Abstract
Our recent studies suggest a role for the proteasome activator REG (11S regulatory particles, 28-kDa proteasome activator)γ in the regulation of tumor protein 53 (p53). However, the molecular details and in vivo biological significance of REGγ-p53 interplay remain elusive. Here, we demonstrate that REGγ-deficient mice develop premature aging phenotypes that are associated with abnormal accumulation of casein kinase (CK) 1δ and p53. Antibody array analysis led us to identify CK1δ as a direct target of REGγ. Silencing CK1δ or inhibition of CK1δ activity prevented decay of murine double minute (Mdm)2. Interestingly, a massive increase of p53 in REGγ−/− tissues is associated with reduced Mdm2 protein levels despite that Mdm2 transcription is enhanced. Allelic p53 haplodeficiency in REGγ-deficient mice attenuated premature aging features. Furthermore, introducing exogenous Mdm2 to REGγ−/− MEFs significantly rescues the phenotype of cellular senescence, thereby establishing a REGγ-CK1-Mdm2-p53 regulatory pathway. Given the conflicting evidence regarding the “antiaging” and “proaging” effects of p53, our results indicate a key role for CK1δ-Mdm2-p53 regulation in the cellular aging process. These findings reveal a unique model that mimics acquired aging in mammals and indicates that modulating the activity of the REGγ-proteasome may be an approach for intervention in aging-associated disorders.
Keywords: casein kinase 1, PA28γ
Premature aging refers to unusual acceleration of the natural aging process and is induced by multiple factors such as genetics, environment, and stress conditions. Many biological markers of premature aging have been described over the past century, including blindness, gray/yellow hair, ear atrophy, osteoporosis, lordokyphosis of the spine, reduced hair regrowth, delayed wound healing, and a shortened lifespan (1, 2). Recently, progress has been made in understanding some of the mechanisms of premature aging (3, 4). DNA damage, oxidative stress, and mitochondrial DNA (mtDNA) mutations are associated with premature aging and may be contributing agents. Furthermore, abnormalities in several cancer-related proteins such as cyclin-dependent kinase inhibitor 1 (p21), tumor protein 53 (p53), and E2F family of transcription factors (retinoblastoma-associated protein; E2F1) also are known to cause premature aging phenotypes (5–8). Given that longer lifespan is mostly associated with an increased cancer incidence, maintaining the balance between longevity and reduced risk of cancer remains a formidable task.
Discrepancies between proaging and antiaging effects of p53 were observed in different experimental systems. A p53 hypermorphic mouse model that harbored a mutant p53 allele (m-p53) displayed resistance to spontaneous cancers, a shortened lifespan, and premature aging phenotypes (2). The role of p53 in promoting aging is supported by a different mouse model, in which a 44-kDa truncated naturally occurring isoform of p53 (p44+/+) is expressed (7). The p44+/+ mice displayed enhanced p53 activity and phenotypes similar to those in the p53+/m mice. In contrast to the p53+/m and p44+/+ mouse models, a “super p53” mouse model, with one or two extra copies of genomic p53 along with flanking regulatory sequences, showed enhanced p53 response to DNA damage, resistance to both spontaneous and carcinogen-induced tumors, but a normal lifespan compared with wild-type mice (9). A murine double minute (Mdm)2 hypomorphic mouse model (10), that had increased p53, showed a normal lifespan but did not age prematurely compared with wild-type mice. It seems likely that aberrantly regulated and constitutively enhanced p53 activity may promote aging through Mdm2 because both p53+/m and p44+/+ mice lack domains required for interaction with Mdm2. The complexity of p53 regulation is demonstrated by the identification of numerous regulators of Mdm2–p53 interaction, including the recently discovered REGγ proteasome activator (11, 12).
REGγ [also known as 28-kDa proteasome activator (PA28γ), proteasome (prosome, macropain) activator subunit 3 (PSME3), and a 32KD antigen identified by an anti-Ki antibody (Ki)] belongs to the REGγ or 11S family of proteasome activator ‘‘caps’’ that have been shown to bind to and activate the proteasome core (20S) proteasome. It regulates a group of growth-related proteins in a ubiquitin- and ATP-independent manner (13, 14). Previous reports showed that cells in REGγ knockout mice displayed reduced growth, decreased proliferation, and increased apoptosis (15, 16). REGγ also was shown to promote the degradation of several important regulatory proteins, including steroid receptor coactivator 3 (SRC-3), cyclin-dependent kinase inhibitors p21, p16, and p19, in a ubiquitin- and ATP-independent manner (14, 17). More recently, REGγ was known to regulate p53 stability/activity in an Mdm2-dependent manner in vitro (11, 12). Overexpression of REGγ has been linked to progression of some cancers (18, 19). REGγ-dependent regulation of p53 prompted us to investigate whether REGγ−/− mice may display premature aging.
In this study, we demonstrate that depletion of REGγ in mice results in a massive increase of p53 in multiple tissues/cell types and ultimately induces premature aging in a p53-dependent manner. Mechanistically, REGγ was found to directly degrade casein kinase 1 (CK1), which negatively regulates Mdm2. Our findings are in agreement with a recent study (20) revealing a mechanism in the control of Mdm2 stability through joint action of casein kinase 1 (CK1, CK1α, and CK1δ) and Skp, Cullin, F-box containing complex (SCF)beta-TRCP following DNA damage. Our results provide evidence that the REGγ-proteasome system plays a role in the regulation of acquired aging mainly via the CK1-Mdm2-p53 pathway.
Results
Early Aging Phenotypes in REGγ-Deficient Mice.
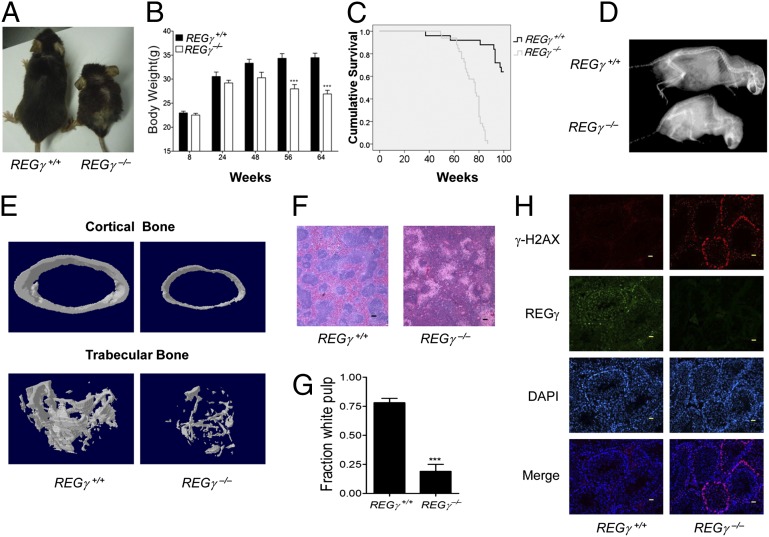
To study the association of REGγ deficiency with aging, we monitored aging-related physical parameters and phenotypes of REGγ+/+ and REGγ−/− mice from birth to death. Up to 12 mo of age, REGγ−/− mice appeared morphologically identical to their REGγ+/+ littermates except for slightly reduced body size and body weight. After 12 mo of age, REGγ−/− mice gradually displayed signs of premature aging (2, 21, 22). The body size and body weight of REGγ−/− male mice were markedly reduced after 56 wk (Fig. 1 A and B), and more than 75% of the aged REGγ−/− mice developed blindness compared with 0% in age-matched REGγ+/+ counterparts (Fig. S1A). Similar to the m-p53 mice, REGγ−/− mice had a shortened lifespan compared with REGγ+/+ controls. Comparison of the REGγ+/+ and REGγ−/− survival curves indicated that the average life expectancy of REGγ+/+ mice was approximately 1.5-fold longer than that of the REGγ−/− mice (Fig. 1C). By 18 mo, signs of lordokyphosis (hunchback spine) were obvious in REGγ−/− mice as shown by X-ray analysis (Fig. 1D). Histological examination of cross-sections from 20-mo-old REGγ+/+ and REGγ−/− mice showed clear reduction in cortical and trabecular bone mineral density in the REGγ−/− tibia (Fig. 1E and Fig. S1B).
Fig. 1.
Early aging phenotypes in REGγ-deficient mice. (A) Photograph of a pair 24-mo-old REGγ+/+ and REGγ−/− male mice. (B) Body weights of age-matched male REGγ+/+ (n = 7) and REGγ−/− (n = 11) mice after 56 wk. *P < 0.05; **P < 0.01; ***P < 0.001. (C) Longevity in REGγ+/+ (n = 25) and REGγ−/− (n = 33) mice. (D) Whole-body radiograph of 20-mo-old REGγ+/+ (n = 20) and REGγ−/− (n = 28) mice. (E) Images representing cortical (Upper) and trabecular (Lower) microstructures of the femurs from 20-mo-old REGγ+/+ and age-matched REGγ−/− mice. (F) Hematoxylin-eosin (HE)-stained cross-section of 20-mo-old REGγ+/+ and REGγ−/− mouse spleen showing the T- and B-cell containing white pulp regions. (G) The mean fraction of white pulp in the spleen of 20-mo-old REGγ+/+ and REGγ−/− mice (n = 5, P < 0.001). (H) Determination of γ-H2AX–positive cells in testis crytosections of 20-mo-old mice. (Magnification: 20×.) (Scale bars: 100 μm.)
It has been reported that the development of T and B cells declines with age (23). The spleen is the largest secondary immune organ and plays important roles in the immune system (24). In this study, the T-cell and B-cell containing white pulp regions in the spleen of 20-mo-old REGγ−/− mice were obviously reduced compared with age-matched REGγ+/+ male mice (Fig. 1F). Statistical analysis showed significant reduction in the white pulp regions in the 20-mo-old REGγ−/− mice (Fig. 1G). Histone phosphorylation of histone H2A (H2AX) phosphorylation, a marker for DNA damage, has been shown to accumulate in senescent human cells and aged mice (21, 25). Thus, we characterized γ-H2AX in 20-mo-old REGγ−/− and REGγ+/+ mice. Testes from REGγ−/− mice displayed age-dependent accumulation of γ-H2AX–positive cells (Fig. 1H). Western blot analysis revealed a large increase of γ-H2AX expression in multiple tissues from 20-mo-old REGγ−/− mice, including kidney, testis, and skin (Fig. S1C). Furthermore, numerous REGγ−/− mice began to develop ear atrophy and gray/yellow hair at the age of 12–15 mo. The aging-associated phenotypes in REGγ+/+ and REGγ−/− mice are summarized in Table S1. These data clearly demonstrate that REGγ deficiency can promote premature aging in mice.
Aging-Related Anomalies in REGγ−/− Skin.
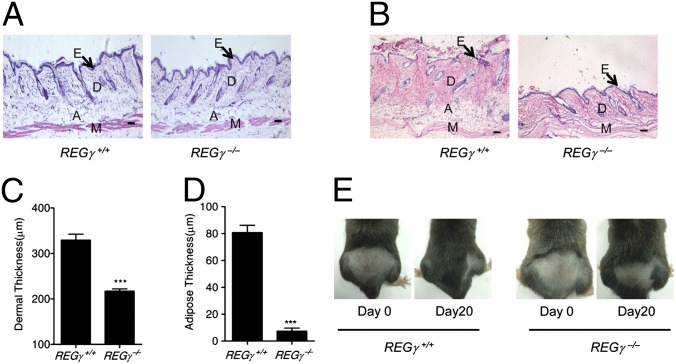
Skin changes are among the most visible signs of aging. Reduced dermal thickness and s.c. adipose tissues are two markers for aged skin in humans (2). Although there were no significant abnormal skin phenotypes in REGγ knockout mice at the age of 2 mo (Fig. 2A), analysis of hematoxylin/eosin (H&E)-stained sections of skin from older (22-mo-old) mice revealed an obvious reduction in the dermal thickness and s.c. adipose of the REGγ−/− skin in comparison with REGγ+/+ controls (Fig. 2B). Differences in the skin anatomy between the age-matched groups were statistically validated (Fig. 2 C and D).
Fig. 2.
Aging related anomaly in REGγ−/− skin. (A) Cross-sections of dorsal skin from 2-mo-old REGγ+/+ and REGγ−/− mice. (Magnification: 10×.) Epidermis (E), dermis (D), adipose under the dermis (A), and muscle (M) are indicated. (B) Cross-sections of dorsal skin from a 22-mo-old REGγ+/+ and an age-matched REGγ−/− mouse. (Magnification: 10×.) Arrows denote the epidermis (E). Dermis (D), adipose under the dermis (A), and muscle (M) are indicated. (C and D) Average dermal thickness (C) in REGγ+/+ and REGγ−/− mice and average thickness of s.c. adipose (D) in REGγ+/+ (n = 4) and REGγ−/− (n = 4) mice (P < 0.001). (E) Representative photos of 20-mo-old REGγ+/+ and REGγ−/− male mice at 20 d after hair removal on a dorsal area. (Scale bars: 50 μm.)
Hair regrowth declines linearly as a function of age in mice (2). A hair regrowth assay was performed to understand the full impact of REGγ deficiency during aging. Almost no hair regrowth was observed in 22-mo-old REGγ−/− mice, whereas REGγ+/+ mice exhibited normal hair regrowth 20 d after shaving a dorsal segment of skin (Fig. 2E and Fig. S1D). It is obvious that hair regrowth is delayed upon loss of REGγ function, indicating that REGγ deficiency exacerbates this aging-related disorder.
REGγ Interacts with CK1δ and Promotes Its Degradation.
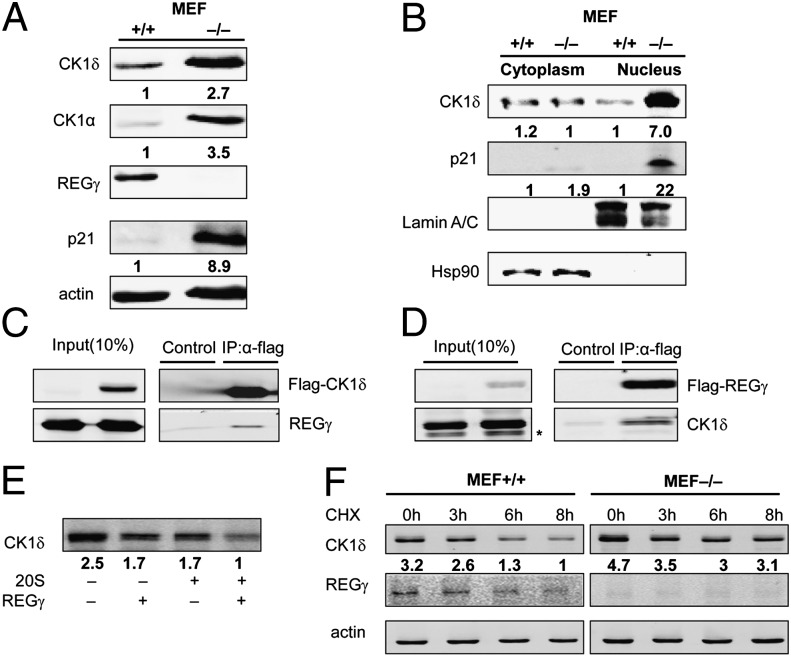
To understand the molecular basis of REGγ-mediated regulation of target proteins such as Mdm2/p53, we carried out a large-scale proteomic screen by using antibody arrays (Full Moon BioSystems) to identify proteins differentially regulated in REGγ-positive and REGγ-null MEF cells. The Full Moon arrays contain antibodies against phospho and total proteins (26), including nearly 1,300 proteins in more than 30 different regulatory pathways. In these antibody arrays, we discovered an increased expression of CK1α in REGγ−/− MEFs. Coincidentally, a recent study has revealed a new mechanism in the control of Mdm2 stability by joint action of CK1 (CK1α and CK1δ) and SCFβ-TRCP (20). To test whether REGγ is involved in this pathway to regulate Mdm2/p53 stability, CK1 protein and mRNA levels were examined by using cell extracts from different cell types or mouse tissues. The results showed that CK1 protein levels were higher in REGγ-deficient cells (Fig. 3A), whereas CK1 mRNA expression was not changed in REGγ+/+ and REGγ−/− MEFs (Fig. S2A). CK1δ is more important than other CK1 isoforms in the regulation of Mdm2 (20), so our subsequent studies have focused mainly on CK1δ.
Fig. 3.
REGγ can interact with CK1δ and directly degrade CK1δ. (A) Expression of REGγ, p21, and CK1α, CK1δ in REGγ+/+, and REGγ−/− MEF cells. (B) Depletion of REGγ blocks nuclear decay of CK1δ. Equal amount of cell extracts was subjected to cytoplasmic and nuclear fractionation, followed by Western blot analysis using indicated antibodies. Lamin A/C and Hsp90 was used as a marker for nuclear and cytoplasm fractions. (C) Interaction between REGγ and CK1δ in 293T cells was determined by coimmunoprecipitation and Western blot analysis following transient transfection of 1μg of FLAG-CK1δ, 10 µg of REGγ, or 10 µg of FLAG-vector control into 293T cells. (D) Reciprocal interaction between REGγ and CK1δ was performed by coimmunoprecipitation as indicated following transient transfection of 2 μg of FLAG-REGγ, 2 µg of CK1δ, or 2 µg of FLAG-vector control into 293T cells. *, nonspecific bands. (E) In vitro proteolytic analysis of REGγ-mediated degradation of CK1δ. Purified REGγ, 20S proteasome, and in vitro-translated CK1δ were incubated as indicated and described in Materials and Methods. (F) Stability of endogenous CK1δ in REGγ+/+ and REGγ−/− MEFs. MEFs were treated with CHX (100 µg/mL) for indicated times followed by Western blotting.
Because REGγ is mostly localized in nucleus and some CK1δ are sequestered in cytoplasm, we carried out cell fractionation to show that depletion of REGγ mainly blocked degradation of CK1δ in the nuclear fractions comparing the REGγ+/+ and REGγ−/− MEFs (Fig. 3B). Consistently, there were increases in CK1δ in multiple tissues from 20-mo-old REGγ+/+ and REGγ−/− mice (Fig. S2B). We previously generated stable HeLa cell lines constitutively expressing either a control nonspecific shRNA (shN) or a REGγ-specific shRNA (shR). The HeLa-shR cells displayed a higher expression of CK1δ compared with HeLa-shN cells (Fig. S2C). We also observed an augmented CK1δ expression when REGγ was transiently silenced in an human colon carcinoma (HCT116) cell line (Fig. S2D). Furthermore, we detected physical interactions between REGγ and CK1δ by transiently expressing combinations of FLAG (a small peptide tag recognized by anti-Flag)-CK1δ/REGγ or CK1δ/FLAG-REGγ along with a FLAG-vector control followed by reciprocal immunoprecipitation in 293T cells. As a result, the FLAG-tagged CK1δ or REGγ successfully coimmunoprecipitated untagged REGγ or CK1δ, whereas the FLAG vector failed to pull down proteins (Fig. 3 C and D). These results further indicate that CK1δ could be a direct target of REGγ.
To gain additional insight into the mechanism of REGγ-mediated CK1δ degradation, we examined the capacity of REGγ to direct cell-free proteolysis (13, 14). Incubation of purified CK1δ with latent 20S proteasome or REGγ alone exhibited no significant degradation of CK1δ beyond nonspecific decay. However, a combination of REGγ and 20S proteasome promoted marked degradation of CK1δ in the absence of additional ATP (Fig. 3E). CK1δ also was degraded faster in REGγ+/+ MEF cells than in REGγ−/− MEFs (Fig. 3F and Fig. S2E). To further demonstrate that CK1δ is degraded by functional REGγ, degradation dynamics were analyzed in 293 cells inducibly expressing a wild-type REGγ or an enzymatically inactive mutant (N151Y) REGγ. In the presence of cycloheximide (CHX), CK1δ decayed faster in the 293 cells expressing wild-type REGγ than in the 293 cells with mutant (N151Y) REGγ (Fig. S2F). Therefore, we conclude that CK1δ is a direct target of REGγ.
REGγ Regulates Mdm2 in a CK1δ-Dependent Manner.
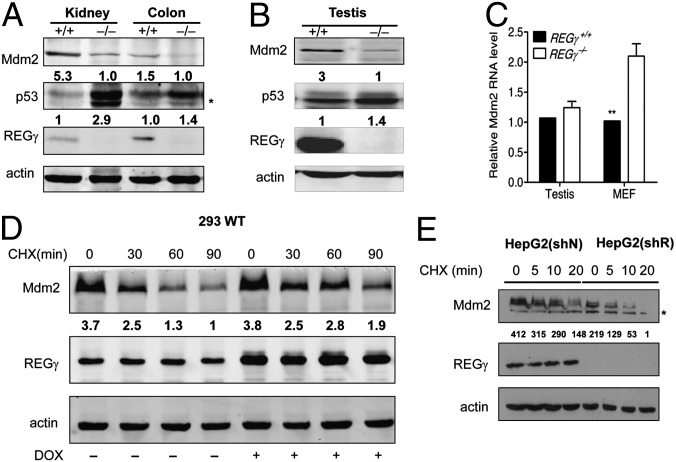
It is known that REGγ can regulate p53 stability/activity in an Mdm2-dependent manner, but the molecular mechanism is unclear. We validated REGγ-mediated dysregulation of p53 levels in vivo by observing large increases in p53 in multiple tissues/cell types in REGγ−/− mice (Fig. S3 A and B). To our surprise, there was a concomitant reduction of Mdm2 in MEF cells depleted of REGγ (Fig. S3C), suggesting that REGγ may regulate Mdm2 stability before its impact on p53. Because Mdm2 is abundant in testis, kidney, and colon tissues (27), we compared Mdm2 expressions in these tissues from REGγ−/− and REGγ+/+ mice. More than 50% reduction of Mdm2 protein level was observed in multiple tissues in young and aged REGγ−/− mice (Fig. 4 A and B). To ensure that REGγ also regulates Mdm2 in a similar fashion in human cell lines, we silenced REGγ in A549 and liver hepatocellular cells (HepG2) cells and also observed a significant reduction in Mdm2 protein levels (Fig. S3 D and E). Interestingly, Mdm2 mRNA levels in REGγ−/− testis and REGγ−/− MEF cells were generally higher compared with those in wild type (Fig. 4C), reflecting a feedback regulation of gene expression by the augmented p53. The seemingly contradictory phenomenon of lower Mdm2 protein and concomitant higher Mdm2 mRNA level in REGγ−/− cells indicates that a faster degradation in Mdm2 protein overrides the mRNA accumulation in the absence of REGγ.
Fig. 4.
REGγ regulates Mdm2 stability. (A) Protein expression of REGγ, p53, and Mdm2 in 22-mo-old REGγ+/+ and REGγ−/− mouse kidney and colon tissues. (B) Expression of REGγ, p53, and Mdm2 in 24-mo-old REGγ+/+ and REGγ−/− testis tissues by Western blotting. (C) Mdm2 mRNA levels in mouse testis and MEF cells were higher in REGγ−/− mice compared with those in REGγ+/+ mice (n = 3) (P < 0.01). (D) Exogenous Mdm2 proteins were measured in 293 cells inducibly expressing REGγ following transiently transfected 0.5 μg of Mdm2 and doxycycline treatment for 48 h, along with CHX (100 µg/mL) for the indicated times. (E) Endogenous Mdm2 proteins were measured in HepG2 cells depleted of REGγ. HepG2 cells stably expressed a control shRNA (HepG2 shN) or an shREGγ (HepG2 shR) were treated with CHX (100 µg/mL) for indicated times before Western blot analysis (*, nonspecific bands).
To verify that REGγ stabilizes Mdm2, we tested Mdm2 stability following overexpression of REGγ in a 293-inducible cell line (14). Exogenously expressed Mdm2 proteins were much more stable in the presence of CHX when REGγ was induced (Fig. 4D). Likewise, endogenous Mdm2 degradation was markedly accelerated in REGγ-diminished HepG2 (shR) cells, which contain a stably integrated shRNA against REGγ, compared with the HepG2 (shN) cells containing a control shRNA (Fig. 4E). These results indicate that REGγ stabilizes Mdm2 and promotes p53 degradation. Given the inability of REGγ to regulate p53 in cells lacking Mdm2 (11), we believe that REGγ modulates Mdm2-p53 expression in a linear order.
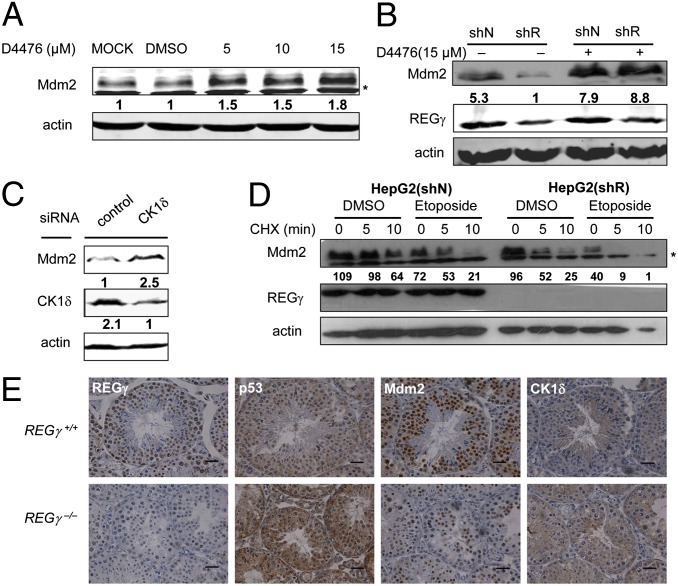
To determine the causal relationships among REGγ, CK1δ, and Mdm2, REGγ-mediated regulation of Mdm2 expression was analyzed before and after treatment with a cell-permeable inhibitor of CK1 (D4476), a chemical inhibitor of CK1. D4476 has been demonstrated to have pronounced specificity for CK1 at lower concentrations (28, 29). With increasing concentrations of D4476, endogenous Mdm2 accumulated in HeLa cells (Fig. 5A), substantiating negative regulation of Mdm2 by CK1. Blocking CK1 activity by D4476 treatment significantly alleviated REGγ deficiency-induced destabilization of Mdm2 in HCT116 (shR) cells (Fig. 5B), indicating that REGγ regulates Mdm2 in a CK1-dependent manner. To exclude the possibility that the CK1 inhibitor could influence Mdm2 stability by targeting other kinases, CK1δ was knocked down by using siRNA in HepG2 cells (Fig. 5C). Silencing CK1δ elevated Mdm2 expression, further suggesting that CK1δ is the major CK1 subtype specifically regulating the Mdm2-p53 pathway.
Fig. 5.
REGγregulate Mdm2 in a CK1δ-dependent manner. (A) Inhibition of CK1 activity enhances Mdm2 levels. HeLa cells were treated with vehicle (DMSO) or the CK1 inhibitor D4476 at different concentrations as indicated for 24 h. Whole-cell lysates were prepared for Western blot analysis (*, nonspecific bands). (B) Blockage of CK1 activity alleviates REGγ-deficiency induced destabilization of Mdm2. HCT116 (ShN) and HCT116 (ShR) cells were treated with vehicle or 15 µM D4476 for 24 h. Mdm2 levels were examined by Western blot analysis. (C) Gene silence of CK1δ elevates Mdm2 level. HepG2 cells were transfected with small interfering casein kinase 1 (siCK)1δ followed by immunoblot analysis of Mdm2 protein expression as indicated. (D) Depletion of REGγ further accelerates DNA damage-induced Mdm2 degradation. HepG2 (ShN) and HepG2 (ShR) cells were treated with 25 µM etoposide or DMSO for 6 h to induce DNA damage. Then the cells were treated with CHX (100 μg/mL) for indicated times followed by Western blot analysis. (E) REGγ-dependent regulation of CK1δ, Mdm2, and p53. IHC staining was performed by using adjacent sections of the same sample to detect the relation between REGγ, CK1δ, Mdm2, and p53 in 20-mo-old REGγ+/+ and REGγ−/− testis. (Scale bars: 50 μm.)
Because Mdm2 protein is quickly degraded in response to DNA damage (20, 30, 31) leading to stabilization and activation of p53, we examined whether REGγ deficiency could expedite DNA damage-induced Mdm2 decay. DNA damage was induced by treating cells with etoposide, a topoisomerase inhibitor producing DNA strand breaks (32), to enhance degradation of Mdm2 (Fig. S4A). We then evaluated dynamic changes of Mdm2 before and after etoposide treatment in HepG2 (shR) and HepG2 (shN) cells. The results revealed an accelerated Mdm2 turnover upon DNA damage in cells with REGγ silencing (Fig. 5D). Given that DNA damage usually accumulates over time, REGγ deficiency in aged mice should have a more profound effect on Mdm2 destruction. In fact, immunohistochemistry (IHC) analysis of REGγ regulated proteins revealed augmented CK1δ, reduced Mdm2, and enhanced p53 expression in testis (Fig. 5E), skin (Fig. S4B) and colon (Fig. S4C) from 20-mo-old REGγ−/− mice, substantiating a linear REGγ-CK1-Mdm2-p53 regulatory pathway.
REGγ Deficiency-Induced Senescence Depends on Dysregulation of Mdm2 and p53 Elevation.
Next, we asked whether REGγ may regulate additional targets other than the Mdm2-p53 pathway to modulate cellular senescence. If regulation of Mdm2-p53 levels by REGγ contributes the major impact on cellular senescence, we expect that manipulation of Mdm2 expression or p53 levels may “rescue” the senescent phenotypes in REGγ−/− cells or tissues. REGγ−/− primary MEFs display a marker of senescence (33, 34) (positive SA-β-galactosidase staining; Fig. S5A) at passage three showing significantly higher numbers of SA-β-gal–positive cells compared with littermate REGγ+/+ MEFs (Fig. S5B). Transducing virally expressed Mdm2 into the REGγ−/− MEF cells significantly reversed the phenotype by reducing the SA-β-galactosidase–positive cells, indicating a key role of Mdm2 in the regulation of cellular aging. Western blot analysis confirmed overexpression of Mdm2 in Mdm2-lentivirus transduced REGγ−/− MEF cells (Fig. S6A).
Because p53−/− mice die early from various tumors, we generated p53+/− REGγ−/− mice to compare with p53+/+REGγ−/− mice for correlations between p53 levels and premature aging phenotypes. Western blot analysis of 20-mo-old testis and skins from littermate p53+/−REGγ−/− and p53+/+REGγ−/− mice always indicated higher p53 levels in p53+/+REGγ−/− tissues than those in p53+/−REGγ−/− tissues (Fig. S5C). IHC analysis of p53 and Mdm2 proteins in the same samples revealed results consistent with that in Fig. S5C (Fig. S6B). To understand the contribution of p53 levels in aging, we analyzed age-associated phenotypes in p53+/−REGγ−/− and p53+/+REGγ−/− mice. The physical parameters and the incidence of premature aging-associated phenotypes, including blindness, gray/yellow hair, ear atrophy, damaged white pulp in spleen, and dermal thickness, were reduced in p53 haplodeficient REGγ−/− mice compared with those in p53+/+REGγ−/− mice up to 16 mo (Fig. S5 D and E). These results demonstrate that p53 dosage-dependent premature phenotypes occur when Mdm2 is deregulated. Taken together with the finding of Mdm2-dependent regulation of p53 by REGγ, we have validated a pathway in which CK1-Mdm2-p53 is sequentially regulated by REGγ. It is a pathway that impacts aging significantly.
Discussion
This study describes a mechanism responsible for REGγ-mediated regulation of aging. We demonstrate that REGγ deficiency causes premature aging phenotypes including blindness, reduced body mass, shortened lifespan, lordokyphosis, and aging-related skin anomalies. We provide evidence that REGγ binds to and directs the degradation of CK1, which promotes degradation of Mdm2 and subsequent dysregulation of p53 (Fig. 6). We established functional roles for components of the REGγ-CK1-Mdm2-p53 pathway in the regulation of aging. Our results emphasize CK1-Mdm2 as the critical players in this pathway and provide an explanation for the dispute over the antiaging and proaging effects of p53.
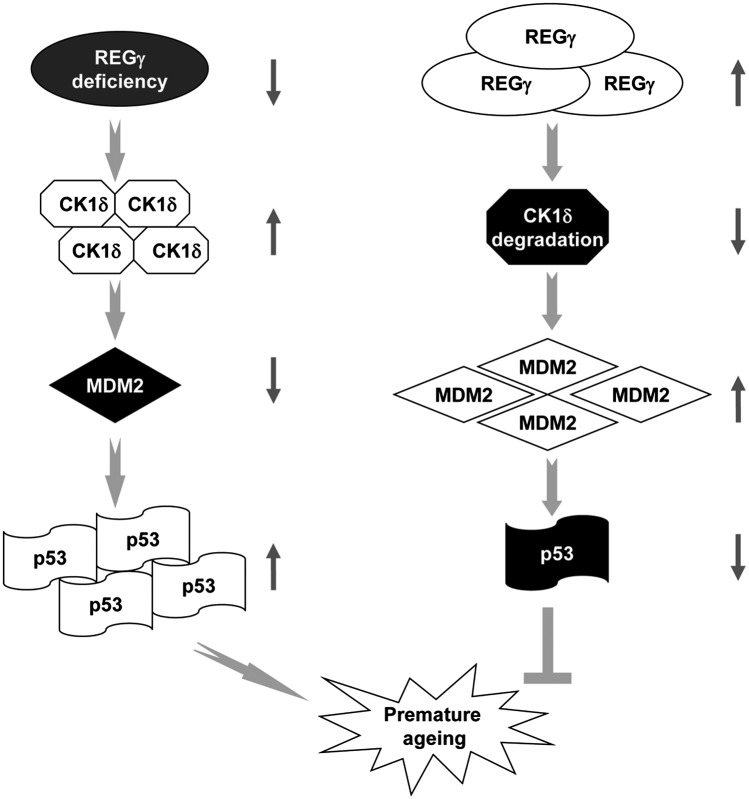
Fig. 6.
Proposed model for the involvement of REGγ in the regulation of premature aging. REGγ deficiency promotes premature aging via accelerated destruction of Mdm2 protein and abnormal accumulation of p53 in a CK1δ-dependent manner. Steady-state levels of REGγ can direct the degradation of CK1δ, which leads to Mdm2 accumulation and p53 degradation, thus results in normal aging. The REGγ-CK1-Mdm2-p53 pathway provides a unique mechanism in the regulation of aging.
Recent studies indicate that blindness, lordokyphosis, reduced body weight, shortened longevity, and increased DNA damage are some of the best-known phenotypes of premature aging (2, 21). In addition to these prominent age-related phenotypes such as lordokyphosis (35), aged REGγ−/− mice display reduction of the white pulp regions in the spleen, indicative of deregulated immune response (23, 36); skin anomalies including reduced dermal thickness, impaired hair regrowth, and wound healing were noted. We found that all REGγ knockout mice developed at least some of these phenotypes characteristic of premature aging, implicating REGγ as a potential target in the control of aging related disorders. Interestingly, none of the aging phenotypes has 100% penetrance in all REGγ−/− mice, which is consistent with cell and tissue specific expression of REGγ in mice (37). It is likely that REGγ deficiency prevents the proliferation of stem cells important to maintain organ homeostasis although further studies are needed to test the effects of REGγ on stem cells. Interestingly, REGγ deficiency barely promotes premature aging in young mice. The same phenomenon also was found in p53 hyperactive mutant mice, in which aging-associated phenotypes are mostly striking in 24-mo-old mice but not in 3-mo-old mice (2).
It already is known that the level of p53 is largely controlled by Mdm2 while Mdm2 expression can be regulated by p53 in an autoregulatory negative feedback loop (38, 39). The distinct longevity between the p53+/m/p44+/+ mice and the super p53 models raise obvious questions about the molecular mechanisms of p53 in aging (2, 7, 10, 21). One possibility is that aberrantly regulated and constitutively enhanced p53 activity may promote aging in cells lacking sufficient or accessible Mdm2. Our results support this hypothesis. The REGγ−/− mouse serves as a true hypomorphic Mdm2 model, where Mdm2 steady-state levels are less than 50% of those in wild-type cells. Therefore, REGγ−/− mice are reminiscent of p53+/m and p44+/+ mice where p53 is elevated because of lack of control from Mdm2 signaling. Interestingly, a recent mouse model lacking Mdm2 in the epidermis showed aging phenotypes in the skin of mice (6). Given the report that Mdm2+/− mice with higher p53 expression do not have aging (10), REGγ−/− mice provide an independent demonstration of the concept that p53-induced premature aging only occurs when Mdm2 is lower than 50% of its normal level during homeostasis. The importance of Mdm2-p53 signaling in aging also is illustrated with the differential aging phenotypes in p53+/−REGγ−/− and p53+/+REGγ−/− mice, highlighting a p53-dependent effect in aging when deregulated.
CK1 is a family of serine-threonine protein kinases that function as regulators of signal transduction pathways in most eukaryotic cells. CK1 was reported to control the phosphorylation of p53 at Ser-20 and has been implicated in aging (40). In addition, CK1δ was shown to phosphorylate p53 at the threonine 18 site of its N terminus upon DNA damage or DNA virus infection (41), inhibiting Mdm2-p53 binding and, therefore, stabilizing p53. CK1 is a major enzyme that mediates TGF-β–dependent activation of p53 with phosphorylation at Ser6/9 in the transactivation domain (42). Furthermore, destructive phosphorylation signals target different sites in Mdm2 via CK1 to promote its turnover (20). CK1 has been implicated in an aging-associated disease, namely Alzheimer’s disease. Indeed, the expression of CK1 has been shown to be up-regulated in the brain of Alzheimer patients (43, 44), and CK1 has been implicated in the phosphorylation of the proteins tau and β-secretase that have been linked to Alzheimer’s disease (45, 46). However, there were reports that several serine residues of Mdm2 in the acidic domain, including Ser-240, Ser-242, and Ser-246, as well as Ser-383 in the C-terminal region, can be phosphorylated by CK1 (47). The phosphorylation of Mdm2 at these sites appears to maintain it in the active form and permit its binding with p53, and causing p53 degradation (38), suggesting complicated regulatory mechanisms for the regulation of Mdm2 function and stability. Our discovery that REGγ regulates the CK1δ-Mdm2-p53 signaling pathway provides insight into the molecular mechanism by which REGγ deficiency and CK1 activation induce premature aging. Identification of CK1δ as a unique target of the REGγ-proteasome system not only expands our knowledge on REGγ substrate spectrum and function, but also substantiates CK1 as a main player in the regulation of aging, further explaining the accelerated degradation of Mdm2 in REGγ−/− cells. Interestingly, we found that blocking CK1δ activity could enhance expression of Mdm2 in steady state or upon DNA damage, indicating that this REGγ-CK1δ signaling may be in play over the entire lifespan. Importantly, we demonstrated that REGγ-mediated regulation of Mdm2 is CK1δ dependent. This result further validated the specificity for the REGγ-CK1δ-Mdm2-p53 pathway in the regulation of aging. However, our study by no means excludes the possibility that other REGγ target proteins could be involved in the regulation of aging.
In summary, this study identifies the REGγ-proteasome as a unique, important pathway for regulation of aging (Fig. 6), demonstrates that REGγ directly promotes degradation of CK1δ, reveals that REGγ stabilizes Mdm2 and further regulates p53 in a CK1δ-dependent manner, and defines the underlying molecular mechanisms by which REGγ deficiency induces early senescence through the CK1δ/Mdm2/p53 pathway.
Materials and Methods
Mice.
REGγ−/− mice with C57BL/6 genetic background were acquired from John J. Monaco (University of Cincinnati College of Medicine, Cincinnati) (15), and p53+/− wt C57BL/6 mice were purchased from Jackson Laboratory. Mice were bred in the Animal Core Facility by following procedures approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. To generate the animals required in this study, we maintained REGγ+/− mice and kept intercrosses between males and females for more than generations. Genotyping of REGγ+/+ and REGγ−/− mice was carried out by PCR analysis of genomic DNA as described (15).
Protein Profiling by the Antibody Microarray.
The Phospho Explorer antibody microarray, which was designed and manufactured by Full Moon BioSystems, contains 1,318 antibodies in duplicate.
Immunoprecipitation, Western Blot Analysis, in Vitro Proteolytic Analysis, and Cell Fractionation.
Immunoprecipitation and Western blot analysis for cells were performed as described (14). The in vitro proteolytic analysis was performed as described (14), and cell fractionation assay were performed as described (11).
SA-β-Galactosidase Staining and Immunostaining.
SA-β-galactosidase staining was performed by using a SA-β-gal staining kit (Genmed Scientifics) as described (48). The staining of γ-H2AX–positive cells were analyzed as described (9).
Hair Regrowth.
The hair regrowth assay was carried out as described (21) except that plucking dorsal skin from a square of ∼3 cm × 3 cm rather than 1.5 cm × 1.5 cm.
Plasmids, Reagents, Cell Culture, RNA Interference and RNA Analyses, Immunohistochemistry Analysis, Bone Density Measurement, and Data Analysis.
Details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Pumin Zhang and Dr. Zhengfeng Yang (Baylor College of Medicine) for their help in this study. This work was supported by National Institutes of Health Grants HD08818, 1R01CA131914, and P30 CA125123 and Norman Hackerman Advanced Research Program Grants 1082318401 and PN004949-0012-2009; and supported in part by Pilot/Feasibility Program Grant P30-DK079638 of the Diabetes and Endocrinology Research Center at Baylor College of Medicine. This manuscript was also funded in part by National Natural Science Foundation of China Grants 81261120555, 81071657, 81272943, 31100946, and 31200878; Science and Technology Commission of Shanghai Municipality Grants 11DZ2260300, 10JC1404200, 11ZR1410000, and 12ZR1409300; National Basic Research Program Grants 2009CB918402 and 2011CB504200; and Fundamental Research Funds for the Central Universities Grants 78210101 and 78210139. This manuscript was also partially supported by a joint grant from the Canadian Institutes of Health Research/the National Natural Science Foundation of China.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308497110/-/DCSupplemental.
References
- 1.Sun LQ, et al. Growth retardation and premature aging phenotypes in mice with disruption of the SNF2-like gene, PASG. Genes Dev. 2004;18(9):1035–1046. doi: 10.1101/gad.1176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyner SD, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 3.Norddahl GL, et al. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8(5):499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227(9):671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 5.Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol. 2000;20(1):273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannon HS, Donehower LA, Lyle S, Jones SN. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev Biol. 2011;353(1):1–9. doi: 10.1016/j.ydbio.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18(3):306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, et al. Cellular senescence and organismal ageing in the absence of p21(CIP1/WAF1) in ku80(-/-) mice. EMBO Rep. 2009;10(1):71–78. doi: 10.1038/embor.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Cao I, et al. Increased p53 activity does not accelerate telomere-driven ageing. EMBO Rep. 2006;7(5):546–552. doi: 10.1038/sj.embor.7400667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendrysa SM, et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20(1):16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, et al. REGgamma modulates p53 activity by regulating its cellular localization. J Cell Sci. 2010;123(Pt 23):4076–4084. doi: 10.1242/jcs.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhang R. Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation. EMBO J. 2008;27(6):852–864. doi: 10.1038/emboj.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124(2):381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Li X, et al. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell. 2007;26(6):831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Barton LF, et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172(6):3948–3954. doi: 10.4049/jimmunol.172.6.3948. [DOI] [PubMed] [Google Scholar]
- 16.Murata S, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274(53):38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell. 2007;26(6):843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao I, Liu J, Li X, Luo H. REGgamma, a proteasome activator and beyond? Cell Mol Life Sci. 2008;65(24):3971–3980. doi: 10.1007/s00018-008-8291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roessler M, et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics. 2006;5(11):2092–2101. doi: 10.1074/mcp.M600118-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Inuzuka H, et al. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell. 2010;18(2):147–159. doi: 10.1016/j.ccr.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matheu A, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448(7151):375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 22.Chen YF, et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23(10):1183–1194. doi: 10.1101/gad.1779509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isobe K, Nishio N, Ito S. [Age-related decline of immune function and age-related diseases] Nihon Rinsho. 2009;67(7):1327–1331. Japanese. [PubMed] [Google Scholar]
- 24.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34(5):455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 25.Sedelnikova OA, et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6(2):168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 26.Bernier M, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286(22):19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry ME, Mendrysa SM, Saucedo LJ, Tannous P, Holubar M. p76(MDM2) inhibits the ability of p90(MDM2) to destabilize p53. J Biol Chem. 2000;275(8):5733–5738. doi: 10.1074/jbc.275.8.5733. [DOI] [PubMed] [Google Scholar]
- 28.Huart AS, MacLaine NJ, Meek DW, Hupp TR. CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability. J Biol Chem. 2009;284(47):32384–32394. doi: 10.1074/jbc.M109.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rena G, Bain J, Elliott M, Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5(1):60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stommel JM, Wahl GM. A new twist in the feedback loop: Stress-activated MDM2 destabilization is required for p53 activation. Cell Cycle. 2005;4(3):411–417. doi: 10.4161/cc.4.3.1522. [DOI] [PubMed] [Google Scholar]
- 31.Stommel JM, Wahl GM. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 2004;23(7):1547–1556. doi: 10.1038/sj.emboj.7600145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stathopoulou A, et al. Cdt1 is differentially targeted for degradation by anticancer chemotherapeutic drugs. PLoS ONE. 2012;7(3):e34621. doi: 10.1371/journal.pone.0034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia L, Li H, Sun Y. Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia. 2011;13(6):561–569. doi: 10.1593/neo.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss A, Arbell I, Steinhagen-Thiessen E, Silbermann M. Structural changes in aging bone: Osteopenia in the proximal femurs of female mice. Bone. 1991;12(3):165–172. doi: 10.1016/8756-3282(91)90039-l. [DOI] [PubMed] [Google Scholar]
- 36.Gress RE, Deeks SG. Reduced thymus activity and infection prematurely age the immune system. J Clin Invest. 2009;119(10):2884–2887. doi: 10.1172/JCI40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu G, et al. Comparative analysis of REGgamma expression in mouse and human tissues. J Mol Cell Biol. 2010;2(4):192–198. doi: 10.1093/jmcb/mjq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1(14):1001–1008. [PubMed] [Google Scholar]
- 39.Zhang R, Wang H, Agrawal S. Novel antisense anti-MDM2 mixed-backbone oligonucleotides: Proof of principle, in vitro and in vivo activities, and mechanisms. Curr Cancer Drug Targets. 2005;5(1):43–49. doi: 10.2174/1568009053332663. [DOI] [PubMed] [Google Scholar]
- 40.Maclaine NJ, Hupp TR. The regulation of p53 by phosphorylation: A model for how distinct signals integrate into the p53 pathway. Aging (Albany, NY Online) 2009;1(5):490–502. doi: 10.18632/aging.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi K, et al. Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. Effect on Mdm2 binding. J Biol Chem. 2000;275(13):9278–9283. doi: 10.1074/jbc.275.13.9278. [DOI] [PubMed] [Google Scholar]
- 42.Cordenonsi M, et al. Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315(5813):840–843. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- 43.Ghoshal N, et al. A new molecular link between the fibrillar and granulovacuolar lesions of Alzheimer’s disease. Am J Pathol. 1999;155(4):1163–1172. doi: 10.1016/S0002-9440(10)65219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasojima K, Kuret J, DeMaggio AJ, McGeer E, McGeer PL. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 2000;865(1):116–120. doi: 10.1016/s0006-8993(00)02200-9. [DOI] [PubMed] [Google Scholar]
- 45.Li G, Yin H, Kuret J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. J Biol Chem. 2004;279(16):15938–15945. doi: 10.1074/jbc.M314116200. [DOI] [PubMed] [Google Scholar]
- 46.Pastorino L, Ikin AF, Nairn AC, Pursnani A, Buxbaum JD. The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total A(beta) Mol Cell Neurosci. 2002;19(2):175–185. doi: 10.1006/mcne.2001.1065. [DOI] [PubMed] [Google Scholar]
- 47.Meek DW, Knippschild U. Posttranslational modification of MDM2. Mol Cancer Res. 2003;1(14):1017–1026. [PubMed] [Google Scholar]
- 48.Zhang DY, Wang HJ, Tan YZ. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS ONE. 2011;6(6):e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.