2 A and B). IKKε was originally discovered as an innate immune kinase induced by inflammatory cytokines (26). To examine the impact of kGPCR on IKKε expression, we established SVEC cells (a simian virus 40-transformed mouse microvascular endothelial cell line) stably expressing kGPCR (Fig. 2C) and examined IKKε expression by quantitative RT-PCR (qRT-PCR). kGPCR increased IKKε mRNA levels by twofold in stable SVEC cells, whereas that of the closely related TBK1 was not affected (Fig. S2A).
Fig. 2.
kGPCR interacts with and up-regulates IKKε. (A and B) Co-immunoprecipitation of 293T cells transfected with indicated plasmids. (C) Mouse SVEC endothelial cells stably expressing kGPCR were fixed and analyzed by immunofluorescence microscopy. (D) IKKβ and IKKε were precipitated from SVEC/Vec or SVEC/kGPCR stable cells and analyzed by in vitro kinase assay using GST–IκBαNT. Autoradiography results were quantified with ImageJ. One of three representative experiments was shown. (E and F) Total RNA was extracted and the relative mRNA levels of IFN-β (E) and IL-6 (F), without and with Sendai virus (SeV) infection, were analyzed by qRT-PCR.
Kinases are often latent in resting cells and activated upon stimulation (e.g., treatment with inflammatory cytokines). Because endogenous IKKε in SVEC is too low to detect by immunoblot, we established a SVEC stable cell line that expresses a Flag-tagged IKKε at a low level by lentivirus transduction. In doing so, the expression of ectopic IKKε is not influenced by kGPCR. In vitro kinase assay showed that kGPCR activated IKKε kinase activity by about threefold, whereas IKKβ was not significantly affected (Fig. S2 B and D). Quantitative real-time PCR (qRT-PCR) analysis assessing proinflammatory cytokines downstream of IKKε showed that, in response to Sendai virus infection, kGPCR further enhanced the accumulation of IFN-β and IL-6 mRNAs by four- and threefold, respectively (Fig. 2 E and F). Notably, kGPCR expression did not significantly alter the basal levels of IFN-β mRNA in SVEC cells (Fig. 2E). Taken together, these results demonstrate that kGPCR interacts with and activates IKKε.
IKKε Kinase Activity Is Crucial to Enable kGPCR-Induced NF-κB Activation and Tumorigenesis.
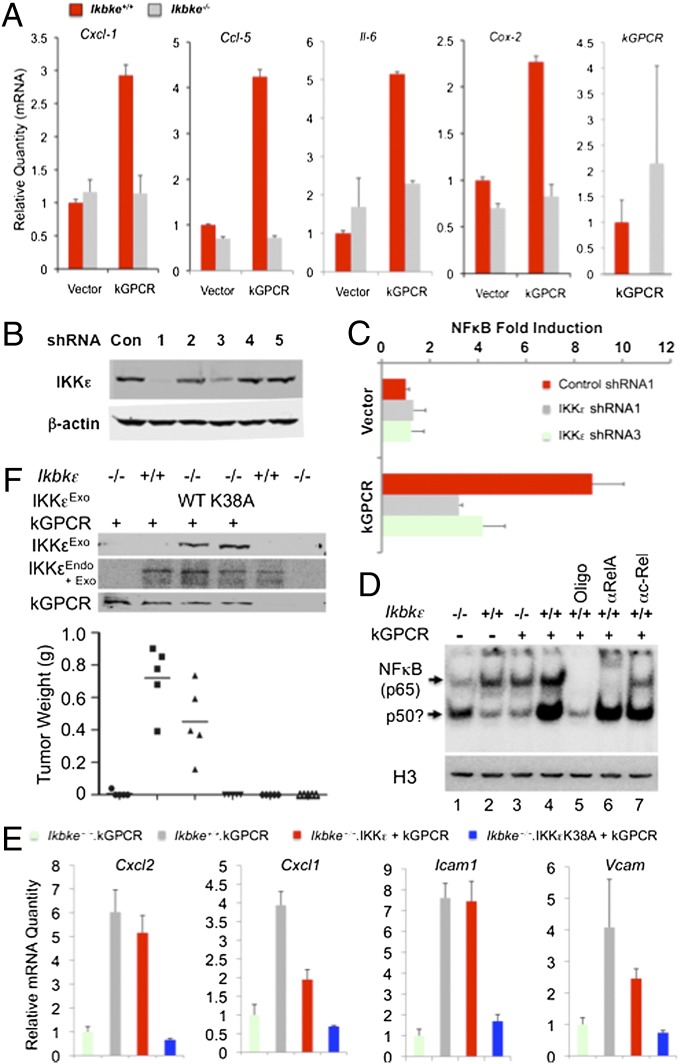
Although NF-κB activation is necessary for kGPCR tumorigenesis (16), how kGPCR activates NF-κB remains unknown. To query the roles of IKKε in kGPCR-induced NF-κB activation, we have examined the expression of selected genes that are NF-κB–dependent and kGPCR-induced. qRT-PCR analysis indicated that loss of IKKε completely abolished kGPCR-induced expression of chemokine C-X-C motif ligand 1 (Cxcl-1), chemokine C-X-C motif ligand 2 (Cxcl-2), chemokine C-C motif ligand 5 (Ccl-5), Il-6, cytochrome c oxidase subunit II (Cox-2), and intercellular adhesion molecule 1 (Icam-1) (Fig. 3A and Fig. S3A). To test whether IKKε is necessary for kGPCR-dependent NF-κB activation, we knocked down IKKε expression with a set of shRNAs (Fig. 3B, sequences as shown in Table S1) and assessed NF-κB activation by reporter assay. IKKε knockdown reduced kGPCR-dependent NF-κB activation by ∼60% (Fig. 3C). Furthermore, exogenous WT IKKε, but not the kinase-dead IKKεK38A, synergized with kGPCR to promote NF-κB activation by reporter assay (Fig. S3B). The electrophoresis mobility shift assay showed that IKKε deficiency abrogated the nuclear DNA-binding ability of NF-κB induced by kGPCR (Fig. 3D). The nuclear DNA-binding ability of NF-κB factors was lower in Ikbke−/− (IKKε knockout) than that in Ikbke+/+ MEFs, suggesting that IKKε is important for basal NF-κB activity as well. Supershift experiment demonstrated that antibody against RelA (p65), but not that against c-Rel, retarded the migration of the NF-κB–containing complex, indicating that the nuclear NF-κB dimer contains RelA, but not c-Rel (Fig. 3D). Finally, kGPCR potently induced RelA nuclear translocation determined by immunofluorescence microscopy and exogenous IKKε cooperated with kGPCR in doing so (Fig. S3C).
Fig. 3.
Loss of IKKε impairs kGPCR-induced NF-κB activation. (A) The mRNA levels of indicated factors were analyzed by qRT-PCR. (B and C) IKKε knockdown by shRNA was analyzed by immunoblot (B). NF-κB fold induction was determined by luciferase reporter assay in 293T cells (C). (D) Nuclear extracts from corresponding cell lines were prepared for electrophoresis mobility shift assay with an NF-κB–specific probe. Cold probe (Oligo) and antibodies against RelA or c-Rel were added. An aliquot of nuclear extract was analyzed by immunoblot with anti-Histone H3 antibody. P50? denotes a possible p50 dimer. (E and F) Ikbke−/− MEFs were “reconstituted” with lentivirus expressing WT IKKε or IKKεK38A, and infected with lentivirus containing kGPCR. Total RNA was extracted for qRT-PCR analysis (E). MEFs mixed with SVEC were injected s.c. into nude mice. Tumor weight was determined when mice were killed (F, Bottom).
To test whether IKKε kinase activity is important for kGPCR-induced NF-κB activation and tumorigenesis, we “reconstituted” IKKε expression, with either WT IKKε or the kinase-dead K38A mutant, by lentivirus infection in Ikbke−/− MEFs. qRT-PCR analysis of the NF-κB–dependent genes (primer sequences as shown in Table S2) indicated that WT IKKε, but not IKKεK38A, restored NF-κB–induced gene expression in Ikbke−/− MEFs (Fig. 3E). Surprisingly, the “reconstituted” expression of IKKε or IKKεK38A reduced the mRNA levels of these NF-κB–dependent genes (Fig. S3D). Tumor formation in nude mice showed that WT IKKε, but not IKKεK38A, restored kGPCR-induced tumorigenesis (Fig. 3F). We noted that the restoration of tumorigenesis by exogenous IKKε was not complete, although exogenous IKKε expression was higher than endogenous IKKε expression. IKKε and IKKεK38A were expressed at similar levels (Fig. 3F). Collectively, these results support the conclusion that IKKε and its kinase activity are essential for kGPCR-induced NF-κB activation and tumorigenesis.
kGPCR Induces RelA Phosphorylation at Ser468 Through IKKε.
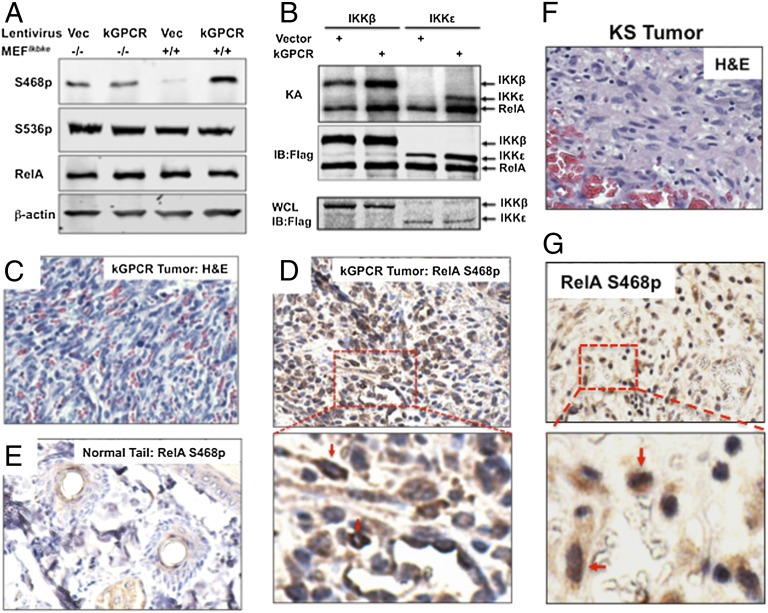
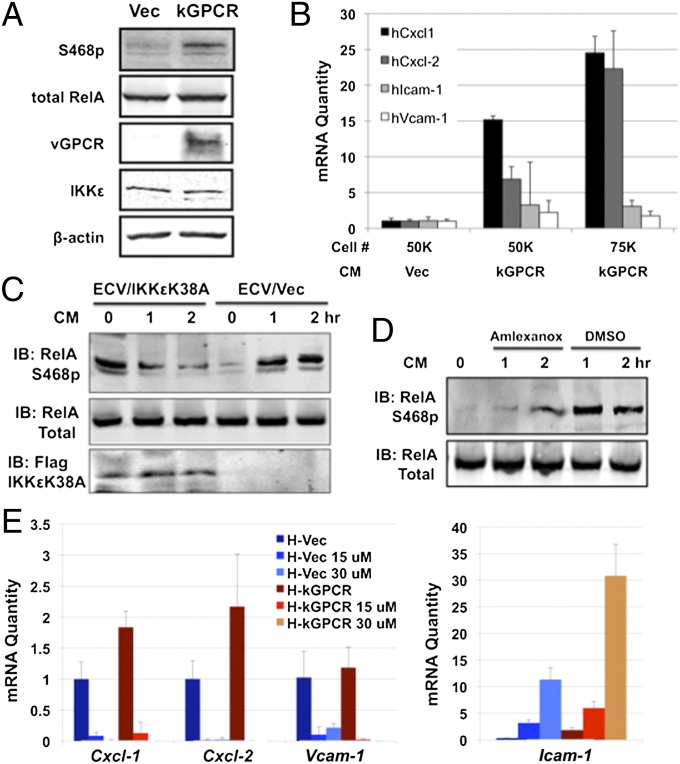
RelA is a key subunit of the transcriptionally active NF-κB dimer, and our EMSA supershift experiment showed that RelA is the major subunit of kGPCR-activated NF-κB (Fig. 3D). We thus investigated the roles of RelA in kGPCR-dependent NF-κB activation and tumorigenesis. To date, multiple forms of activated RelA have been induced by various stimuli, including those modified by phosphorylation at serine 468 (RelA S468p) and serine 536 (RelA S536p) (32, 33). Immunoblot analysis using phospho-specific antibodies indicated that kGPCR elevated the level of RelA S468p in Ikbke+/+ MEFs, whereas kGPCR had no detectable effect on RelA S468p in Ikbke−/− MEFs (Fig. 4A). Interestingly, loss of IKKε slightly elevated the level of RelA S468p, implying that IKKε reduces the steady level of RelA S468p. kGPCR expression and loss of IKKε had no detectable effect on that of RelA S536p, total RelA, or phosphorylated IκBα, the inhibitor of NF-κB (Fig. 4A and Fig. S4A). In vitro kinase assay, using purified RelA and IKK, showed that, in the presence of kGPCR, IKKε and IKKβ were more potent to phosphorylate RelA and that IKKε was more robust than IKKβ (Fig. 4B). Despite the same level of IKKε expression in SVEC/Vec and SVEC/kGPCR cells, we consistently precipitated more IKKε from cell lysate of SVEC/kGPCR than from that of SVEC/Vec, whereas IKKβ was equally precipitated. This effect of kGPCR on IKKε requires further investigation. Nevertheless, these results indicate that IKKε promoted RelA phosphorylation at serine 468.
Fig. 4.
IKKε phosphorylates RelA at Serine 468. (A) Ikbke+/+ and Ikbke−/− MEFs expressing kGPCR were analyzed for RelA phosphorylation by immunoblot with indicated antibodies. (B) IKKβ and IKKε were precipitated from stable SVEC/Vec and SVEC/kGPCR cells. Purified RelA was added to precipitated IKKs for in vitro kinase assay. Proteins were analyzed by autoradiography or immunoblot. Whole-cell lysates (WCL) were analyzed by immunoblot for IKKs. KA, kinase assay. (C–E) Tumors derived from TIE2-tva mice (C and D) and normal tails (E) were analyzed by H&E staining and IHC staining for RelA S468p. Enlarged image of the boxed region in D is shown, and arrows indicate RelA S468p-positive cells. (F and G) KS tumors were analyzed by H&E and IHC staining (F) for RelA S468p (G). The enlarged image of the boxed region in G is shown (Lower).
To inquire the possible roles of RelA S468p in kGPCR tumorigenesis, we examined RelA Ser468p in tumor lesions derived from TIE2-tva mice, tissue composed primarily of spindle cells (Fig. 4C). Immunohistochemistry staining demonstrated that RelA S468p was grossly elevated in most cells of KS-like tumors (Fig. 4D and Fig. S4B), but not in tissues of control mouse tails (Fig. 4E). RelA S468p resided predominantly in the nucleus. A minor subset of cells had nuclei light blue in color, serving as an internal negative control for RelA S468p staining. Within cells that are highly positive for RelA S468p, the RelA S468p concentrated in the proximity of the perinuclear region (Fig. 4D, Lower). We have further examined the status of RelA S468p in human KS tumor. In the majority of human KS tumor cells that include both spindle-shaped cells and cells of round nuclei (Fig. 4F), RelA S468p was robustly detected (Fig. 4G and Fig. S4C). RelA S468p was clearly visible in both the cytoplasm and the nucleus, with more punctate staining within the nucleus (Fig. 4G and Fig. S4C). These findings indicate that kGPCR promotes RelA phosphorylation at serine 468 via IKKε.
Paracrine Activation of IKKε and RelA by kGPCR.
KS tumors are largely composed of KSHV latently infected cells. The abundant expression of IKKε and RelA S468p in KS tumors raises the possibility that latent gene products, including viral FLICE-inhibitory protein (vFLIP), viral cyclin (vCyclin), LANA, and Kaposin, activate NF-κB via IKKε. Particularly, vFLIP activates NF-κB to enable B-cell survival (34). Thus, we expressed all four latent genes, along with kGPCR, in 293T/IKKε cells (Fig. S5A) and examined their ability to activate IKKε by in vitro kinase assay. Surprisingly, vFLIP and LANA had a marginal effect on IKKε, whereas Kaposin B and, to a lesser extent, vCyclin, activated IKKε (Fig. S5 B and C). However, when NF-κB was examined by reporter assay, except for vFLIP, none of the three latent proteins activated NF-κB. More importantly, unlike kGPCR, none of the latent proteins cooperated with IKKε to activate NF-κB (Fig. S5D). These results indicate that, although KSHV latent proteins may contribute to IKKε and NF-κB activation, kGPCR is the most potent activator.
Next, we examined the paracrine effect of kGPCR on IKKε and NF-κB activation. We first confirmed that kGPCR increased RelA Ser468p in human umbilical vein endothelial cells (HUVEC) (Fig. 5A). Assessing the expression of NF-κB–dependent genes by qRT-PCR, we found that incubation with conditioned medium of HUVEC/kGPCR for 24 h, compared with that of HUVEC/Vec cells, up-regulated the mRNA levels of Cxcl-1 and Cxcl-2 by 5- to 25-fold, and Icam-1 and vascular cell adhesion molecule (Vcam) by approximately threefold (Fig. 5B). This effect was more pronounced when treatment was extended to 48 h (Fig. S5E). Owing to the toxicity of lentivirus infection in HUVEC cells, we have used human ECV-304 endothelial cells to express IKKεK38A. We examined the levels of RelA S468p in ECV cells and found that, when treated with HUVEC/kGPCR-conditioned medium, RelA Ser468p levels were increased (Fig. S5F). Compared with control lentivirus, IKKεK38A expression greatly reduced RelA S468p levels, incubated with HUVEC/kGPCR-conditioned medium (Fig. 5C). IKKεK38A expression elevated the basal RelA S468p levels, similar to what was observed in Ikbke−/− MEFs (Fig. 4A). Moreover, treatment with amlexanox, an inhibitor specific for IKKε and TBK1 (35), also potently reduced RelA Ser468p in ECV cells under similar conditions (Fig. 5D). We further assessed the expression of NF-κB–dependent genes by qRT-PCR in HUVEC cells treated with HUVEC/kGPCR-conditioned medium and amlexanox. Despite the weak induction of NF-κB–dependent gene expression, likely due to a short incubation time, amlexanox greatly reduced the mRNA levels of Cxcl-1, Cxcl-2, and Vcam in cells treated with conditioned medium of HUVEC/Vec and those of HUVEC/kGPCR (Fig. 5E). Similar results were observed in SVEC cells (Fig. S5G). Surprisingly, the mRNA levels of Icam-1 were increased by amlexanox treatment in a dose-dependent manner. This observation suggests that, although these genes are regulated by NF-κB, the regulatory mechanisms may be distinct. Collectively, our data indicate that IKKε-mediated NF-κB activation is necessary for kGPCR-mediated paracrine stimulation.
Fig. 5.
Paracrine activation of IKKε and NF-κB by kGPCR. (A) Whole-cell lysates of HUVECs were analyzed for RelA S468p with indicated antibodies by immunoblot. (B) HUVECs were stimulated with conditioned medium (CM) of HUVEC/Vec or HUVEC/kGPCR cells for 24 h. Total RNA was extracted and analyzed by qRT-PCR. (C and D) Human ECV endothelial cells, infected with control (Vec) or IKKεK38A-expressing lentivirus (C), or treated with amlexanox (15 μM) (D), were stimulated with HUVEC/kGPCR-conditioned medium and analyzed by immunoblot for RelA. (E) HUVECs were treated with conditioned medium of HUVEC/Vec (H-Vec) or HUVEC/kGPCR (H-kGPCR), without or with amlexanox, at indicated concentrations for 18 h. Total RNA was extracted and analyzed by qRT-PCR.
Kinase-Dead IKKεK38A Inhibits NF-κB Activation and Tumorigenesis Induced by kGPCR.
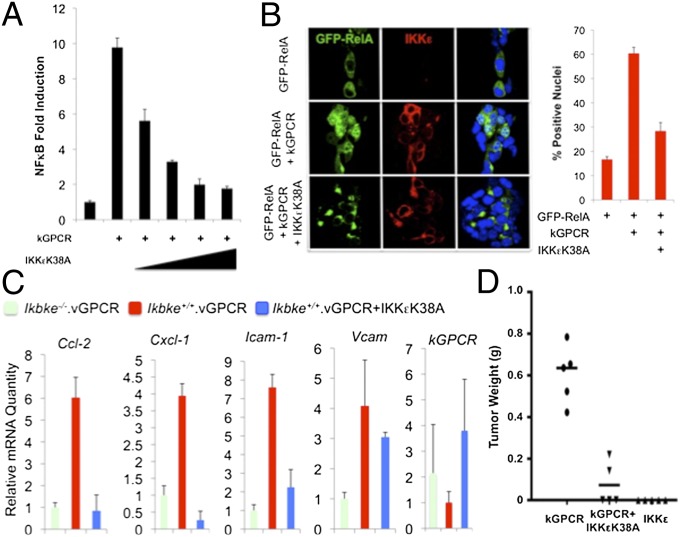
Our results indicate that the kinase activity of IKKε is necessary for kGPCR-induced NF-κB activation. We reasoned that IKKεK38A could have a dominant negative effect on kGPCR-mediated NF-κB activation. To test this hypothesis, we first examined the effect of IKKεK38A on kGPCR-dependent NF-κB activation by a reporter assay. In 293T cells, IKKεK38A expression diminished kGPCR-induced NF-κB activation in a dose-dependent manner (Fig. 6A and Fig. S6A). Taking advantage of the fluorescent GFP–RelA fusion protein, we analyzed RelA nuclear translocation by immunofluorescence microscopy. Whereas kGPCR potently promoted RelA nuclear translocation, IKKεK38A sequested RelA in the cytoplasm (Fig. 6B). Semiquantitative analysis indicated that kGPCR expression increased RelA nuclear staining from ∼15–60%, and IKKεK38A reduced RelA nuclear staining to 30% of all cells positive for GFP–RelA. Finally, we infected kGPCR-expressing MEFs with lentivirus containing IKKεK38A (Fig. S6B) and examined kGPCR-induced gene expression. qRT-PCR analysis showed that IKKεK38A effectively inhibited three of four NF-κB–dependent genes to levels below or similar to that of Ikbke−/− MEF expressing kGPCR, including Cxcl-1, Cxcl-2, and Icam-1 (Fig. 6C). Interestingly, the expression of Vcam resisted the inhibition by IKKεK38A, implying the differential requirement of IKKε in kGPCR-induced gene expression.
Fig. 6.
IKKεK38A inhibits kGPCR-induced NF-κB activation and tumorigenesis. (A) kGPCR-induced NF-κB activation was determined by luciferase reporter assay in 293T cells. (B) The 293T cells transfected with plasmids containing GFP-RelA, kGPCR, and IKKεK38A were fixed, stained, and analyzed by immunofluorescence microscopy. Representative images were shown (Left). More than 300 cells were counted for statistical analysis (Right). (C) Total RNA extracted from MEFs of indicated genotypes was analyzed by qRT-PCR for the expression (mRNA) of selected NF-κB–dependent genes. (D) MEFs as in C, mixed with SVEC endothelial cells were injected s.c. into nude mice. Tumor weight was recorded when mice were killed.
To test whether IKKεK38A thwarts kGPCR tumorigenesis, we injected nude mice with MEFs expressing kGPCR alone or kGPCR and IKKεK38A and monitored tumor formation. Additionally, we determined whether IKKε overexpression is sufficient to promote tumor formation (Fig. S6B). We found that exogenous IKKεK38 expression significantly reduced the average tumor weight of ∼600 mg to∼50 mg, induced by kGPCR (Fig. 6D). Notably, exogenous IKKε expression did not induce tumor formation in nude mice. Together with findings from IKKε-deficient MEFs, these results support the conclusion that IKKε is critically required for kGPCR-dependent NF-κB activation and tumorigenesis.
Discussion
In this study we identified the IKKε kinase that relays signal transduction from the transforming kGPCR to NF-κB activation. GPCRs are the most ubiquitous signaling molecules that transduce messages across membrane in response to diverse physiological stimuli. Although the signaling cascades leading to NFAT activation by GPCRs have been the subject of much research, the components and regulation that govern GPCR-induced NF-κB activation remain unknown. Herpesvirus GPCRs are implicated in viral pathogenesis. kGPCR expression sufficiently activates NF-κB, and NF-κB activation is essential for kGPCR-induced tumorigenesis (16). We demonstrated that kGPCR triggers a series of signature events of NF-κB activation, including IKKε kinase activation, RelA phosphorylation, and downstream proinflammatory cytokine expression. Moreover, key events of NF-κB activation, for example, the drastic up-regulation of IKKε and RelA S468p, were observed in human KS tumor tissue and mouse KS-like lesions. Collectively, these observations support the notion that kGPCR nourishes chronic NF-κB activation, which imposes an inflammatory microenvironment that, in turn, fosters tumor formation in the endothelium, an intimate interface between the highly dynamic immune cells and the differentiated/quiescent fibroblasts.
NF-κB activation is critical for a plethora of fundamental biological processes ranging from development and immune responses to carcinogenesis. Persistent or chronic NF-κB activation is one recurrent theme found in a broad spectrum of human cancers (36). The “acute” NF-κB activation by cytokine (such as TNFα) stimulation and pathogen infection is chiefly achieved through a K63-linked ubiquitin chain-mediated activation of the IKK complex, consisting of IKKγ and IKKα and/or IKKβ (17). The inducible IKKε was originally implicated in oncogenesis of breast cancer by an siRNA screen, via inducing NF-κB activation (28). Accumulating studies indicate that elevated IKKε expression is consistently observed in multiple forms of malignant tumors (29), such as prostate and ovarian cancers. However, the mechanism by which NF-κB is activated by IKKε remains largely unknown. We found that IKKε, when activated by kGPCR, phosphorylated RelA at Ser468 and promoted its nuclear translocation, agreeing with the findings that RelA Ser468p up-regulates the expression of a subset of inflammatory genes (32). Interestingly, previous reports, including our findings from virus-infected cells (37), showed that the Ser468 phosphorylation primes RelA for degradation (38, 39). By contrast, kGPCR expression activates IKKε that phosphorylates and activates RelA. These observations suggest that additional signaling events, downstream of kGPCR, may switch RelA toward NF-κB activation.
The IKK-related IKKε was originally discovered as being inducible by inflammatory cytokines (26). The involvement of IKKε in the carcinogenesis of KS fits with the inflammatory and angiogenic nature of KS tumors. The induction of IKKε expression is more robust in human and mouse tumors than in kGPCR-expressing endothelial cells, suggesting that in vivo paracrine mechanisms of immune activation of IKKε are crucial and dominant in kGPCR tumorigenesis. Given the inducible nature of IKKε, it is a general belief that induction of IKKε kinase activity largely stems from an up-regulation of protein expression. The molecular mechanisms governing IKKε activation at the posttranslational level, in analogy to IKKβ activation, remain unknown. IKKε is highly expressed in immune cells, including B and T lymphoid cells (26). Our observation that IKKε is very abundant in tumor cells of mouse KS-like lesions suggests that nonimmune cells, such as endothelial cells, can be primed for IKKε expression. The intracellular distribution of IKKε is remarkably diverse in tumor cells, ranging from being plasma membrane-associated, cytosolic punctate, and nuclear. Similar patterns have been reported previously (28). High levels of IKKε were also observed in human KS tumors, although tumor cells with nuclear IKKε were predominant. Whereas vGPCR is predominantly expressed during lytic infection, KS tumor tissues are mainly composed of endothelial cells that are latently infected with KSHV. Conceivably, latent gene products may alter IKKε to influence KSHV infection and diseases thereof in vivo. Indeed, our results indicate that Kaposin B and, to a lesser extent, vCyclin, activate IKKε, but not NF-κB. Conversely, vFLIP activates NF-κB, but not IKKε. These observations suggest that latent gene products may function together to instigate IKKε and NF-κB activation. In sum, our study identifies IKKε as a key molecule linking kGPCR to NF-κB activation and tumorigenesis. The potent inhibition of the kinase-dead IKKεK38A on kGPCR tumorigenesis suggests that IKKε represents a potential drug target to treat human KS.
Materials and Methods
Information on reagents and experimental procedures is given in SI Materials and Methods. Included topics are animal experiments, immunoblotting, qRT-PCR, immunofluorescence microscopy, EMSA, and H&E. All statistical analyses were done with unpaired, two-tailed Student's t test.
Supplementary Material
Acknowledgments
We thank Ms. Yuqi Wang and Lisa Arneson for assistance with the maintenance of mouse colonies and John Shelton and Lillian Young for histology. This work was supported by National Cancer Institute Grant R01 CA134241, National Institute of Health DE021445, American Cancer Society Grant RSG-11-162-01-MPC (to P.F.), and National Institute of Health U19:A1083025 (to J.U.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219829110/-/DCSupplemental.
References
- 1.Soulier J, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332(18):1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Nador RG, Cesarman E, Knowles DM, Said JW. Herpes-like DNA sequences in a body-cavity-based lymphoma in an HIV-negative patient. N Engl J Med. 1995;333(14):943. doi: 10.1056/NEJM199510053331417. [DOI] [PubMed] [Google Scholar]
- 5.Rezza G, et al. Human herpesvirus 8 seropositivity and risk of Kaposi’s sarcoma and other acquired immunodeficiency syndrome-related diseases. J Natl Cancer Inst. 1999;91(17):1468–1474. doi: 10.1093/jnci/91.17.1468. [DOI] [PubMed] [Google Scholar]
- 6.Moore PS, Chang Y. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356(1408):499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montaner S, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3(1):23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 8.Sun R, et al. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol. 1999;73(3):2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang TY, et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191(3):445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wess J. G-protein-coupled receptors: Molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11(5):346–354. [PubMed] [Google Scholar]
- 11.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 12.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385(6614):347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 13.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 14.Sodhi A, et al. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. 2006;10(2):133–143. doi: 10.1016/j.ccr.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Martin D, et al. PI3Kγ mediates kaposi’s sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell. 2011;19(6):805–813. doi: 10.1016/j.ccr.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin D, Galisteo R, Ji Y, Montaner S, Gutkind JS. An NF-kappaB gene expression signature contributes to Kaposi’s sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene. 2008;27(13):1844–1852. doi: 10.1038/sj.onc.1210817. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006(357):re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 19.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 20.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 22.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11(8):1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 27.Tenoever BR, et al. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315(5816):1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 28.Boehm JS, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Clément JF, Meloche S, Servant MJ. The IKK-related kinases: From innate immunity to oncogenesis. Cell Res. 2008;18(9):889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 30.Hayward GS. Initiation of angiogenic Kaposi’s sarcoma lesions. Cancer Cell. 2003;3(1):1–3. doi: 10.1016/s1535-6108(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 31.Cesarman E, Mesri EA, Gershengorn MC. Viral G protein-coupled receptor and Kaposi’s sarcoma: A model of paracrine neoplasia? J Exp Med. 2000;191(3):417–422. doi: 10.1084/jem.191.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno R, Sobotzik JM, Schultz C, Schmitz ML. Specification of the NF-kappaB transcriptional response by p65 phosphorylation and TNF-induced nuclear translocation of IKK epsilon. Nucleic Acids Res. 2010;38(18):6029–6044. doi: 10.1093/nar/gkq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattioli I, et al. Inducible phosphorylation of NF-kappa B p65 at serine 468 by T cell costimulation is mediated by IKK epsilon. J Biol Chem. 2006;281(10):6175–6183. doi: 10.1074/jbc.M508045200. [DOI] [PubMed] [Google Scholar]
- 34.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199(7):993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Reilly SM, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 37.Dong X, et al. Murine gammaherpesvirus 68 evades host cytokine production via replication transactivator-induced RelA degradation. J Virol. 2012;86(4):1930–1941. doi: 10.1128/JVI.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao X, et al. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-kappaB/RelA. Genes Dev. 2009;23(7):849–861. doi: 10.1101/gad.1748409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong X, Feng P. Murine gamma herpesvirus 68 hijacks MAVS and IKKβ to abrogate NF-κB activation and antiviral cytokine production. PLoS Pathog. 2011;7(11):e1002336. doi: 10.1371/journal.ppat.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.