Dopamine (DA) disruption is implicated in the neuropathology of multiple brain disorders (schizophrenia, addiction, attention deficit hyperactivity disorder, Parkinson disease) and it is the main target of antipsychotics, stimulants, and antiparkinson medications. Although with PET and single-photon emission computed tomography one can measure DA signaling in the human brain, the application of these methodologies has been limited by its cost (PET), limited access to radiotracers, and the restrictions imposed by the use of radiation. Thus, alternative imaging modalities that overcome these limitations could expand their utilization in clinical research and would bring us closer to a more accessible biomarker for assessing brain DA activity in the clinical setting. In PNAS, Sander et al. (1) demonstrate a dynamic coupling between DA D2 receptor (D2R) occupancy level, a marker of DA neurotransmission, and the concomitant regional cerebral blood volume (rCBV) changes in the primate brain.
Using simultaneous PET and MRI with pharmacological doses of a DA D2/D3 antagonist (raclopride), Sander et al. (1) demonstrate that MRI can provide a good surrogate for the D2R measures traditionally obtained with PET. From a technical perspective, the authors’ findings help clarify the nature of the coupling between striatal D2R blockade and the consequent cerebrovascular responses that presumably reflect changes in neuronal/glial activity, which has been an area of intense investigation in the functional MRI (fMRI) literature (2). Furthermore, the approach could be extended to interrogate different neurotransmitter receptor systems using the appropriate PET radiotracers and pharmacological interventions to explore the functional brain networks that they modulate. From a neuroscience perspective, the report by Sander et al. (1) touches on the role of D2R on striatal activation. The findings of a rCBV increase in the striatum after antagonism of D2R is consistent with the cerebral blood flow increases in the striatum of primates after administration of D2R antagonists (3), as well as with the opposite findings (decreases in striatal activity) after administration of dopamine agonists (4). Thus, the findings of Sander et al. (1) are consistent with the notion that inhibitory D2R signaling predominates over stimulatory D1R signaling in the striatum of primates (5). Taken together with prior results, these findings indicate that inhibition of D2R signaling in the primate brain leads to striatal activation.
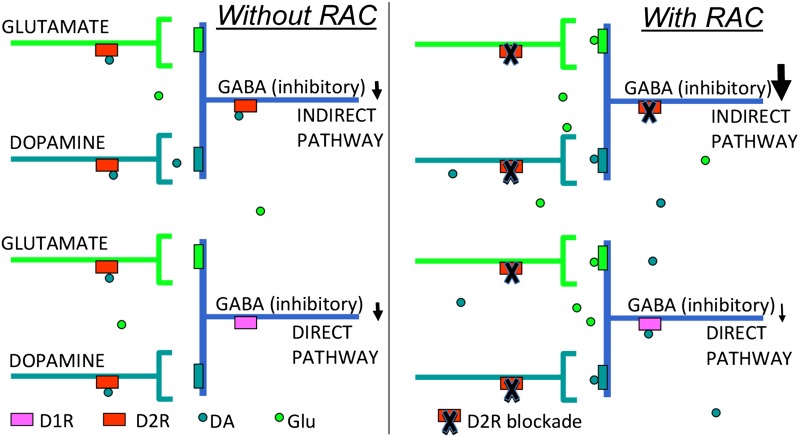
Studies on brain glucose metabolism have demonstrated responses in projection areas rather than in the stimulated area (6). Sander et al. (1) document a regional association between the D2R blockade and concomitant CBV increases in the striatum, which indicates that local processes mediate these effects. In the striatum, D2R are predominantly expressed in postsynaptic GABAergic neurons (middle spiny neurons or MSN), which project to the external globus pallidum, where DA binding to D2R inhibits MSN activity (Fig. 1). However, D2R are also expressed in DA and cortico-striatal glutametergic terminals, where DA binding, respectively, inhibits DA and glutamate release (7). Thus, the increased CBV in striatum with D2R blockade could reflect not only disinhibition of D2R-expressing MSN but also enhanced cortico-striatal glutamatergic signaling, as well as increased DA release with a resultant increase in stimulatory D1R signaling. In addition, D2R are also expressed in acetyl choline and GABA interneurons (2–4% of the total neuronal population in striatum), which could also contribute to the striatal signals. The association between D2R blockade and CBV was limited to the striatum but D2R are also expressed in the cerebral cortex and in midbrain. Failure to observe a similar association in these brain regions may reflect the poor sensitivity of [11C]raclopride for monitoring D2R availability in extrastriatal regions (8).
Fig. 1.
(Left) Schematics for the synaptic neuroransmission in the striatum. GABAergic neurons (MSN) that project to the external globus pallidum express D2R. DA binding to D2R inhibits neuronal activity. Glutametergic and dopaminergic terminals also express D2R, and DA binding to D2R in these terminals inhibits DA and glutamate release, respectively. (Right) Pharmacologic doses of raclopride (RAC) block D2Rs in GABAergic, dopaminergic, and glutamatergic terminals, increasing DA and glutamate release in the basal ganglia with a resultant increase in stimulatory D1R signaling.
Blood vessels in the brain also express D2R, which modulates vascular reactivity. Thus, one could question whether the temporal association could reflect vascular responses resulting from direct D2R blockade in blood vessels. However, this result is unlikely because the relative concentration of D2R in endothelial cells is minimal compared with that in striatal neurons, and as a result the PET signal predominantly reflects [11C]raclopride binding to D2R receptors in neurons. Detection of rCBV changes produced by raclopride is challenging using the endogenous blood-oxygen level dependent (BOLD) fMRI contrast because small CBV changes (<10%) are expected to cause even smaller BOLD signals (<1%) (9). To enhance the rCBV-fMRI signals, Sander et al. (1) inject coated superparamagnetic iron oxide nanoparticles in the blood stream of the primates (10). Although not currently feasible, this approach might prove practical for human studies in the near future, as the contrast agent and MRI technologies constantly evolve to reduce toxicity and improve signal detection levels.
The report by Sander et al. (1) also shows how the integrated use of PET and MRI technologies can be useful for addressing relevant questions regarding the role of striatal DA activity in the primate brain. The study also illustrates the power of simultaneous PET/MRI imaging in helping develop surrogate markers of DA activity (or other brain neurotransmitters) that could expand their use in clinical research and eventually lead to biomarkers for use in clinical practice.
Footnotes
The authors declare no conflict of interest.
See companion article on page 11169.
References
- 1.Sander CY, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci USA. 2013;110:11169–11174. doi: 10.1073/pnas.1220512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30(3):700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 2005;180(4):705–715. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- 4.Mandeville JB, et al. fMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology. 2011;36(6):1187–1198. doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: Studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22(1):294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz WJ, et al. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979;205(4407):723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21(4):519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, et al. Predominance of D2 receptors in mediating dopamine’s effects in brain metabolism: effects of alcoholism. J Neurosci. 2013;33(10):4527–4535. doi: 10.1523/JNEUROSCI.5261-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge: Cambridge Univ Press; 2002. [Google Scholar]
- 10.Mandeville JB. IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage. 2012;62(2):1000–1008. doi: 10.1016/j.neuroimage.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]