Abstract
High surface expression of programmed death 1 (PD-1) is associated with T-cell exhaustion; however, the relationship between PD-1 expression and T-cell dysfunction has not been delineated. We developed a model to study PD-1 signaling in primary human T cells to study how PD-1 expression affected T-cell function. By determining the number of T-cell receptor/peptide-MHC complexes needed to initiate a Ca2+ flux, we found that PD-1 ligation dramatically shifts the dose–response curve, making T cells much less sensitive to T-cell receptor–generated signals. Importantly, other T-cell functions were differentially sensitive to PD-1 expression. We observed that high levels of PD-1 expression were required to inhibit macrophage inflammatory protein 1 beta production, lower levels were required to block cytotoxicity and IFN-γ production, and very low levels of PD-1 expression could inhibit TNF-α and IL-2 production as well as T-cell expansion. These findings provide insight into the role of PD-1 expression in enforcing T-cell exhaustion and the therapeutic potential of PD-1 blockade.
Keywords: TCR signaling, peptide counting, HIV-1 specific T cell response
T cells are well designed to fight invading pathogens. The relatively few naive T cells that recognize a pathogen undergo a massive differentiation and expansion program that tailors the T-cell response to control a particular infection throughout the body (1). These pathogen-specific T cells produce cytokines that limit pathogen replication and engage other arms of the immune response and also up-regulate their lytic machinery to trigger destruction of cells harboring the foreign antigen. Once the antigen is cleared, a small subset of these pathogen-specific T cells differentiates into a highly functional, long-lasting memory T-cell pool that is poised to clear a subsequent encounter with that pathogen rapidly. However, pathogens such as HIV-1, HCV, and tuberculosis as well as tumors avoid immune clearance and persist. This continued presence of antigen subverts normal memory T-cell generation and leads effector T cells down a path of exhaustion in which they sequentially lose effector function (2). Proliferative capacity is generally the first function T cells lose in the presence of chronic antigen, followed by the lack of cytotoxic ability and IFN-γ production upon T-cell receptor (TCR) engagement, and eventually these nonfunctional T cells are eliminated (3). Although T-cell exhaustion limits immune pathology (4, 5), it also results in loss of immune control, potentially having devastating consequences to the host. Therapeutics reviving exhausted T cells would be attractive to treat chronic infection and cancer and are being pursued actively (6, 7).
Programmed death-1 (PD-1, CD279) is a cell-surface protein expressed as a monomer (8) on a wide array of immune cells (9). Engagement of PD-1 by either of its two ligands, PD-L1 (B7-H1 or CD274) or PD-L2 (B7-DC or CD273), inhibits T-cell activation (10). Interest in PD-1 biology was fueled most recently by the observations that exhausted T cells express high levels of PD-1 and, furthermore, that PD-1 blockade was able to restore function to these exhausted T cells (4, 11, 12). Moreover, antibodies targeting the PD-1 pathway are proving to be effective cancer therapeutics (13, 14). However, the relationship between PD-1 expression and T-cell exhaustion has not been defined clearly. Although it is clear that most, if not all, exhausted cells express high levels of PD-1, not all cells expressing high levels of PD-1 are exhausted (15). Other molecules such as 2B4 (CD244), lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin domain and mucin domain 3 (Tim-3), and B- and T-lymphocyte attenuator (BTLA, CD272) also have been implicated as regulators of exhaustion (16–18). Although PD-1 blockade was able to restore function impressively to exhausted cells in mice infected with lymphocytic choriomeningitis virus (LCMV) (4), the effect of PD-1 blockade in humans has been less dramatic, and some T-cell functions were affected by PD-1 ligation much more profoundly than others (19–21). Thus, the extent to which PD-1 is an enforcer of or a simply a marker of T-cell exhaustion is unclear.
Attempts to understand the role that PD-1 signaling plays in mediating T-cell tolerance and exhaustion have been hampered by models of T-cell activation that rely upon nonphysiologic agents such as agonist antibody and pervanadate (22). Moreover, comparisons of various T-cell populations within individuals or within mice that differentially express PD-1 are complicated because each of the T cells has a distinct replicative history and expresses a wide array of costimulatory and coinhibitory molecules and because T-cells are difficult to acquire in quantities that permit robust biochemical investigations. Thus, we developed a system that allows us to modulate PD-1 expression in otherwise identical cells so we could determine how the strength of PD-1 signaling affects a variety of T-cell functions. Use of the model revealed that high levels of PD-1 expression were sufficient to block T-cell function. Additionally, we observed that various T-cell effector functions show differential susceptibility to PD-1 signaling. We demonstrate that production of IFN-γ and β-chemokines is relatively resistant to PD-1 engagement, whereas Ca2+ flux and cytotoxicity are more sensitive to PD-1 engagement. T-cell expansion and IL-2 and TNF-α production proved to be the functions most sensitive to PD-1 signaling. These results provide insight into what can and cannot be expected in terms of immune reconstitution from therapeutics that target the PD-1 pathway.
Results
Development of a Model to Study PD-1 Signaling and Function in Primary Human T Cells.
Previous models used to investigate PD-1 signaling and function have relied on the use of magnetic beads coated with agonist antibody and/or pervanadate (23–27). We wished to develop a model to study the effect of PD-1 ligation on T-cell activation that relied only on natural ligands. Unfortunately, most forms of gene transfer into primary human T cells require T-cell activation to observe high levels of expression of the introduced gene in a majority of the T cells. This requirement precludes the study of T cells as they transition from G0 to G1, which is likely a key target of PD-1–mediated inhibition (28, 29). To determine whether RNA transfection of freshly isolated, resting primary human CD8 T cells could be used to study T-cell activation, we introduced RNA encoding a TCRα and TCRβ chain linked by a T2A sequence that conferred specificity to the SLYNTVATL (HIV-1GAG 77–85) peptide presented by HLA-A2 (30). We observed both high and uniform A2-SL9 tetramer staining (Fig. 1A), indicating that RNA transfection is an efficient way to generate antigen-specific T cells for signaling and functional studies. To analyze the functional properties of these antigen-specific T cells, we generated a series of artificial antigen-presenting cells (aAPCs) that all express HLA-A2 and are divergent for expression of the HIV-1GAG minigene that contains the A2-SL9 epitope or PD-L1 (Fig. 1B). To validate that we could observe antigen-specific activation of these TCR-transduced T cells, we mixed carboxyfluorescein succinimidyl ester (CFSE)-labeled, TCR-transfected T cells with aAPCs expressing either HLA-A2 (K.A2) or HLA-A2 and the SL9 minigene (K.A2.SL9). We observed no T-cell expansion in cultures that received no aAPCs or in those that were mixed with K.A2 aAPCs (Fig. 1C). T-cell expansion was observed in cultures that were stimulated with aAPCs that expressed the cognate peptide-MHC complexes (pMHC), although this expansion was less pronounced than in cultures stimulated with nonphysiologic CD3/28-coated beads. Because the strength of T-cell activation alters the expression of many negative regulators of T-cell activation and thus could confound our studies, we compared the expression of Tim-3, BTLA, cytotoxic T-lymphocyte antigen 4 (CTLA-4, CD152), 2B4, and PD-1 after stimulation with antigen-specific or CD3/28-coated beads (Fig. 1D). As above, we noted antigen-specific stimulation led to much lower levels of induction of these negative regulators of T-cell activation. These studies indicate that antigen-specific activation of primary human T cells can be investigated using this model and that the overall signal strength is much less than that observed with CD3/28-coated beads.
Fig. 1.
Development of a model to study PD-1 signaling and function in primary human T cells. (A) RNA encoding both chains of the A2-SL9–specific TCR was transfected into resting primary human CD8 T cells, and, after overnight culture, the ability to bind SL9 tetramer was measured by flow cytometry. (B) aAPCs were generated by transducing K562s with HLA.A2, the DsRed -SL9 minigene, or PD-L1 as indicated. Single-cell clones of each aAPC were expanded, and expression of each introduced construct was validated by flow cytometry. K.A2 cells are depicted as a long dashed line, K.A2.SL9 DsRed as a short dashed line, and the K.A2.SL9-DsRed.PD-L1 as a solid line. Isotype staining (gray shading) served as a negative control for A2 staining. (C) Cells described in A were labeled with CFSE and cocultured with the indicated aAPC at a 2:1 ratio. CFSE dilution was measured by flow cytometry after 5 d of culture. As a positive control, T cells also were stimulated with CD3/CD28 beads at a 3:1 ratio. (D) T cells described in A were cocultured with K. A2 DsRed. SL9 (solid lines) at a 1:2 ratio or with CD3/CD28 beads (long dashed lines) at a 1:3 ratio for 3 d and were stained with indicated antibodies. Mock TCR-transfected T cells incubated with KT. A2 DsRed. SL9 aAPCS served as control (gray shading).
PD-1 Inhibits Ca2+ Flux in a Dose-Dependent Manner.
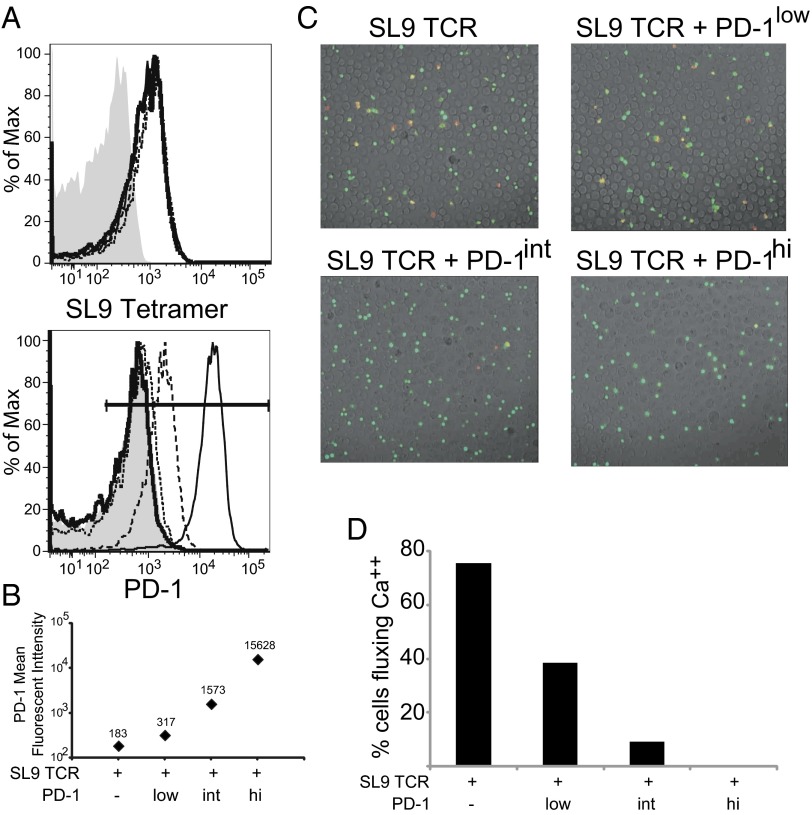
TCR signaling results in a rapid flux of intracellular Ca2+ that activates a number of signaling pathways crucial for T-cell activation and differentiation (31). To ascertain how the level of PD-1 expression affects the ability of TCR engagement to alter Ca2+ signaling, we transfected uniform levels of the A2-SL9–specific TCRs and variable amounts of PD-1–encoding mRNA into primary human CD8 T cells so that we could compare SL9-specific T cells with endogenous [183 mean fluorescence intensity (MFI)], low (317 MFI), intermediate (Int, 1,573 MFI), and high (15,628 MFI) PD-1 expression (Fig. 2A). These T cells were loaded with the Ca2+-sensitive dye Fura-2 and were placed on a monolayer of aAPCs expressing K.A2.SL9 or K.A2.SL9.PD-L1, and changes in intracellular Ca2+ levels were measured over time using time-lapse ratio-imaging microscopy. In the absence of PD-L1 expression on aAPCs, all the T cells except those expressing high levels of PD-1 showed equally robust Ca2+ flux (Fig. S1). In the presence of PD-L1 expression on aAPCs, we observed a clear inverse correlation between the level of PD-1 expression and the number of T cells fluxing Ca2+. Doubling the level of PD-1 expression (low) over the endogenous level reduced the number of T cells fluxing Ca2+ by twofold (Fig. 2 B and C and Movies S1–S4). T cells expressing an approximately fivefold additional PD-1 (Int) showed a corresponding reduction in the number of T cells fluxing Ca2+. Finally, T cells expressing very high levels of PD-1 were completely unable to flux Ca2+. These studies are consistent with the notion that PD-1 ligation can interfere with the most membrane-proximal signaling events (32) and clearly demonstrate that the ability of PD-L1–expressing aAPCs to inhibit Ca2+ is directly proportional to the amount of PD-1 on the T-cell surface.
Fig. 2.
PD-1 inhibits Ca2+ flux in a dose-dependent manner. (A) RNA (10 µg) encoding both chains of the A2-SL9–specific TCR was mixed with 0 (thin solid line), 0.125 (short dashed line), 1 (long dashed line), or 10 (thick solid line) µg RNA encoding PD-1 and was transfected into resting primary human CD8 T cells. After overnight culture, the ability to bind SL9 tetramer (Upper) and PD-1 expression (Lower) were measured by flow cytometry. Untransfected cells served as negative control (gray shading). (B) PD-1 geometric mean fluorescent intensities are plotted from the data gathered in A. (C) T cells pulsed with the calcium-sensitive dye Fura-2 were injected into the chamber containing poly-l-lysine–anchored K.A2.SL9.PD-L1 aAPCs, and the 510-nm emissions excited by 340-nm and 380-nm light were captured immediately for 45 min at 5-s intervals. Images show the composition of a bright-field image overlaid with a transparent color scale of the ratio of fluorescence emissions at 340 and 380 nm of Fura-2. The ratio correlates with intracellular calcium concentration: Green indicates resting (low) concentration, and red indicates high intracellular calcium concentration. The pictures show 200-s snapshots after mixing of cells transfected with A2-SL9–specific TCRs alone or with a mixture of different amounts of PD-1 with K.A2 DsRed.PD-L1 (see Movies S1–S4). (D) Plot showing the number of T cells that fluxed Ca2+ at least once during the 45-min experiment as a function of PD-1 expression. An increase in the ratio of 510-nm emission excited by 340 nm to that excited by 380 nm indicated an increased intracellular calcium level. T cells with a ratio more than two times that before stimulation were considered to have fluxed calcium. Data are representative of three individual experiments.
PD-1 Signaling Increases the Number of Engaged TCRs Required to Initiate a Ca2+ Flux.
To understand better how PD-1 ligation alters the ability of pMHC recognition to initiate a Ca2+ flux, we modified a peptide-counting system that originally was developed to study murine TCR transgenic T cells (33–35) to query how the number of pMHC/TCR complexes recruited to the immunological synapse alters Ca2+ signaling in primary human T cells. The technique detects single molecules of biotinylated peptides complexed with phycoerythrin-tagged streptavidin molecules bound by an MHC by imaging with a cooled, charge-coupled device camera. Initially, we found that K562-based aAPCs were not compatible with this approach because the biotin-labeled peptide did not load efficiently. Therefore, we switched to a transporter associated with antigen processing (TAP)-deficient aAPC system in which PD-L1 was introduced lentivirally into HLA-A2+ T2 cells to favor loading of the exogenous peptide (Fig. 3A). As with the K562-based aAPC system, we were able to observe antigen-specific T-cell activation and PD-L1–dependent suppression of T-cell activity by measuring the amount of IFN-γ secreted after incubation of the aAPCs and T cells (Fig. 3B). Next, we introduced SL9-specific TCR and an intermediate amount of PD-1 RNA (1 µg/10 M T cells) into primary human CD8 T cells. This amount of PD-1–specific mRNA was chosen because it approximated the amount of PD-1 on the surface of exhausted T cells and enabled us to observe some of the T cells still undergoing a Ca2+ flux in the presence of PD-L1 ligation (Fig. 2B). These T cells were labeled with Fura-2 and mixed with T2 or T2 PD-L1 aAPCs loaded with biotin-labeled SL9 peptide. T-cell–aAPC conjugates were evaluated by the degree to which changes in Ca++ flux were observed and by the number of peptides present in the T-cell synapse.
Fig. 3.
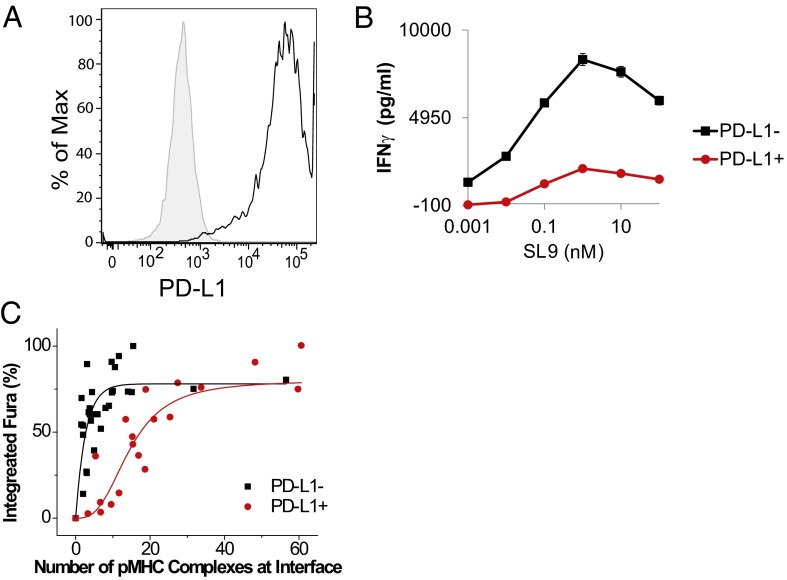
PD-1 signaling increases the number of engaged TCRs required to initiate a Ca2+ flux. (A) T2 cells were transduced with a lentiviral vector encoding PD-L1, and a single clone was expanded and evaluated for PD-L1 expression by flow cytometry. (B) T2 cells were loaded with the indicated concentrations of biotinylated labeled SL9 peptide and were mixed with CD8 T cells coexpressing intermediate levels of PD-1 and SL9-specific TCR. IFN-γ secretion was measured by ELISA from supernatant collected after 24 h of culture. (C) Dose response of calcium signals to the number of pMHC complexes at the T-cell/APC interface in the presence and absence of PD-L1. Fura-2 ratios were integrated every 20 s for 10 min after initial calcium release. The integrated calcium signals were normalized to the maximal signal. The number of pMHC complexes was quantified as described in Methods. Each point represents data from a single cell. Data were pooled from more than seven experiments carried out on different days using T cells independently prepared from different donors. The two sets of data (T cells stimulated by PD-L1− and PD-L1+ aAPCs) are statistically different (P = 0.008, Mann–Whitney test).
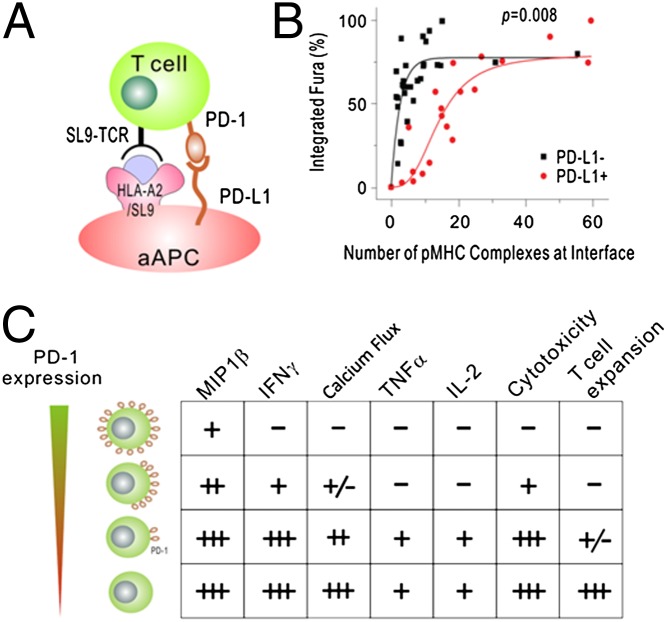
Previously, it has been reported in mouse CD4+ and CD8+ T cells (33–35) that a single pMHC complex can trigger a transient calcium signal. The strength of the calcium signal increases with additional ligand and reaches its maximum at ∼10 complexes, at which point the mature immunological synapse is formed. Here, we observed that a similar number of pMHC complexes were needed to induce calcium response in our human T-cell model (Fig. 3C, black curve), demonstrating that human and mouse T cells have similar antigen sensitivity. In the presence of PD-L1, the antigen sensitivity is greatly reduced, as demonstrated by a shift in the dose–response curve (Fig. 3C, red curve). We estimated that ∼30 complexes are required to trigger maximal calcium signals in the presence of PD-L1, a threefold increase compared with PD-L1− cells. Our data suggest that the recruitment of PD-1 at the synapse greatly reduces T-cell antigen sensitivity, contributing to the inhibitory effect of PD-1.
T-Cell Proliferation Is Highly Sensitive to PD-1–Mediated Inhibition.
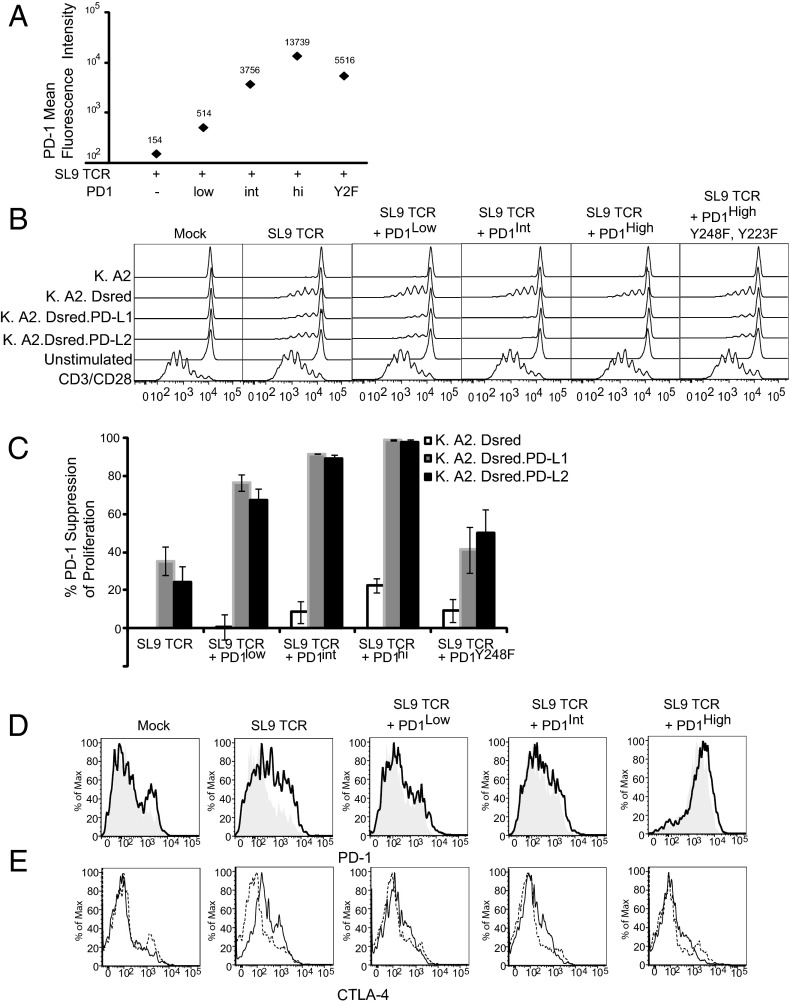
Upon pMHC recognition, T cells proliferate, produce cytokines, and mediate cytotoxic effects. All these functions are impaired in exhausted T cells, and we were interested in elucidating the extent to which high levels of PD-1 expression contribute to mediating the exhausted phenotype. To study the effects of PD-1 expression on T-cell proliferation, we labeled the A2-SL9–specific T cells expressing varying amounts of PD-1 (Fig. 4A) with CFSE, mixed them with K.A2, K.A2.SL9, K.A2.SL9.PD-L1, or K.A2.SL9.PD-L2 aAPCs, and measured CFSE dilution 5 d later. Surprisingly, we observed that T-cell proliferation was more sensitive to PD-1–mediated signals than Ca2+ flux, because we observed a reduction in the proliferative index of ∼40% when the PD-L1 aAPCs were used to stimulate the resting SL9 TCR-expressing T cells with low, barely detectable levels of endogenous PD-1. When PD-1 expression was increased slightly (by approximately threefold), we observed a nearly complete block in proliferative capacity, demonstrating that T-cell expansion is very sensitive to PD-1–mediated inhibition (Fig. 4 B and C), consistent with its ability to target early cell-cycle regulators (28, 29) We hypothesized that introduction of PD-1 Y223F, Y248F RNA would function as signaling-defective, dominant-negative version of PD-1. Surprisingly, although there was ∼35-fold more of the signaling-defective form of PD-1 on the surface of the T cell, we still observed significant inhibition of proliferation when the T cells were stimulated by the PD-L1–expressing aAPCs. This finding shows that minute amounts of PD-1 signaling can effectively inhibit T-cell proliferation. Last, as further evidence supporting the notion that proliferation is highly sensitive to PD-1 signals, we were able to observe that T cells expressing high levels of PD-1 in the absence of PD-L1 aAPCs had reduced proliferative activity. We speculate that the subtle amount of PD-1 ligands that can be expressed on primary human CD8 T cells (36) is sufficient to mediate suppression of T-cell expansion in a manner dependent on T–T-cell interaction when PD-1 expression is high.
Fig. 4.
T-cell proliferation is highly sensitive to PD-1–mediated inhibition. (A) RNA (10 µg) encoding of the A2-SL9–specific TCR was mixed with 0, 0.125, 1, or 10 µg RNA encoding PD-1 and was transfected into equally mixed resting primary human CD8 and CD4 T cells. After overnight culture, PD-1 expression was measured by flow cytometry. Data are representative of three individual experiments. (B) Cells described in A were stained with CFSE and cocultured with the indicated aAPCs at a 2:1 ratio for 5 d, and CFSE dilution was measured by flow cytometry. As a positive control, T cells also were stimulated with CD3/CD28 beads at a 3:1 ratio. (C) Percent suppression of T-cell proliferation was calculated as described in Methods and averaged from three independent experiments. Error bars indicate SD (n = 3). White bars indicate T cells stimulated by K.A2.SL9-dsRED, gray bars indicate T cells stimulated by K.A2.SL9-dsRED PD-L1, and black bars indicate T cells stimulated with K.A2.SL9-dsRED PD-L2. (D and E) PD-1 (D) and CTLA-4 (E) expression was measured after 3-d stimulation with K.A2.SL9 (solid lines) or K.A2.SL9.PD-L1 (long dashed lines).
We also examined how PD-1 expression was modulated during the time course of the assay. After 3 d, the overall hierarchy of PD-1 expression was maintained, but the differences were less pronounced (Fig. 4D and Fig. S2). We observed higher PD-1 expression in T cells that received no additional PD-1 and that were stimulated in the absence of PD-L1, suggesting that higher PD-1 expression on resting T cells was able to block the induction of PD-1 upon antigen recognition. We also evaluated how the level of PD-1 expression affected the regulation of other coinhibitory factors to determine the extent to which PD-1 expression altered the ability of other negative regulators to limit T-cell function. When no additional PD-1 was added to the T cells and in the absence of PD-L1 on the aAPC, we observed significant up-regulation of CTLA-4 (Fig. 4E). However, in the presence of PD-1 signaling the amount of CTLA-4 up-regulation was muted, suggesting that PD-1 signaling could suppress CTLA-4. Examination of how Tim-3, BTLA, and 2B4 expression was affected by the PD-1 expression revealed no differences (Fig. S3).
IL-2 and TNF-α Expression Are More Sensitive to PD-1–Mediated Inhibition than IFN-γ or Macrophage Inflammatory Protein 1 Beta Production.
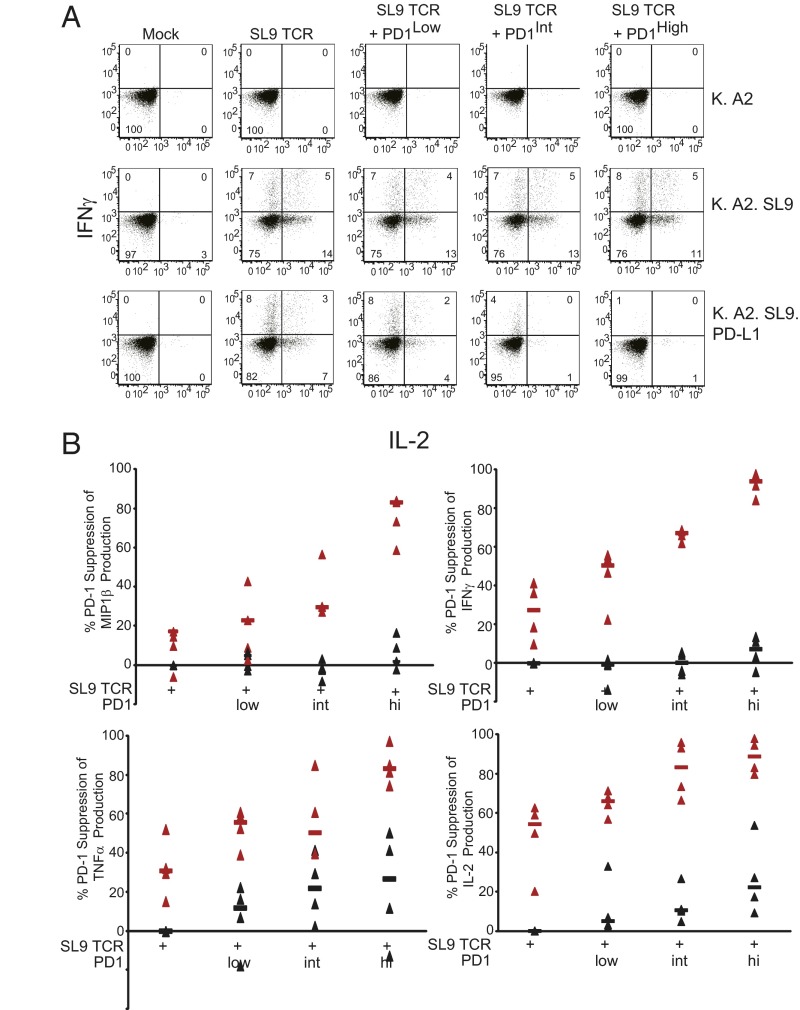
Many studies have reported that exhausted T cells have a reduced cytokine response to cognate pMHC and that the addition of PD-1– or PD-L1–blocking antibodies restores some of this cytokine production (2). What is unclear from these studies is the degree to which PD-1 blockade rescues cytokine production. That is, because cells expressing PD-1 are likely to express multiple inhibitory receptors (16) and have a more extensive replicative history than T cells expressing low levels of PD-1 (37), it is difficult to ascertain how much of the maximal cytokine production profile is restored by PD-1 blockade and whether the expression of some cytokines is more sensitive than others to PD-1 signals. An additional caveat of many studies which show restored cytokine production upon PD-1 blockade is that blocking interactions between PD-1 and PD-L1 may promote cell survival (20, 38). Thus, it is not clear—especially in a 3–6 d assay—whether increased cytokine production after PD-1 blockade is the direct result of interfering with PD-1 signaling or whether the promotion of cell survival simply enables cells to produce more cytokines. Our model allows us to ask this question directly. In preliminary studies, we could not obtain robust, polyfunctional cytokine responses consistently in a 4-h cytokine release assay using freshly isolated T cells. Therefore, we introduced SL9 TCRs and varying levels of PD-1 into previously CD3/28-coated bead-activated T cells, which were expanded for 9–10 d before RNA transfection. These cells then were mixed with aAPCs to determine how PD-1 signaling affects cytokine production. We observed differential sensitivity to PD-1 signaling among the various cytokines. In the absence of antigen (K.A2) or SL9 TCR (mock), we did not observe any cytokine production (Fig. 5A). T cells expressing the SL9 TCR with no additional PD-1 added (248 MFI), produced both INF-γ and IL-2 when stimulated by the K.A2.SL9 aAPCs. Interestingly, when stimulated by the K.A2.SL9.PD-L1 aAPCs, the number of IL-2–producing T cells was reduced from 19% to 10% (52%), whereas the number of IFN-γ–producing cells remained largely the same (12% vs. 11%). This preferential dampening of IL-2 production also was evident when PD-1 expression was modulated to increasingly higher levels. When low levels of PD-1 were added (MFI 409), the number of IL-2–producing cells was reduced to 6% (69% suppression), whereas 10% of the cells were able to produce IFN-γ (16% suppression). At intermediate (MFI 2,062) PD-1 expression, we observed a greater than 90% reduction in the number of IL-2–producing cells, whereas under the same conditions the number of IFN-γ–producing T cells was reduced by only 60%. High levels of PD-1 (MFI 16,846) effectively inhibited both IL-2 and IFN-γ production, suggesting that sufficiently strong PD-1 signaling can block all cytokine production. Analysis of cytokine production in T cells expressing high levels of PD-1 that see cognate pMHC in the absence of PD-L1 also provides insight into the differential sensitivity cytokines show in response to PD-1 signaling. Although IFN-γ production is unaffected by PD-1 expression in the absence PD-L1 engagement, our data show that IL-2 production is reduced in a manner that is dependent on the PD-1 dose, with 19% of the cells making IL-2 in the absence of PD-1 RNA transfection but 16% of T cells expressing high levels of PD-1 producing IL-2. To illustrate this point better, we compiled the data from all four cytokines from four different donors (Fig. 5B). From these data, one can observe that macrophage inflammatory protein 1 beta (MIP-1β) expression is least affected by PD-1 signaling and that only T cells expressing high levels of PD-1 that come in contact with aAPCs expressing PD-L1 dramatically reduce MIP-1β expression. IFN-γ production is more sensitive to PD-1 than MIP-1β, but TNF-α and IL-2 are clearly the most sensitive to PD-1–mediated inhibition. Moreover, examination of cytokine production in T cells expressing high levels of PD-1 that have been stimulated by K.A2.SL9 aAPCs (with no PD-L1) reveals that MIP-1β and IFN-γ are largely insensitive to high levels of PD-1 expression, whereas IL-2 and TNF-α are sensitive to the low levels of PD-1–binding proteins present on CD8 T cells. These findings reveal that cytokines are differentially susceptible to PD-1–mediated inhibition.
Fig. 5.
IL-2 and TNF-α expression are more sensitive to PD-1–mediated inhibition than IFN-γ or MIP-1β production. (A) Previously stimulated and expanded primary human T cells were transfected with A2-SL9–specific TCR and 0, 0.125, 1, or 10 µg RNA encoding PD-1. After overnight culture, cells were stimulated with the indicated aAPCs for 5 h. The ability of these stimulated CD8 cells to produce MIP1β, IFN-γ, TNF-α, and IL-2 was measured by intracellular cytokine staining. Representative data from donor 1 showing IFN-γ and IL-2 production is plotted in A, and complete data from four donors are plotted in B. Red triangles indicate the samples with K.A2.SL9.PD-L1. Black triangles indicate samples with K. A2. SL9.
High Levels of PD-1 Are Required to Interfere with Cytotoxicity.
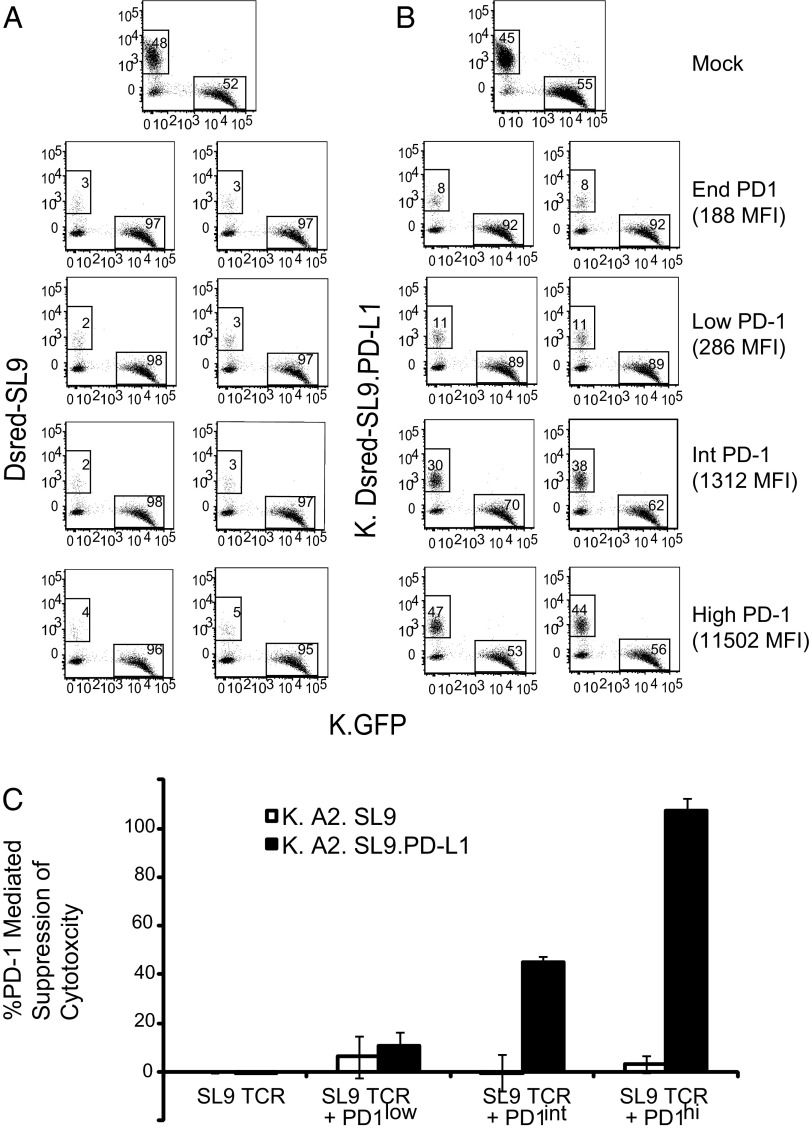
Exhausted T cells exhibit modest to no cytotoxic activity (39, 40). To determine the role that PD-1 expression plays in this T-cell dysfunction, we set up a cytotoxicity assay that would allow us to measure antigen-specific killing in the presence or absence of PD-L1 engagement. In this assay, we mixed HLA-A2–expressing aAPCs in which one line expressed SL9-DsRed minigene fusion and the other line expressed GFP. After 24 h incubation in the presence of non–A2-SL9 TCR-transduced CD8 T cells, we observed that 48% of the aAPCs were red, and 52% of the aAPCs were green. Introduction of A2-SL9 TCR-expressing CD8 T cells having endogenous PD-1 resulted in selective killing of the SL9-DsRed aAPCs regardless of PD-1 expression (Fig. 6A). Incubating the K.GFP and K.A2. DsRed SL9.PDL1 aAPCs with nontransduced T cells for 24 h resulted in a nearly 1:1 ratio (Fig. 6B). SL9-specific TCR-expressing CD8 T cells expressing endogenous or low levels of PD-1 could kill K.A2. DsRed SL9. PDL1 aAPCs. efficiently and selectively Augmenting the expression of PD-1 in these T cells resulted in an ∼50% loss of cytotoxic activity, and T cells expressing high levels of PD-1 lost all cytotoxic activity (Fig. 6C). These results indicate that that only T cells expressing intermediate levels of PD-1 are sensitive to PD-L1–mediated inhibition of cytotoxicity.
Fig. 6.
High levels of PD-1 are required to interfere with cytotoxicity. (A and B) An equal mixture of (A) K.A2.DsRed SL9 and K.A2.GFP or (B) K.A2.DsRed SL9.PD-L1 and K.A2.GFP was cocultured with primary CD8 T cells transfected with A2-SL9–specific TCRs and 0, 0.125, 1, or 10 µg RNA encoding PD-1 at a 1:5 target–to–T-cell ratio in duplicate. After 24 h of culture, cells were stained by eFluor 780 viability dye to exclude dead cells, and GFP and DsRed expression were measured by flow cytometry. (C) The percent suppression of T-cell cytotoxicity was calculated as described in Methods and averaged from three independent experiments. Error bars indicate SD (n = 3).
Discussion
The ability of chronic antigen exposure to induce T-cell dysfunction, often referred to as “T-cell exhaustion,” has been observed in numerous viral and parasitic infections as well as in cancer (2). T-cell exhaustion often leads to disease progression, because the ability of the immune system to keep an infection or tumor in check wanes as T-cell functionality disappears. Key to our understanding of T-cell exhaustion is unraveling the role that negative regulators of T-cell activation (such as PD-1) play in enforcing T-cell exhaustion. Is T-cell exhaustion a differentiation state analogous to Th1, Th2, and so forth, in which high PD-1 expression is largely a marker for exhausted T cells and T-cell dysfunction is programmed by a series of linage-specific transcription factors analogous to T box expressed in T-cells (Tbet), retinoic acid-related orphan receptor γT (RORγt), and forkhead box P3 (Foxp3)? Or is T-cell exhaustion simply the result of the overexpression of negative regulatory molecules such as PD-1 that render otherwise functional T cells less responsive to cognate antigen? One way this question has been addressed is by blocking interactions between negative regulatory molecules and their ligands and asking if the T-cell functional response is improved. If PD-1 blockade quickly reverses T-cell dysfunction, then the role of negative regulators of T-cell activation as enforcers of T-cell exhaustion is supported. If, instead, it takes several days to observe improved T-cell function, then a reasonable interpretation is that PD-1 blockade helped expand the few nonexhausted T cells, and the improvement of T-cell function is the result of selection rather than restoration of T-cell function to terminally exhausted T cells. Studies in LCMV demonstrate that high levels of PD-1 expression are responsible for the dysfunction in some but not all exhausted T cells, because a significant fraction of the T cells does not respond to PD-1 blockade (38). Whether these cells are under the control of additional negative regulators of T-cell activation such as 2B4, Tim-3, and LAG-3 (16) or have terminally differentiated to a state of complete nonresponsiveness is unclear.
Investigations on whether the same dichotomy exists in humans exposed to chronic antigens have been inconclusive thus far. In HIV-1, a series of studies found that in vitro expansion of T cells in the presence in PD-1 blockade resulted in more functional T cells (19–21, 41). Interestingly, in vitro stimulation was required to see the beneficial effects of PD-1 blockade in terms of cytokine secretion, suggesting that PD-1 blockade was promoting the expansion of functional T cells rather than restoring function to fully exhausted T cells. Other studies, however, noted that cytokine production could be elevated immediately after PD-1 blockage, suggesting that PD-1 and PD-L1 interactions were the primary cause of the lack of cytokine production (42, 43). Studying the effects of PD-1 blockade in restoring function to tumor-specific T cells also has resulted in inconsistent and conflicting data. In one study, interfering with PD-1/PD-L1 interactions resulted in augmented numbers of NY-ESO-1–specific T cells isolated from melanoma patients after 6 d of in vitro stimulation, and these T cells had a greater potential to produce effector cytokines than those expanded with PD-1 blockade (44, 45). However, in vitro stimulation was required to observe the beneficial effects of PD-1 blockade, because no differences were noted when studying recently isolated PD-1 high NY-ESO-1–specific T cells, suggesting that the majority of tumor-specific T cells are unresponsive to PD-1 blockade. However, in a similar study examining MART-specific T cells, significant restoration of cytokine production was evident in freshly isolated T cells after PD-1 blockade, suggesting that overexpression of PD-1 was the primary cause of T-cell dysfunction (46). Previously, the association of PD-1 expression and T-cell dysfunction has been investigated by comparing cells expressing low and high levels of PD-1 within a person (47). The difficulty with such comparisons is that cells expressing high levels ofPD-1 likely have a much more extensive replicative history and are more likely to express additional negative regulatory markers, making it difficult to ascertain whether differences in function are the direct result of higher PD-1 expression.
Our studies have approached this question from a different angle. Instead of isolating antigen-specific T cells from patients who vary in disease course, HLA, and medications and asking how PD-1 blockade restores T-cell function, we used RNA engineering to endow T cells from healthy individually with a desired specificity and measured how varying levels of PD-1 expression interfered with T-cell function. This approach allows us to probe exactly how PD-1 expression contributes to T-cell dysfunction without any other complicating factors. Most studies examining T-cell populations isolated from the peripheral blood of patients show that exhausted T cells have approximately three- to 10-fold more PD-1 than effector T cells (19, 44, 48). However, PD-1 expression on exhausted T cells can be modulated further by the severity of the infection, the size of the tumor (38), and the location of the T cell within the body (12, 39). Therefore, to account for these various scenarios, we studied a wide range of PD-1 expression. Our studies clearly show that high levels of PD-1 expression are fully capable of blocking all T-cell functions and that medium levels of PD-1 expression, comparable to those observed on exhausted T cells isolated from the peripheral blood, are able to reduce severely most but not all of a T cell’s effector functions. This result suggests that elevated levels of PD-1 expression are sufficient to render fully functional T cells nonresponsive when stimulated by an APC expressing PD-L1. This result, of course, does not exclude the role other negative regulatory molecules may play in enforcing T-cell exhaustion, nor does it rule out that PD-1 blockade could select positively for pathogen-specific T cells with low levels of PD-1 expression, but it does demonstrate that high levels of PD-1 expression play a prominent role in mediating the T-cell exhaustion phenotype. Moreover, our data examining how the strength of PD-1 signaling differentially affects T-cell function provide insight into the studies that have examined PD-1 blockade in vivo. Several in vivo models in which PD-1 antibodies were used to treat LCMV (4), toxoplasma (49), malaria (50), and simian immunodeficiency virus (51, 52) infection show in an increase of antigen-specific T cells after PD-1 antibody blockade. The blocking of T-cell expansion by very low levels of PD-1 suggests that the amount of PD-1 antibody used in vivo was saturating and was able to interfere with the great majority of PD-1 interactions with APCs. In contrast, restoration of IL-2 expression in exhausted T cells was rarely, if ever, observed with short-term (less than 6 h) PD-1 blockade. This result suggests that, in addition to PD-1, other negative regulatory pathways or T-cell differentiation programs are involved in suppressing IL-2 production. Thus, the value of these studies is that they define effect of PD-1 engagement, allowing one to start to investigate the role of other pathways and molecules in mediating T-cell exhaustion. In particular, these studies should aid in the analysis of PD-1 blockade in human clinical trials (13, 14), which are showing promise in cancer and likely other clinical settings.
Last, our studies provide some mechanistic insight into how PD-1 ligation alters a T-cell response to pMHC. Previous studies by our group and others demonstrated that PD-1 interfered with TCR-generated signals (25, 53, 54). By studying the effects of PD-1 engagement on the single-cell basis, we are able to characterize further how PD-1 signaling interferes with T-cell activation. Several years ago, the Davis laboratory developed a technique using a time-lapse 3D fluorescence microscopy and fluorescently labeled peptides to count the number of pMHC complexes colocated at a particular murine APC and T-cell intersection (33–35). These studies have established a hierarchy of T-cell effector functions in which Ca2+ flux can be elicited with approximately one pMHC complex, killing requires approximately three pMHC complexes, and formation of a stable immunological synapse and cytokine production requires more than ∼10 pMHC complexes. We have found that murine and human T cells trigger Ca2+ with as little as one single pMHC/TCR interaction, and maximal Ca++ flux was observed when 10 pMHC engaged their cognate TCRs. Engagement of PD-1 by its ligand PD-L1 dramatically shifted this dose–response curve, with maximal Ca2+ flux requiring 30 engaged TCRs. The ability of PD-1 to alter a T cell’s sensitivity to TCR signals provides insight into the strength of TCR signaling required to induce cytokine production. We found that IL-2 and TNF-α production is the most sensitive to PD-1 signals and thus likely requires strong TCR-mediated signals to be produced. Thus, by measuring how resistant a particular T-cell function is to PD-1 signaling, we gain insight into the strength of the TCR signaling required to induce this T-cell function.
Methods
Cell Isolation and aAPC Preparation.
De-identified, purified human CD8 T cells were obtained from the Human Immunology Core of the University of Pennsylvania under an Institutional Review Board-approved protocol. aAPCs were generated by transducing lentiviral vectors encoding the desired genes into either K652 or T2 cells which then were sorted to isolate single clones as previously described (55).
RNA in Vitro Transcription and Electroporation of T Cells.
The RNA was generated in vitro by mMESSAGE mMACHINE T7 Ultra per the manufacturer’s suggestions (Life Technologies) and was purified by the RNeasy Mini Kit (Qiagen). Before electroporation, T cells were washed three times with Opti-MEM (Invitrogen) and were resuspended in Opti-MEM to a final concentration of 1–2 × 108/mL. Subsequently, 0.1 mL of the cells was mixed with 10 µg (or as otherwise indicated) in vitro-transcribed RNA and was electroporated in a 2-mm cuvette using BTX EM830 (Harvard Apparatus BTX) as previously described (56).
Calcium Imaging.
To generate a monolayer, the indicated aAPCs were attached to poly-l-lysine–coated coverslips by incubation at 37 °C for 40 min and extensive washing. The coverslip then was assembled in a FCS2 flow chamber (Bioptech) in which a 0.3-mm space was formed between the aAPC-coated coverslip and another coverslip on top. The chamber was mounted onto a Zeiss Axioplan2 microscope under a 20× air objective (NA = 0.75), and the temperature was maintained at 37 °C. T cells pulsed with the Ca2+-sensitive dye Fura-2 AM (Life Technologies) were injected into the chamber, and the 510-nm emissions excited by 340 nm and 380 nm were captured immediately for 45 min at 5-s intervals. The narrow space between the two coverslips of the chamber facilitated nearly simultaneous contact of injected T cells with the aAPC monolayer within 1 min of T-cell injection. Images were captured and analyzed using SlideBook 5 software (Intelligent Imaging Innovations). The intensity of emissions of individual T cells was followed throughout the time course and was analyzed using masking and particle tracking based on 340-nm intensity. An increase in the ratio of 510-nm emission excited by 340 nm to that excited by 380 nm indicated increased intracellular Ca2+ level. T cells with a ratio more than two times greater than before stimulation were considered to have fluxed Ca2+.
Surface and Intracellular Cytokine Staining.
For intracellular cytokine staining, T cells (CD4 mixed with CD8 cells at a ratio of 1:1) were activated by CD3CD28 beads at a ratio of 1:3. Beads were removed at day 3, and culture continued until day 9. Then the T cells were cryopreserved until the day before the experiment. After thawing, T cells were electroporated, cultured overnight, and then were mixed with K562 at a ratio of 1:2 and were incubated for 4 h with GolgiStop (BD). Surface staining was performed, followed by intracellular staining, as described previously (30), with an LSR II flow cytometer (BD Biosciences) and FlowJo software (Tree Star). PD-1 was stained with APC anti-PD1 antibody (J105; eBioscience), and SL9 TCRs were stained with PE-A2-SL9–specific tetramers (Beckman Coulter). The following antibodies were used for intracellular staining: APC-H7-CD8 (clone SK1), APC-IL-2 (clone MQ1-17H12), V450-IFN-γ (clone B27), PE-Cy7-TNF (clone MAb11), PerCP-Cy5.5 -MIP-1β (clone D21-1351), PE-Tim-3 (F38-2E2; Biolegend), PE-BTLA (MIH26; Biolegend), PE-CTLA-4 (L3D10; Biolegend), and PE-2B4 (C1.7; Biolegend).
Peptide-Counting Assay.
APCs and T cells were prepared as previously described (33, 34) with some modifications. APCs [T2 (57) or T2 PD-L1] were pulsed with 0.1–50 μM SLYNTVATL (SL9) peptide with a linker and biotinylation site on the C terminus, GGGSGGGSGGGSGGGSK-biotin (synthesized by Bio-Synthesis Inc.) for 2 h at 37 °C, 5% CO2 in RPMI medium (RPMI 1640, 10% FBS, Glutamax-I, sodium pyruvate, non-essential amino acids and penicillin-streptomycin). Cells were washed twice in PBS and resuspended in RMPI medium with 5 μg/mL Fc receptor blocker (BD Biosciences) for 20 min at room temperature. Cells were washed once in PBS and resuspended in staining solution [1% Imject BSA (Thermo Scientific), 5 μg/mL Streptavidin-PE (BD Biosciences), and 0.5% fresh NaN3] for 20 min at room temperature. Cells were washed twice in imaging medium (RPMI medium without Phenol Red), resuspended in ice-cold imaging medium with 0.5% fresh NaN3, and placed on ice until use. T cells expressing SL9 TCR and PD-1 were labeled with 5 μM Fura-2 AM (Molecular Probes) in imaging medium for 20 min at room temperature, washed once, and resuspended in imaging medium. The fluorescent imaging was done on a Zeiss Axiovert microscope controlled by Metamorph (Molecular Devices) with a 40× objective and a XL-3-TIRF Incubator (PeCon) kept at 37 °C, 5% CO2. Data acquisition and analysis were performed as previously described (33, 34). For statistical analysis, a square-root transformation was applied to the data followed by a linear fit. The residues of the fit were compared using the Mann–Whitney test.
Proliferation Assay.
RNA-transfected T cells were labeled with CFSE and stimulated with the indicated aAPCs. Five days later, CFSE dilution was analyzed as previously described (58). Data were acquired on a LSR II flow cytometer using the FACSDiva software. Percent suppression was calculated using the formula: percent T-cells responding in unsuppressed condition minus percent T-cells responding in suppressed condition divided by percent T-cells responding in unsuppressed condition × 100.
Cytotoxicity Assay.
The ability of T cells to kill target cells expressing cognate pMHC was measured as previously described (59) with some modifications. Instead of labeling target cells with dyes, lentiviral vectors that expressed a minigene that fused the 50 amino acids surrounding the SL9 epitope coupled to DsRed (60) or GFP were used. K562-based targets were washed twice and suspended in cytotoxicity medium (R10 medium without antibiotics). Effector T cells were washed and suspended at 5 × 106 cells/mL An equal mixture of K.A2.SL9 or K.A2.SL9.PD-L1 and K.GFP cells were mixed with T cells at a ratio of five T cells to one aAPC (5:1). After 24 h of culture, CountBright Absolute Counting Beads (Life Technologies) were added to each well. Cells then were transferred to FACS tubes and stained by Fixable Viability Dye eFluor 780 as described by the manufacturer (eBioscience). Analysis was gated on viability dye-negative (live) cells, and the percentages of live green and red aAPCs were determined. Percent suppression was calculated using the formula: percent T-cell cytotoxicity in unsuppressed condition minus percent T-cell cytotoxicity in suppressed condition divided by percent T-cell cytotoxicity in unsuppressed condition × 100.
Supplementary Material
Acknowledgments
We thank the Center for AIDS Research Immunology Core for providing the purified immune cells used in this study; John Wherry and Mike Dustin for helpful suggestions; and Caitlin Baiduc and Julie Jadlowsky for proofreading. This work was supported by the National Institutes of Health Grants P01 P01AI080192 (to J.L.R, M.K., G.J.F., and R.A.) and U01CA137070 (to M.K.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 10892 (volume 110, number 27).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305394110/-/DCSupplemental.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 5.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31(2):309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley JL, June CH. The road to recovery: Translating PD-1 biology into clinical benefit. Trends Immunol. 2007;28(2):48–50. doi: 10.1016/j.it.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20(3):337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radziewicz H, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81(6):2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brahmer JR, et al. (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28(19):3167–3175. [DOI] [PMC free article] [PubMed]
- 14. Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opinion Immunol 2:207–212. [DOI] [PMC free article] [PubMed]
- 15.Duraiswamy J, et al. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186(7):4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahan RH, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120(12):4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 20.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 22.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett F, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: Attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170(2):711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 24.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 25.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98(24):13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J Immunol. 2006;176(11):6603–6614. doi: 10.4049/jimmunol.176.11.6603. [DOI] [PubMed] [Google Scholar]
- 28.Patsoukis N, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11(23):4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varela-Rohena A, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14(12):1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallo EM, Canté-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7(1):25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 32.Wang S-F, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS ONE. 2011;6(3):e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419(6909):845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 34.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5(5):524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 35.Li QJ, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5(8):791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 36.Messal N, Serriari NE, Pastor S, Nunès JA, Olive D. PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011;48(15-16):2214–2219. doi: 10.1016/j.molimm.2011.06.436. [DOI] [PubMed] [Google Scholar]
- 37.Lichterfeld M, et al. Telomerase activity of HIV-1-specific CD8+ T cells: Constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008;112(9):3679–3687. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci USA. 2008;105(39):15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn SD, et al. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: Implications for CD8 T-cell exhaustion. J Virol. 2010;84(4):2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urbani S, et al. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: Effect of viremia levels and antiviral treatment. J Hepatol. 2008;48(4):548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 41.D’Souza M, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179(3):1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J-Y, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109(11):4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 43.Porichis F, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118(4):965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourcade J, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182(9):5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong RM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, et al. Dynamic decrease in PD-1 expression correlates with HBV-specific memory CD8 T-cell development in acute self-limited hepatitis B patients. J Hepatol. 2009;50(6):1163–1173. doi: 10.1016/j.jhep.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 48. Nakamoto N, et al. (2008) Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 134(7):1927–1937. [DOI] [PMC free article] [PubMed]
- 49.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci USA. 2011;108(22):9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velu V, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dyavar Shetty R, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122(5):1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175(11):7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheppard KA, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 55. Suhoski MM, et al. (2007) Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther 15(5):981–988. [DOI] [PMC free article] [PubMed]
- 56.Zhao Y, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 58.Golovina TN, et al. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS ONE. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermans IF, et al. The VITAL assay: A versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J Immunol Methods. 2004;285(1):25–40. doi: 10.1016/j.jim.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 60.Plesa G, et al. TCR affinity and specificity requirements for human regulatory T-cell function. Blood. 2012;119(15):3420–3430. doi: 10.1182/blood-2011-09-377051. [DOI] [PMC free article] [PubMed] [Google Scholar]