Abstract
ArfGAP With Coiled-Coil, Ankyrin Repeat And PH Domains 4 (ACAP4) is an ADP-ribosylation factor 6 (ARF6) GTPase-activating protein essential for EGF-elicited cell migration. However, how ACAP4 regulates membrane dynamics and curvature in response to EGF stimulation is unknown. Here, we show that phosphorylation of the N-terminal region of ACAP4, named the Bin, Amphiphysin, and RSV161/167 (BAR) domain, at Tyr34 is necessary for EGF-elicited membrane remodeling. Domain structure analysis demonstrates that the BAR domain regulates membrane curvature. EGF stimulation of cells causes phosphorylation of ACAP4 at Tyr34, which subsequently promotes ACAP4 homodimer curvature. The phospho-mimicking mutant of ACAP4 demonstrates lipid-binding activity and tubulation in vitro, and ARF6 enrichment at the membrane is associated with ruffles of EGF-stimulated cells. Expression of the phospho-mimicking ACAP4 mutant promotes ARF6-dependent cell migration. Thus, the results present a previously undefined mechanism by which EGF-elicited phosphorylation of the BAR domain controls ACAP4 molecular plasticity and plasma membrane dynamics during cell migration.
Many cellular processes are orchestrated by dynamic changes in the plasma membrane to form membrane projections and endocytic vesicles. Cell migration is necessary for tissue development and wound repair. During cell migration, the coordination of membrane traffic, actin skeleton remodeling, and formation of new adhesion complexes is required for protrusive activities at the leading edges of the migrating cells (1, 2). Some small GTPases, such as ADP-ribosylation factors (ARFs) and Rhos, are involved in coupling actin dynamics to trafficking of vesicular membranes (3, 4) and in control of membrane curvature. In mammals, the six ARF proteins belong to three classes, based on sequence homology: class I (ARF1–3), class II (ARF4 and 5), and class III (ARF6). The sole member of class III, ARF6, functions in plasma membrane dynamics (5) and in promoting endocytic recycling (6). ARF6 resides on endosomal and plasma membranes to regulate membrane trafficking between these compartments (7–10). Activation of ARF6 promotes cortical actin assembly (9) and plasma membrane remodeling (10). Aberrant expression of ARF6 has been implicated in tumor invasion and metastasis (11).
Key determinants of ARF6 function are the lifetime and the subcellular locations of the GTP-bound active state, which is orchestrated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) (12). Our proteomic analyses identified ArfGAP With Coiled-Coil, Ankyrin Repeat And PH Domains 4 (ACAP4) as a GAP protein that selectively binds to active ARF6 and catalyzes GTP hydrolysis (13). The ACAP4 gene encodes 903 amino acids and contains a catalytic core of a pleckstrin homology (PH) domain, a GAP motif, and two ankyrin (ANK) repeats. The GAP activity is regulated by phosphatidylinositol 4,5-biphosphate [PI(4,5)P2] via binding to the PH domain. The Arg469 in the GAP domain is necessary for its activity in GTP hydrolysis (13). The crystal structure of the catalytic core of ACAP4 in a complex with ARF6 reveals the structural determinants underlying ACAP4 selectivity and specificity as an ARF6 GTPase-activating protein (14). ACAP4 also associates with focal adhesions and with cytoplasmic membranes at the circular dorsal ruffles, where the actin skeleton is remodeled (15). Depletion of ACAP4 by RNA interference suppresses cell migration in wound healing (13, 15).
Dynamic changes in the plasma membrane and vesicular trafficking are achieved by recruitment of membrane shaping proteins such as those in the Bin, Amphiphysin, and RSV161/167 (BAR) superfamily. The BAR domain is characterized as a crescent-shaped dimer composed mainly of three long, kinked α-helices. Structural studies indicate that all BAR domains have a concave membrane-binding interface that interacts with negatively charged membranes (16–20). The BAR domain, also found in other contexts in a wide variety of proteins (17, 19), is believed to be responsible for generating, stabilizing, or sensing curvature (18). The concave shape of the BAR domain appears to be responsible for the tubulation of membranes in intracellular trafficking processes (18). The BAR domain also contributes to the membrane scission of budding vesicles (21).
Several proteins containing the BAR domain regulate actin-based cytoskeleton dynamics and cell migration (22, 23). The I-BAR protein, MIM, also promotes Arp2/3-mediated assembly of actin filaments at adherent junctions (24). Sensing of membrane curvature is coupled with enzymatic activities, directly, as with GAPs and GEFs, and, indirectly, as in the case of synaptojanin bound to endophilin (18). However, the mechanism underlying the membrane association of these proteins has remained undefined.
The present report demonstrates that ACAP4, which contains a BAR domain, is associated with membrane binding and bending activity in vitro and in vivo. The BAR domain is necessary for the recruitment of ACAP4 to membrane structures and for its GAP activity. Importantly, Tyr34 in the BAR domain is phosphorylated in response to EGF stimulation. Phosphorylation of Tyr34 promotes the migratory activity of MDA-MB-231 cells. Thus, phosphorylation of Tyr34 in the BAR domain modulates the membrane-binding capacity of ACAP4. Thus, this study revealed a previously undefined role for ACAP4 in linking the ARF6-mediated vesicular membrane dynamics to EGF-elicited cell migration.
Results
Identification of a BAR Domain in ACAP4.
Computational analysis of the primary sequence of ACAP4, in comparison with APPL1 (adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1), ASAP (ArfGAP with SH3 domain, ankyrin repeat and PH domain)1, and ASAP2, suggested that the N terminus of ACAP4 (7–271 amino acids) contains a typical BAR domain (Fig. 1A), a structure element responsible for membrane binding and deformation. Modeling of the X-ray structure of the homologous APPL1 indicated that the ACAP4 BAR domain forms a bundle of three major α helices that dimerize into a crescent-shaped structure (Fig. 1B). Indeed, a gel filtration assay showed that the BAR domains of ACAP4 form homodimers in vitro (Fig. S1 A and B), and a reconstitution assay coupled with electron microscopic analyses demonstrated that the recombinant BAR domain of ACAP4 elicited membrane tubulation (Fig. 1D).
Fig. 1.
Membrane sculpture capacity of the ACAP4 BAR domain. (A) Structure-based sequence alignment of the ACAP4 BAR domain with its homologs. The clustalW server (www.ebi.ac.uk/Tools/msa/clustalw2/) was used to align the sequences of APPL1 (accession Q9U.K.G), ASAP1 (accession Q9ULH1), ASAP2 (accession O43150), and ACAP4 (accession Q8TDY4). The Phyre webserver was used for structure prediction (www.sbg.bio.ic.ac.uk/~phyre/index.cgi). Blue triangles indicate the positive-charged residues predicted to be important in membrane binding: K56E+K57E (mutA) and R165E+R167E+R169E (mutB). The red triangle indicates the Tyr34 phosphorylation site. (B) Modeling of the N terminus of ACAP4. A model of residues 7–271 of ACAP4 was derived from the structure of the BAR domain of APPL1 using the Pymol software and the 3D structure prediction website Phyre. (C) BAR domain proteins were copelleted with LUVs made from total brain lipids. The pellet (P) and supernatant (S) components were fractionated by SDS/PAGE followed by Coomassie blue staining. (D) Electron micrographs of LUVs made from total brain lipids and incubated with the indicated proteins (5 μM). The WT BAR (WT), but not mutants, induces buds and tubular structures, whereas mutants exhibit no distinction from WT protein in lipid binding in vitro (Fig. S8). (Scale bar, 100 nm.) (E) Quantitative analyses of liposome binding activity. Data represent means ± SE (P < 0.05; n = 3). (F) ACAP4 BAR domain tubulates membranes. HepG2 cells overexpressing GFP-BAR (WT or the mutA or mutB). (Scale bar, 10 μm.)
As shown in Fig. 1A, several positively charged residues were considered to be necessary for membrane binding. To perturb the electrostatic interaction of ACAP4 with the membrane, some of these residues were mutated to glutamates: Lys56 and Lys57 on the putative helix 1 (mutA) and Arg165, Arg167, and Arg169 on the disordered loop between helices 4 and 5 (mutB) (Fig. 1 A and B). A binding assay indicated that none of the mutants altered the dimerizing capacity of the BAR domain (Fig. S2A). To determine whether these mutants retained their interactions with the membrane, liposome sedimentation assays were conducted. The associations of the BAR domain with brain liposomes [as large unilamellar vesicles (LUVs)] were significantly attenuated (Fig. 1 C and E; P < 0.01; n = 3).
To determine whether the ACAP4 BAR domain exhibits membrane-bending activity, electron microscopic analyses of liposomes incubated with WT and mutant BAR domains were accomplished. As shown in Fig. 1D, the WT BAR domain of ACAP4 had the capacity to tubulate the liposomes. The membrane-bending activity of the BAR domain, however, was abolished by the mutations of positively charged residues (Fig. 1D, Bottom), suggesting that the ACAP4 BAR domain induces changes in membrane curvature. To examine whether the BAR domain is involved in induction of tubulation, HepG2 cells were transiently transfected to express BAR-GFP or its mutants (mutA and mutB). As shown in Fig. 1F, the WT BAR domain was readily apparent at intracellular tubular structures (Fig. 1 F, a; WT). However, mutA and mutB were diffused in the cytoplasm (Fig. 1 F, c and d). In fact, no tubular structures were found in HepG2 cells expressing the mutA or mutB BAR domains. Thus, we conclude that the N-ACAP4 apparently contains a functional BAR domain.
BAR Domain Specifies ACAP4 Localization to the Functional Membrane.
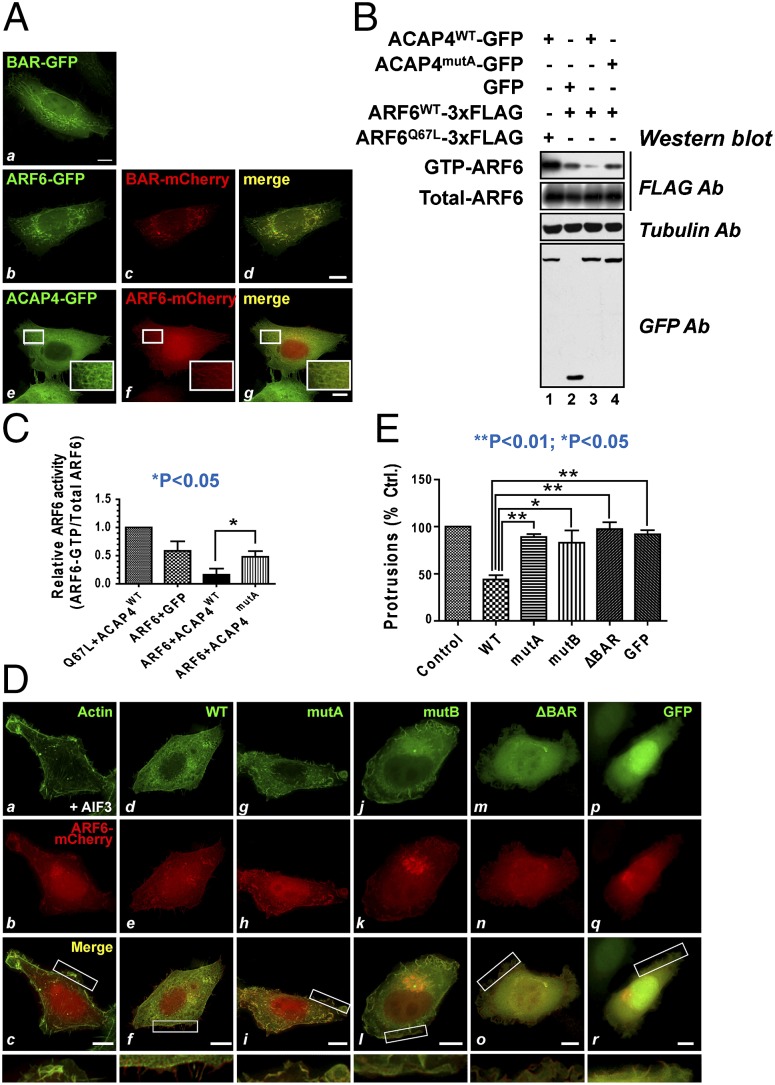
Our previous work showed that ACAP4 is a unique GAP protein of ARF6 that regulates membrane trafficking and the actin cytoskeleton at the cell periphery (13). In HepG2 cells cotransfected with ACAP4-GFP and ARF6-mCherry, these two proteins codistributed at the cell periphery and at perinuclear endosomes (Fig. 2 A, e–g).As the BAR domain has an intrinsic capacity for membrane tubulation, the exogenous ACAP4 BAR domain distributed mostly to the endosomal tubulovesicles rather than to the cell periphery (Fig. 2 A, a). However, the BAR domain distributed to ARF6 resident membrane tubules (Fig. 2 A, b–d), suggesting that it contributes to recruitment of the protein to the ARF6 resident membranes.
Fig. 2.
BAR domain is essential for ACAP4 GAP activity. (A) HepG2 cells were transfected with BAR-GFP (a); ARF6-GFP and the BAR-mCherry domain (b–d); or ACAP4-GFP and the ARF6-mCherry domain (e–g). (Scale bar, 10 μm.) (B) ACAP4-GFP proteins isolated from 293T cell lysates were measured by GST-GGA3 pull-down. Typically, the exogenously expressed ACAP4 proteins are threefold higher those of endogenous ACAP4 (Fig. S9). Aliquots of total cell lysates (20 μg) were used. (C) Quantitative analyses of B. Ratios of ARF6-GTP/total ARF6 were determined by the densities of respective blots and expressed as means ± SE (P < 0.05; n = 3). (D) ACAP4-GFP or its derivatives were cotransfected into HepG2 cells with ARF6-mCherry. At 24 h after transfection, cells were stimulated by 30 mM NaF and 50 μM AlCl3 before fixation and immunostaining. F-actin was visualized by use of FITC-phalloidin in cells expressing only ARF6-mCherry (a–c). ACAP4 and derivatives were marked by GFP (d–r). (Scale bar, 10 μm.) (E) Data are presented as the fractions of cells forming protrusions coexpressing the indicated ACAP4 protein, normalized to the fraction of cells expressing ARF6 alone (mean ± SE; n = 3).
The acute formation of actin-rich membrane protrusions in cells treated with AlF3 has been used to study the role of ARF6 GTPase in membrane-cytoskeleton remodeling (25–27). In our previous research, we found that overexpression of ACAP4 inhibited the formation of protrusions in response to AlF3 (Fig. 2 D, a–f). If ACAP4 functions in membrane recruitment, alteration of the membrane binding capacity of the BAR domain would block ARF6-mediated membrane-cytoskeletal remodeling. Consistent with this prediction, mutants deficient in membrane binding and the BAR domain deletion (indicated as ΔBAR) failed to inhibit AlF3-induced membrane protrusions (Fig. 2 D, g–o). The proportion of cells making protrusions in each case was quantified relative to those of cells expressing ARF6 alone. A quantitative analysis indicated that inhibition of membrane protrusions by ACAP4 depends on the membrane binding capacity of the BAR domain (Fig. 2E).
The regulatory role of ACAP4 on AlF3-induced protrusions depends on its GAP activity toward ARF6 (13). To determine whether the membrane-binding capacity of the BAR domain affects the GAP activity of ACAP4 toward ARF6, an ARF6-GTP activity pull-down assay was performed by using GGA3 as an affinity matrix to isolate active ARF6 (28). To this end, 293T cells were transiently transfected to express ARF6-FLAG plasmids with ACAP4-GFP constructs (WT and mutant ACAP4). Initial experiments showed that exogenously expressed ACAP4 and ARF6 proteins were at comparable levels (Fig. 2B). With the constitutively active mutant of ARF6Q67L as a positive control, the pull-down assay showed that GST-GGA3 absorbed a large amount of ARF6Q67L-FLAG (Fig. 2B, lane 1). In contrast, GST-GGA3 affinity matrixes pulled down little GTP-bound ARF6 protein from cell lysates coexpressing ACAP4-GFP (Fig. 2B, lane 3). GST-GGA3 affinity matrixes absorbed a greater amount of GTP-bound ARF6 protein from cell lysates coexpressing ACAP4mutA-GFP, indicating that the mutant, ACAP4mutA, which is deficient in membrane binding, had a lower level of ARF6 GAP activity. Quantitative analyses showed that ACAP4mutA exhibited a 2.7-fold lower ARF6 GTPase GAP activity (Fig. 2C). These results suggest that membrane association with the BAR domain regulates ACAP4 GAP activity toward ARF6 GTPase.
Phosphorylation of the BAR Domain by EGF Stimulation.
ACAP4 regulates ARF6 activation in EGF-stimulated membrane-cytoskeletal remodeling (13). We assessed the effect of EGF stimulation on distribution of the BAR domain with respect to ARF6 in HepG2 cells. As shown in Fig. 3 A, g–i, EGF stimulation enhanced the distribution of the BAR domain to the membrane ruffles. Consistent with the function of ACAP4 in membrane dynamics, membrane ruffles were also pronounced in cells overexpressing the mutant deficient in membrane binding and the ΔBAR mutant (Fig. 3 A, j–o). Quantitative analyses indicated that, at the localized membrane, ACAP4 participated in the EGF-elicited membrane remodeling by spatial regulation dependent on the BAR domain (Fig. 3B).
Fig. 3.
EGF stimulation elicits phosphorylation of ACAP at Tyr34. (A) HepG2 cells were cotransfected with ARF6-mCherry and ACAP4-GFP constructs. At 24 h after transfection, cells were subjected to serum starvation and EGF stimulation. Treated cells were fixed and then examined under a fluorescence microscope. (Scale bar, 10 μm.) (B) Quantitative data are presented as the fractions of cells forming protrusions, normalized to the fraction of cells expressing ARF6 alone (n = 3). (C) HeLa cells expressing ACAP4-FLAG were serum starved and treated with 5 µM lapatinib or DMSO before EGF stimulation. Clarified cell lysates were immunoprecipitated followed by Western analyses. (D) The ACAP4-FLAG proteins were expressed in HeLa cells. Serum-deprived cells were stimulated by EGF before being lysed in ice-cold lysis buffer. ACAP4-FLAG proteins were isolated and subjected to Western blotting with the pY34 antibody and FLAG antibody. (E) HeLa cells were serum starved for 8 h followed by treatment with lapatinib or DMSO before stimulation with EGF. Treated cells were collected for Western blotting with the anti-pY34 antibody. (F) Tyr34 is a substrate of EGFR. Recombinant histidine-ACAP4 proteins were phosphorylated with [γ-32P]-ATP and EGFR as described in Materials and Methods. Samples were separated by SDS/PAGE followed by Coomassie blue staining of ACAP4 proteins (Upper). The gel was then dried and incubated with X-ray film (Lower).
Activation of the EGF receptor (EGFR) cascade leads to an array of tyrosine-based phosphorylation of proteins including ACAP4 (Fig. 3C). To establish the role of ACAP4 in EGFR signaling, HeLa cells expressing ACAP4-FLAG were serum starved and then stimulated by EGF. These cells were then subjected to immunoprecipitation using anti-FLAG affinity beads. Mass spectrometric analyses of ACAP4 indicated that Tyr733 and Tyr34 were phosphorylated in response to EGF stimulation (29). Because the BAR domain promotes ACAP4 GAP in a membrane-localized manner and because Tyr34 is in the BAR domain, we sought to determine whether Tyr34 is phosphorylated in response to EGF stimulation. To this end, the nonphosphorylatable ACAP4Y34F mutant was generated and transfected with WT ACAP4-FLAG into HeLa cells with and without EGF stimulation followed by isolation of ACAP4-FLAG proteins. FLAG affinity beads absorbed equivalent amounts of ACAP4-FLAG under various conditions (Fig. 3D, Bottom, lanes 1–4). However, only WT ACAP4-FLAG from EGF-stimulated cells exhibited reactivity to anti-phosphorylated Tyr34 blotting (Fig. 3D, Top, lane 1), suggesting that Tyr34 is apparently a substrate for EGF stimulation. To validate whether endogenous ACAP4 phosphorylation of Tyr34 is a function of EGF stimulation, endogenous ACAP4 from EGF-stimulated cells was probed with the anti-pY34 antibody. Tyr34 was phosphorylated (Fig. 3E, Top, lane 3), and the level of phosphorylation was minimized by the EGFR inhibitor lapatinib (lane 1).
To determine whether Tyr34 is a direct substrate of EGFR kinase, phosphorylation of recombinant histidine-ACAP4 fusion proteins was assessed. Incubation of the His-ACAP4 proteins with [γ-32P]-ATP and recombinant EGFR kinase resulted in incorporation of 32P into the WT protein but not into the ACAP4Y34F mutant (Fig. 3F, Lower, lanes 2 and 3). This EGFR-mediated phosphorylation was specific, as incubation of ACAP4 with [γ-32P]-ATP in the absence of EGFR resulted in no detectable incorporation of radioactivity (Fig. 3F; Lower, lane 1). If Tyr34 is a cognate substrate of EGFR in cells, depletion of EGFR would diminish the phosphorylation of Tyr34 on EGF stimulation. Consistent with this hypothesis, suppression of EGFR by siRNA minimized the level of pY34 but not the level of the ACAP4 protein (Fig. S3, lane 2). Thus, we conclude that Tyr34 of ACAP4 is a substrate for EGFR.
Tyr34 Phosphorylation Is Essential for the Recruitment of ACAP4 to the Plasma Membrane.
Next, the role of pY34 in partitioning of the ACAP4 protein into the plasma membrane was assessed using a digitonin protocol to separate the membrane and cytosolic fractions (30). HeLa cells were transiently transfected to express ACAP4-FLAG and ACAP4Y34F-FLAG along with EGFR. At 24 h after transfection, cells were starved of serum for 8 h, followed by EGF stimulation and digitonin extraction (30). EGFR was enriched in the membrane fraction with or without EGF stimulation (Fig. 4A, Middle).
Fig. 4.
Tyr34 phosphorylation is required for recruitment of ACAP4 to plasma membranes. (A) HepG2 cells expressing FLAG-ACAP4WT or FLAG-ACAP4Y34F were stimulated with EGF for 5 min. Cells were permeabilized with 0.1% digitonin in K buffer and pelleted as described in Materials and Methods. The cytosolic components and the pellets were subjected to analyses by anti-FLAG blotting. (B) ACAP4 association with the membrane cytoskeleton as a function of pY34. Data were quantified from the band densities of ACAP4 immunoreactivity in membrane and cytosolic fractions and presented as percentages of the total (mean ± SE; n = 3; *P < 0.01 compared with control and Y34F-expressing cells). (C) WT BAR domain and the BARY34F mutant were cosedimented with LUVs made from total brain lipids. The pellet (P) and supernatant (S) components were subjected to SDS/PAGE and Coomassie blue staining. The ratios of BAR and BARY34F on liposome binding activity are presented as means ± SE (P < 0.05; n = 3). (D) Y34F mutant (b) shows a more diffuse distribution than the WT (a) and the Y34E mutant (c) in HepG2 cells. (Scale bar, 10 μm.) (E) ACAP4WT-FLAG was cotransfected with GFP-ACAP4 (WT or Y34F) in 293T cells and immunoprecipitated followed by Western blotting. (F) HeLa cell homogenates were subjected to differential centrifugation after EGF stimulation. Western blotting analyses show that ACAP4 retention on the plasma membrane is a function of EGF stimulation. (G) Data from F were quantified and presented as relative enrichment (fold) over those of PBS-treated samples. Levels of endogenous ACAP4 and pY34 were elevated in response to EGF stimulation (*P < 0.01, **P < 0.001; n = 3).
As previously reported (31), ACAP4 was present in the microsomal fraction of nonstimulated cells (Fig. 4A, Bottom, lane 2). EGF stimulation, however, elicited translocation of ACAP4-FLAG, but not mutant ACAP4Y34F-FLAG (lanes 5 and 6), from the cytosol to the plasma membrane (lanes 3 and 4). Quantitative Western blotting confirmed that pY34 promoted the retention of ACAP4 in the digitonin-insoluble membrane fraction (Fig. 4B). To evaluate the contribution of Tyr34 to the membrane-binding capacity of the BAR domain, a liposome sedimentation assay was used, in which the BARY34F mutant exhibited a reduced association with the liposomes (Fig. 4C). To determine whether pY34 modulated membrane tubulation, HepG2 cells were transiently transfected to express BAR-GFP and the phospho-mimicking (Y34E) or Y34F mutants. The WT BAR domain was readily apparent at intracellular tubular structures (Fig. 4 D, a; WT). Only limited membrane tubulation was observed in HepG2 cells expressing BARY34F-GFP, suggesting that the phosphorylation of Tyr34 also regulated the membrane tubulation (Fig. S4). In fact, expression of BARY34E promoted a green spot-like distribution of ACAP4 in the cytoplasm of HepG2 cells (Fig. 4 D, c). To rule out the possibility that pY34 disturbed the dimerization of the BAR domain, coimmunoprecipitation was accomplished with lysates from 293T cells transiently transfected to express ACAP4-FLAG with ACAP-GFP or ACAPY34F-GFP. As shown in Fig. 4E, the inhibition of Tyr34 phosphorylation did not disrupt ACAP4 dimerization.
To assess the physiological relevance of Tyr34 phosphorylation in EGF stimulation, the extent of pY34 together with ACAP4 level in plasma membrane fraction was quantified. Quantitative Western blotting of membrane proteins, such as Na,K-ATPase, a plasma membrane marker; EGFR; and β-actin, was accomplished. As shown in Fig. 4F, EGF stimulation did not cause appreciable retention of Na,K-ATPase or β-actin in the plasma membrane fraction. However, the ACAP4 protein and pY34 signal were significantly enriched in this fraction (Fig. 4G; P < 0.05). In addition, ACAP4Y34E-FLAG exhibited a greater ability to associate with plasma membrane (Fig. S5). These results suggest that pY34 is essential for recruitment of ACAP4 to the plasma membrane on stimulation of cells with EGF.
Phosphorylation of Tyr34 Orchestrates EGF-Elicited Cell Motility.
We next sought to examine the function of pY34 in regulating ACAP4 activity in EGF-elicited cell migration. HepG2 cells were transiently transfected to express ARF6-mCherry along with ACAP4-GFP, ACAP4Y34F-GFP, or GFP. Membrane ruffles were readily apparent in cells expressing ACAP4Y34F-GFP but not in cells with WT ACAP4 (Fig. 5A). Quantitative analyses showed that inhibition of pY34 promoted formation of membrane ruffles (P < 0.01; n = 30). To examine whether pY34 modulates the GAP activity toward ARF6, aliquots of 293T cells were transiently transfected to express WT and constitutively active ARF6Q67L tagged with FLAG with ACAP4-GFP constructs followed by GGA affinity pull-down assays. As shown in Fig. 5C, the level of GTP-bound ARF6 was elevated in cells expressing the Y34F mutant compared with that in cells expressing ACAP4-GFP (Top, lanes 3 and 4), suggesting that pY34 promotes ARF6 GTP hydrolysis. Consistent with this concept, ACAP4Y34E reduced the level of GTP-bound ARF6 (lane 5), showing that pY34 is essential for ACAP4 GAP activity. Statistical analyses supported the function of pY34 in regulation of ARF6 GTPase activity. In fact, overexpression of ACAP4Y34F failed to inhibit the formation of protrusions in response to AlF3 treatment (Fig. S6). Thus, we conclude that pY34 regulates GAP activity of ACAP4 at the cell cortex.
Fig. 5.
Phosphorylation of ACAP4 at Tyr34 regulates cell motility. (A) HepG2 cells transfected with ARF6-mCherry or cotransfected with ACAP4-GFP variants with and without EGF stimulation. Cells were either stained with FITC-phalloidin for visualization of actin (a–c) or directly subjected to ACAP4 and ARF6 imaging (d–l). (Scale bar, 10 μm.) (B) Quantitative data are presented as the fractions of cells forming protrusions when coexpressing the indicated protein, normalized to the fraction of cells expressing ARF6 alone (P < 0.05; n = 3). (C) Exogenous ARF6 activities in cotransfected 293T cells were measured by GST-GGA pull-down. Active forms of ARF6 were precipitated followed by anti-FLAG immunoblotting. (D) Quantitative analyses of GST-GGA pull-down described in C. Ratios of ARF6-GTP/total ARF6 were determined by Western blotting. (**P < 0.01; n = 3). (E) Aliquots of MDA-MB-231 cells were transfected with ACAP4-GFP or Tyr-34 mutants followed by serum starvation and EGF stimulation. Paths of the migrating cells were traced by time-lapse microscopy. (F) Quantitative analyses of the velocities. The migration speeds of cells are shown in the bar charts as means ± SE (**P < 0.001, *<0.05; n > 20 cells).
To examine the role of pY34 in cell migration, we monitored the real-time migration velocity of MDA-MB-231 cells seeded on fibronectin-coated Petri dishes (Fig. 5E). As shown in Fig. 5E, cells expressing ACAP4Y34F displayed slower velocities in migration relative to that of cells expressing WT ACAP4 (22.7 ± 1.5 vs. 25.0 ± 1.1 μm/h, respectively). Cells expressing ACAP4Y34E exhibited faster speeds (31.5 ± 0.9 μm/h; P < 0.001, n = 20; Fig. 5F). Active ARF6 (both WT and the Q67L mutant) cooperated with ACAP4Y34E in enhancing HeLa cell migration (41.7 ± 2.3 μm/h) relative to cells expressing ARF6Q67L (27.1 ± 3.3 μm/h; n = 20). These results indicate that phosphorylation of Tyr34 facilitates cell migration.
Discussion
This study establishes that ACAP4 contains a functional BAR domain involved the dimerization of ACAP4 membrane tubulation of the BAR domain that is responsible for localizing ACAP4 to curved membranes and regulating membrane-cytoskeleton dynamics. Interestingly, the BAR is phosphorylated by EGFR at Tyr34, which alters its membrane-binding capacity. Phosphorylation of Tyr34 provides a link between EGF stimulation and membrane dynamics in cell migration. These results establish a previously uncharacterized regulatory mechanism governing ACAP4-associated membrane dynamics through phosphorylation-mediated regulation of the BAR domain.
The function of the phosphorylation site in the BAR domain has not been well understood because there is little conservation in its structure. To date, there are only three proteins, APPL1, Cdc15, and syndapin I, known to contain phosphorylatable sites in their BAR domains (32–34). Phosphorylation of the F-BAR domain in syndapin I is the only characterized example. The two sites for syndapin I phosphorylation, Ser76 and Thr181, are located at the N-terminal helix-capping motifs (N-Cap) of different α-helices that provide hydrogen-bonding partners to stabilize homodimer structures. Ser76 phosphorylation of syndapin I regulates F-BAR homodimer curvature by changing the hinge angle of the dimer. As determined by our computational modeling of the ACAP4 structure, the Tyr34 site is located at the beginning of the ACAP4 BAR domain helix1. Thus, we reason that the phosphorylation-elicited conformational change and destabilization of the BAR domain homodimer would be a common structural feature and regulatory mechanism underlying both ACAP4 and Syndapin I because both Y34 of ACAP4 and S76 of Syndapin I are oriented to the center of the six-helix bundle away from the lipid interacting surface.
In our simulation model, the side chain of residue Tyr34 orients to the side closed to the helix α5 and, in this region, forms multiple contacts, which may contribute to stabilizing the helix α5 in the homodimer (Fig. S9D). Because helix α5 is located at the center of the six-helix bundle, phosphorylation of Tyr34 may cause a destabilization of this region, resulting in a change in structure of the BAR domain. Because modification of the Tyr34 site apparently serves as a regulatory signal to alter the curvature of the BAR domain homodimer, it would be appropriate to validate this model by crystallographic investigations.
It has been reported that the Pleckstrin Homology (PH) or Phox Homology (PX) domain functions together with the adjacent BAR domain (19, 35). A plausible hypothesis is that the BAR+PH domain functions synergistically as a sensor to select the membrane domains based on their lipid composition and to execute spatial regulation in curvature of the membrane (Fig. S7). This mechanism, observed frequently in signaling pathways, gives rise to precise spatial regulation in localizing binding partners and enzymatic activities (18). The experimental results of AlF3-elicited membrane ruffles in this study suggest that the membrane association of ACAP4 regulates intracellular ARF6 activity and thereby regulates actin polymerization and signaling pathways. Perhaps ACAP4 is preferentially enriched within membrane microdomains with high curvature in the presence of components such as PI(4,5)P2. Currently, we are using pair correlation analysis combined with photo-activation localization microscopy, pioneered by Lippincott-Schwartz and colleagues, to annotate a nanoscale organization of the ACAP4 BAR domain with different lipid partition profiles and to determine whether phosphorylation of Tyr34 alters its spatial localization (36). With this approach, we will assess whether phosphorylation of pY34, on association with membrane domains, promotes the recruitment of ARF6 to the membrane in addition to enhancing GAP activity.
The present results demonstrate that phosphorylation at the Tyr34 site in the N-terminal BAR domain of ACAP4 regulates the recruitment of ACAP4 to the plasma membrane. During cell migration, this membrane recruitment by the ACAP4 BAR domain regulates ARF6 GTPase activity and dynamics in EGF-stimulated membrane-cytoskeleton remodeling. Further, Tyr34 phosphorylation promotes the motility of MDA-MB-231 cells. Thus, the results provide insights on the regulation of ARF6 activity by ACAP4 in the EGF signaling pathway.
Materials and Methods
LUVs consisting of total brain lipids (Folch fraction 1; Sigma Chemicals) were analyzed by dynamic light scattering. The sedimentation and tubulation assays were performed as described (19).
Other materials and methods are detailed in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jennifer Lippincott-Schwartz for comments on refinement of the manuscript and Dr. Don Hill for proofreading. This work was supported by Chinese 973 Projects 2010CB912103, 2012CB917204, and 2013CB911203; Chinese Academy of Science KSCX1-YW-R-65; MOST 2009DFA31010; Ministry of Health 2012ZX10002012-013; Ministry of Education 20113402130010; Anhui Project 08040102005; Chinese Natural Science Foundation Grants 90508002, 91129714, 81270466, 31271518, and 90913016; China Fellowship 2012M510210; National Institutes of Health Grants CA164133, DK56292, G12RR03034, and UL1RR025008; and Central University Grants WK2340000032 and WK2340000021. X. Yao is a Cheung Kong Scholar.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217727110/-/DCSupplemental.
References
- 1.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippincott-Schwartz J, Liu W. Membrane trafficking: Coat control by curvature. Nature. 2003;426(6966):507–508. doi: 10.1038/426507a. [DOI] [PubMed] [Google Scholar]
- 5.Jackson TR, et al. ACAPs function in the cell periphery. J Cell Biol. 2000;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai J, et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell. 2004;7(5):771–776. doi: 10.1016/j.devcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267(5201):1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 8.Schafer DA, D’Souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1(11):892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza-Schorey C, et al. ARF6 targets recycling vesicles to the plasma membrane: Insights from an ultrastructural investigation. J Cell Biol. 1998;140(3):603–616. doi: 10.1083/jcb.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters PJ, et al. Overexpression of wild-type and mutant ARF1 and ARF6: Distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128(6):1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabe H, et al. The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic. 2009;10(8):982–993. doi: 10.1111/j.1600-0854.2009.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randazzo PA, Nie Z, Miura K, Hsu VW. Molecular aspects of the cellular activities of ADP-ribosylation factors. Sci STKE. 2000;2000(59):re1. doi: 10.1126/stke.2000.59.re1. [DOI] [PubMed] [Google Scholar]
- 13.Fang Z, et al. Proteomic identification and functional characterization of a novel ARF6 GTPase-activating protein, ACAP4. Mol Cell Proteomics. 2006;5(8):1437–1449. doi: 10.1074/mcp.M600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Ismail SA, Vetter IR, Sot B, Wittinghofer A. The structure of an Arf-ArfGAP complex reveals a Ca2+ regulatory mechanism. Cell. 2010;141(5):812–821. doi: 10.1016/j.cell.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Ha VL, et al. ASAP3 is a focal adhesion-associated Arf GAP that functions in cell migration and invasion. J Biol Chem. 2008;283(22):14915–14926. doi: 10.1074/jbc.M709717200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Zelhof AC. Amphiphysins: Raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp. Traffic. 2002;3(7):452–460. doi: 10.1034/j.1600-0854.2002.30702.x. [DOI] [PubMed] [Google Scholar]
- 17.Habermann B. The BAR-domain family of proteins: A case of bending and binding? EMBO Rep. 2004;5(3):250–255. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallop JL, McMahon HT. BAR domains and membrane curvature: Bringing your curves to the BAR. Biochem Soc Symp. 2005;(72):223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 19.Peter BJ, et al. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 20.Shen H, Pirruccello M, De Camilli P. SnapShot: Membrane curvature sensors and generators. Cell. 2012;150(6):1300–1300, e1–e2. doi: 10.1016/j.cell.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson JC, Legg JA, Machesky LM. Bar domain proteins: A role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16(10):493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Fricke R, et al. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol. 2009;19(17):1429–1437. doi: 10.1016/j.cub.2009.07.058. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, et al. SEX33 induces micronucleated phenotype and actin polymerization by interacting with WASP. J Biol Chem. 2009;284:21659–21669. doi: 10.1074/jbc.M109.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saarikangas J, et al. Missing-in-metastasis MIM/MTSS1 promotes actin assembly at intercellular junctions and is required for integrity of kidney epithelia. J Cell Sci. 2011;124(Pt 8):1245–1255. doi: 10.1242/jcs.082610. [DOI] [PubMed] [Google Scholar]
- 25.Radhakrishna H, Klausner RD, Donaldson JG. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J Cell Biol. 1996;134(4):935–947. doi: 10.1083/jcb.134.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank S, Upender S, Hansen SH, Casanova JE. ARNO is a guanine nucleotide exchange factor for ARF6. J Biol Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Song J, Khachikian Z, Radhakrishna H, Donaldson JG. Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J Cell Sci. 1998;111(Pt 15):2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- 28.Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154(3):599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, et al. ACAP4 protein cooperates with Grb2 protein to orchestrate EGF-stimulated integrin β1 recycling in cell migration. J Biol Chem. 2011;286:43735–43747. doi: 10.1074/jbc.M111.278770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malinowska DH, Koelz HR, Hersey SJ, Sachs G. Properties of the gastric proton pump in unstimulated permeable gastric glands. Proc Natl Acad Sci USA. 1981;78(9):5908–5912. doi: 10.1073/pnas.78.9.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding X, et al. Phospho-regulated ACAP4-Ezrin interaction is essential for parietal cell secretion. J Biol Chem. 2010;285:18769–18780. doi: 10.1074/jbc.M110.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gant-Branum RL, Broussard JA, Mahsut A, Webb DJ, McLean JA. Identification of phosphorylation sites within the signaling adaptor APPL1 by mass spectrometry. J Proteome Res. 2010;9(3):1541–1548. doi: 10.1021/pr901043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts-Galbraith RH, et al. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell. 2010;39(1):86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan A, et al. Phosphorylation of syndapin I F-BAR domain at two helix-capping motifs regulates membrane tubulation. Proc Natl Acad Sci USA. 2012;109(10):3760–3765. doi: 10.1073/pnas.1108294109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlton J, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through high-curvature membranes. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 36.Sengupta P, et al. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods. 2011;8(11):969–975. doi: 10.1038/nmeth.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.