Abstract
S-prenylation is an important lipid modification that targets proteins to membranes for cell signaling and vesicle trafficking in eukaryotes. As S-prenylated proteins are often key effectors for oncogenesis, congenital disorders, and microbial pathogenesis, robust proteomic methods are still needed to biochemically characterize these lipidated proteins in specific cell types and disease states. Here, we report that bioorthogonal proteomics of macrophages with an improved alkyne-isoprenoid chemical reporter enables large-scale profiling of prenylated proteins, as well as the discovery of unannotated lipidated proteins such as isoform-specific S-farnesylation of zinc-finger antiviral protein (ZAP). Notably, S-farnesylation was crucial for targeting the long-isoform of ZAP (ZAPL/PARP-13.1/zc3hav1) to endolysosomes and enhancing the antiviral activity of this immune effector. These studies demonstrate the utility of isoprenoid chemical reporters for proteomic analysis of prenylated proteins and reveal a role for protein prenylation in host defense against viral infections.
Keywords: antiviral effector, bioorthogonal labeling, click chemistry, protein prenylation, lipid chemical reporter
Protein S-prenylation is a covalent isoprenoid (farnesyl or geranylgeranyl) modification on cysteine (Cys) residues at carboxyl-terminal CaaX or C(X)C motifs (Fig. 1A) (1). The lipid modification increases the hydrophobicity of proteins and enhances their affinity for cellular membranes (1). Prenylated proteins have important and diverse roles in eukaryotic biology, as exemplified by small GTPases such as K/H/Ras in cell growth (2), Rab-family proteins in membrane trafficking (3), and lamin isoforms in nuclear matrix homeostasis (4). Because aberrant expression or mutation of prenylated proteins like K-Ras and lamin A are major drivers of human diseases like cancer or progeria, respectively, prenyltransferase inhibitors that interfere with lipidation of these proteins and their function are under clinical development (5, 6). In addition to cellular proteins, virulence factors from viruses (7-10) and bacterial pathogens (11–14) can be S-prenylated by host enzymes to enhance microbial infection. The analysis of S-prenylated proteins is therefore crucial for understanding fundamental mechanisms of cell biology and human disease, as well as the characterization of drugs targeting protein prenylation.
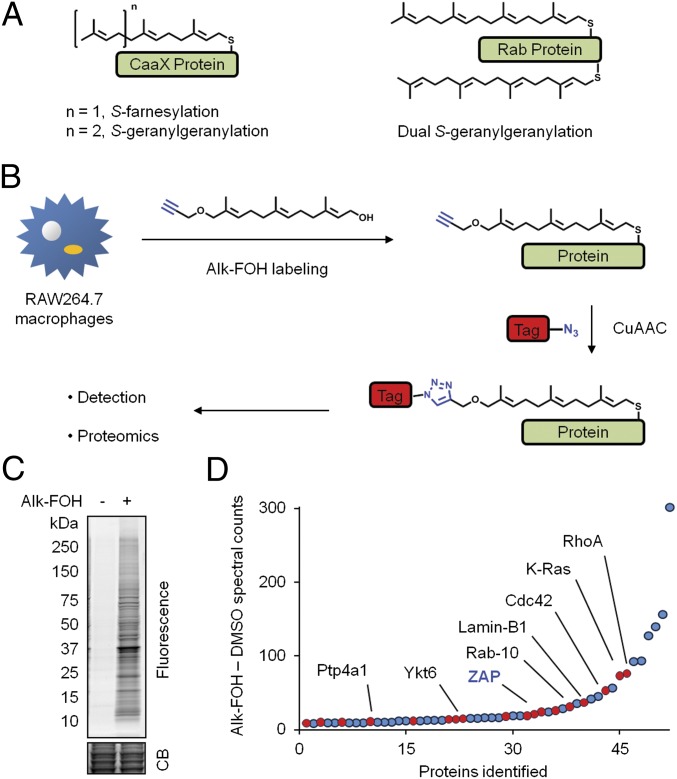
Fig. 1.
Visualization and identification of prenylated proteins in RAW264.7 macrophages. (A) Scheme of S-prenylated proteins with a CaaX motif (Left) and dually S-geranylgeranylated Rab proteins with a C(X)C motif (Right). (B) Metabolic labeling of cells with alk-FOH prenylation reporter and subsequent CuAAC ligation with bioorthogonal detection tags for imaging or proteomics. (C and D) RAW264.7 macrophages pretreated for 12 h with 10 μM lovastatin and then incubated for 12 h with 50 μM alk-FOH or DMSO as a control. In C, cell lysates were reacted with az-rho by CuAAC, and proteins were separated by SDS/PAGE for visualization by fluorescence gel scanning. Coomassie blue (CB) staining demonstrates comparable loading. In D, lysates from lovastatin-treated cells were reacted with az-azo-biotin by CuAAC for enrichment of alk-FOH–labeled proteins with streptavidin beads and identification by mass spectrometry. For each high-confidence identified protein, the difference of assigned peptide spectral counts from the alk-FOH and DMSO samples was plotted. Proteins with a carboxyl-terminal CaaX or C(X)C motif are shown in red. Several known prenylated proteins are labeled in black, and ZAP, the highest ranked protein not known to be prenylated, is labeled in bold blue.
Although bioinformatics predict hundreds of prenylated proteins in eukaryotes based on Cys-Aaa-Aaa-Xaa (CaaX) or Cys-Xaa-Cys [C(X)C] motifs of known substrates (15), only a small fraction has been characterized biochemically and experimentally validated. Although detergent partitioning methods and radioactive lipid labeling have been useful for characterizing prenylated proteins, more sensitive methods for monitoring protein prenylation are still needed (16). To this end, chemical reporters of prenylated proteins that provide better sensitivity than traditional radioactive labeling have now been developed (17, 18). For example, a biotin geranyl diphosphate analog can be used with engineered prenyltransferases to visualize and profile geranylgeranylated proteins in vitro (19). Alternatively, azide-derivatives of farnesol or geranylgeraniol or their diphosphate analogs can be used to detect prenylated proteins after bioorthogonal labeling with fluorescent or affinity tags (20, 21). More recently, alkynyl-isoprenoids have also been developed to enable more efficient labeling and detection of prenylated proteins in mammalian cells compared with their azide-counterparts using Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) (22, 23). The application of these lipid chemical reporters has enabled the enrichment and identification of farnesylated (20) or geranylgeranylated protein subsets (19, 21), but the general proteomic coverage of prenylated proteins has been limited. Using an alkyne-farnesol chemical reporter and improved bioorthogonal proteomic methods, we identified over 100 candidate isoprenoid-labeled proteins in macrophages, including small GTPases as well as previously unannotated S-prenylated substrates such as the zinc-finger antiviral protein (ZAP).
ZAP was originally identified in a rat cDNA overexpression screen for host factors that could significantly impair replication of Moloney murine leukemia virus (MuLV) as an N-terminal protein fused to the Zeocin resistance marker (24). This original ZAP construct consisting of the first 254 amino acids of rat ZAP (rNZAP) fused to the marker (Fig. 2A and Fig. S1) and inhibited the replication of various alphaviruses (25), filoviruses (26) and retroviruses (24, 27), but did not affect host susceptibility to other viruses such as vesicular stomatitis virus, poliovirus, yellow fever virus, and herpes simplex virus type 1 (25). Additional experiments suggested that rNZAP did not interfere with MuLV entry, viral DNA synthesis and integration, and viral RNA production in the nucleus, but decreased the level of posttranscriptional viral mRNA in the cytoplasm (24). Similarly, rNZAP inhibited Sindbis virus (SINV) replication by blocking postentry steps of translation and amplification of incoming viral RNA (25). rNZAP is predominantly localized in the cytoplasm at steady state but shuttles between the cytoplasm and the nucleus in a CRM1-dependent manner (28). rNZAP is also proposed to bind cytoplasmic viral mRNA through its second and fourth CCCH-type zinc-fingers (26, 29) although recent structural studies suggest a role for all four zinc-fingers in forming an RNA binding groove (30). ZAP recruits p72 DEAD-box (31) and DHX30 DEXH-box (32) RNA helicases, and the RNA processing exosome (33) for optimal depletion of viral mRNA. Although early ZAP studies were conducted with rNZAP, the analysis of full-length rat ZAP (rZAP), which bears an additional WWE domain predicted to mediate specific protein–protein interactions in ubiquitin and ADP ribose conjugation systems (34) (Fig. 2A and Fig. S1), suggests similar antiviral activity against MuLV (24). Recent reports have suggested that human ZAP (hZAP) recruits both the 3′ and 5′ mRNA degradation machinery because it binds adenylase poly(A)-specific ribonuclease to remove the poly(A) tail and the decapping complex Dcp1a/Dcp2 to remove the cap structure (27).
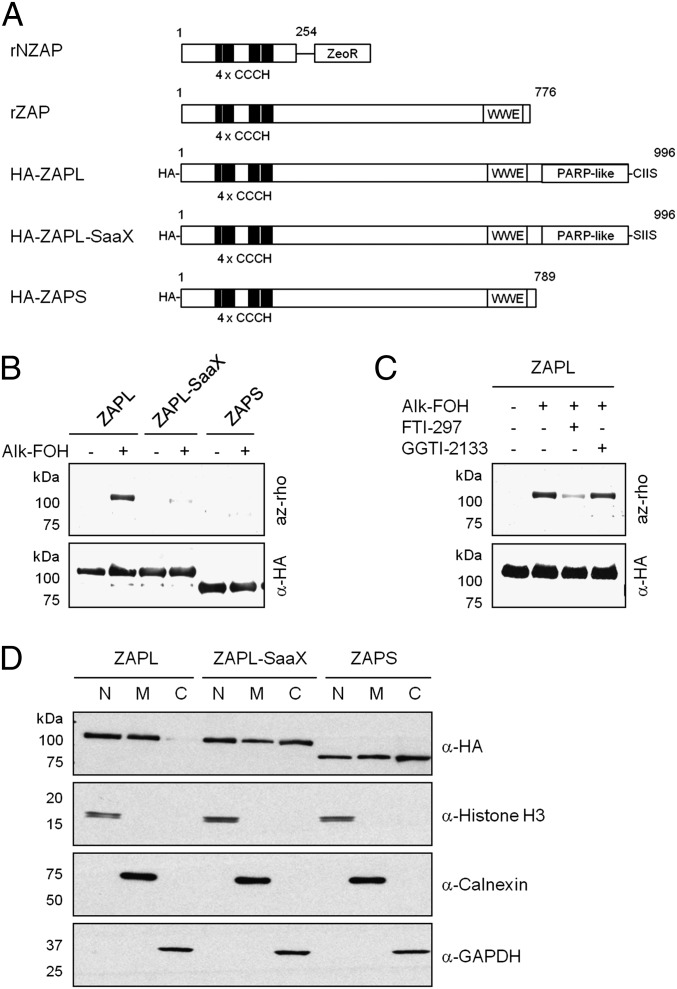
Fig. 2.
S-farnesylation of Cys993 excludes murine ZAPL from the cytosol. (A) Schematic representation of protein domains of rNZAP, rZAP, and mouse HA-tagged ZAP constructs. (B–D) 293T or MEFs were transfected with pCMV-HA-ZAPL, pCMV-HA-ZAPL-SaaX, or pCMV-HA-ZAPS (shown in A) and labeled with 50 μM alk-FOH for 4 h. In B and C, 293T cell lysates were subjected to anti-HA immunoprecipitation, reacted with az-rho by CuAAC, separated by SDS/PAGE, and visualized by fluorescence gel scanning. Comparable protein loading was confirmed by anti-HA Western blotting. In C, cells were pretreated for 1 h with 10 μM prenyltransferase inhibitors (FTI-297, farnesyltransferase inhibitor; GGTI-2133, geranylgeranyltransferase-I inhibitor) as indicated before alk-FOH labeling. In D, MEFs were detergent fractionated into nuclear (N), membrane (M) and cytosolic (C) fractions, and their purity was assessed by anti-histone H3, anti-calnexin, and anti-GAPDH Western blotting, respectively. Cellular localization of HA-ZAP was assessed by anti-HA Western blotting.
There are two ZAP isoforms arising from alternative splicing: ZAP-long (ZAPL) and ZAP-short (ZAPS) (Fig. S1). Both have the same amino acid sequence, but ZAPS lacks the carboxyl-terminal poly(ADP ribose) polymerase (PARP)-like domain present in ZAPL (Fig. 2A) (35). Even though the PARP-like domain of ZAPL is predicted to be inactive, ZAPL/S isoforms have been annotated as PARP-13.1/2 respectively. Human ZAPL exhibits stronger antiviral activity than hZAPS against MuLV expression and Semliki forest virus infection (35) whereas both isoforms prevent HIV-1 infection (27). The ZAP isoforms are relatively broadly expressed in human tissues (35), but the mRNA expression of hZAPS is markedly increased by IFNα treatment (36). hZAPS is also more active than hZAPL in enhancing RIG-I-dependent signaling in response to 5′-triphosphate-modified RNA, suggesting that regulation of ZAP activity can enhance or even amplify IFN responses (36). In addition, ZAPS and ZAPL are both polyADP ribosylated and have been implicated in stabilization of mRNA during cellular stress (37). Interestingly, ZAPS polyADP ribosylation is selectively elevated during cellular stress compared with ZAPL (37). These results highlight a potential duality in the regulation and functions of ZAP isoforms. The role of S-prenylation on ZAPL (PARP-13.1/zc3hav1) localization and antiviral function has not been investigated. Here, we show that S-prenylation enhances the membrane targeting and antiviral activity of ZAPL.
Results and Discussion
Proteomic Analysis of Prenylated Proteins in RAW264.7 Macrophages.
To identify lipid-modified proteins involved in immune responses to microbial infections, we performed a large-scale profiling of prenylated proteins in the mouse macrophage line RAW264.7 (38) using the isoprenoid chemical reporter alkynyl-farnesol (alk-FOH) (22, 23) and CuAAC (Fig. 1B). Alk-FOH targets the substrates of all three prenyltransferases in cells: CaaX S-farnesylated and S-geranylgeranylated proteins, as well as C(X)C RabGTPases (22). In-gel fluorescence profiling of RAW264.7 cell lysates, reacted with an azide-functionalized fluorophore, azido-rhodamine (az-rho) (39), demonstrated that a diverse repertoire of proteins are metabolically labeled by alk-FOH (Fig. 1C). Cell lysates were then reacted with a cleavable affinity tag (az-azo-biotin) (40) for enrichment of alk-FOH–labeled proteins with streptavidin beads, selective elution, and gel-based proteomic identification by mass spectrometry (Fig. 1B). Coomassie blue staining of proteins retrieved with streptavidin beads and sodium dithionite (Na2S2O4) elution demonstrates the specificity of alk-FOH and CuAAC labeling methods (Fig. S2A). Proteins identified in three independent experimental runs were compiled and categorized into high- and medium-confidence lists on the basis of the total number of assigned spectra and the fold-increase above control samples that were not labeled with alk-FOH. For this analysis, we selectively identified 114 proteins by alk-FOH labeling compared with control samples, with 52 and 62 proteins assigned to high- and medium-confidence lists, respectively (Fig. 1D and Tables S1 and S2). The analysis of subcellular distribution suggests that 60% of high-confidence hits were membrane-associated proteins whereas 21% were mitochondrial proteins (Fig. S2B). Of the high-confidence list, 35% (23 + 12) of hits bear a carboxyl-terminal CaaX or C(X)C motif, respectively (Table S1). Of these putative prenylated proteins, 61% of high- and medium-confidence hits have been reported in previous labeling and enrichment studies with biotin, azide, or alkyne chemical reporters in other cell types (Tables S1 and S2). These proteins include K-Ras, Cdc42, Lamin-B1, DnaJA2, Rap2C and Rab proteins (Fig. 1D and Tables S1 and S2). In addition, RhoA, Ptp4A, Ykt6, Rac2, Brox and RhoG, which have predicted prenylation sites, were recovered in our alk-FOH proteomic dataset (Fig. 1D and Tables S1 and S2). In comparison with previous proteomic studies that targeted subsets of S-prenylated proteins (19–21, 23), our proteomic analysis of alk-FOH–labeled proteins recovered both farnesylated and geranylgeranylated proteins, as well as many other candidate isoprenoid-modified proteins (Tables S1 and S2). To validate our alk-FOH–labeled proteins in our dataset, we biochemically characterized a canonical CaaX-containing farnesylated protein (DnaJA2) and an unpredicted substrate (Pcbp1). Analysis of GFP-tagged DnaJA2 constructs demonstrated that alk-FOH labeled this CaaX-containing protein at the predicted site of S-prenylation, which also was uniquely sensitive to the farnesyltransferase inhibitor (FTI-297) (Fig. S3 A and B) and consistent with previous reports of farnesylation on this chaperone protein (20). In addition, we demonstrate that HA-tagged Pcbp1, a protein implicated in nucleic acid binding (41), is also labeled by alk-FOH, the majority of which appears to be on Cys355 at the carboxyl terminus and insensitive to prenylation inhibitors (Fig. 3 C and D). These results demonstrate that proteomic analysis of alk-FOH–labeled proteins enables profiling of known prenylated proteins and discovery of unanticipated isoprenoid-modified proteins. Notably, we identified the long-isoform of the zinc-finger antiviral protein (ZAPL) as a high-confidence hit, which was not previously annotated as a prenylated protein.
Long-Isoform of ZAP is S-Farnesylated.
S-prenylation of ZAP was investigated further to validate our alk-FOH proteomic data and explore the impact of this lipid modification on ZAP subcellular localization and antiviral activity. ZAP amino acid sequence analysis indicates that ZAPL, but not ZAPS, bears a carboxyl-terminal CaaX motif for protein prenylation (Fig. 2A). Alk-FOH labeling of HEK293T and HeLa cells transfected with plasmids expressing hemagglutinin epitope (HA)-tagged mouse ZAP (HA-ZAP) followed by anti-HA immunoprecipitation, and CuAAC with az-rho and in-gel fluorescence scanning, indicated that ZAPL, but not ZAPS, is indeed prenylated (Fig. 2B and Fig. S4A). Because protein S-prenylation is sometimes followed by protein S-palmitoylation to increase membrane affinity (42), we also labeled HA-ZAPL–expressing HeLa cells with alk-16, a chemical reporter of palmitoylation (39), but palmitoylation of ZAPL was not detected (Fig. S4A). We then evaluated whether ZAPL is prenylated at cysteine (Cys) residue 993 of the CaaX motif. Alk-FOH labeling of HEK293T and HeLa cells transfected with a plasmid bearing a Cys-to-serine (Ser) mutation in HA-ZAPL, termed HA-ZAPL-SaaX (Fig. 2A), showed a significant reduction in the level of ZAPL prenylation (Fig. 2B and Fig. S4B). To assess whether ZAPL is farnesylated or geranygeranylated, HEK293T and HeLa cells expressing HA-ZAPL were treated with farnesyltransferase or geranylgeranyltransferase-I inhibitors (FTI-297 or GGTI-2133, respectively) before alk-FOH labeling. Although GGTI-treated cells had the same fluorescence level as nontreated cells, FTI-treated cells showed a marked decrease, suggesting that the long-isoform of ZAP (ZAPL) is S-farnesylated at Cys993 (Fig. 2C and Fig. S4C).
S-Farnesylation Controls Membrane Targeting and Cellular Localization of ZAPL.
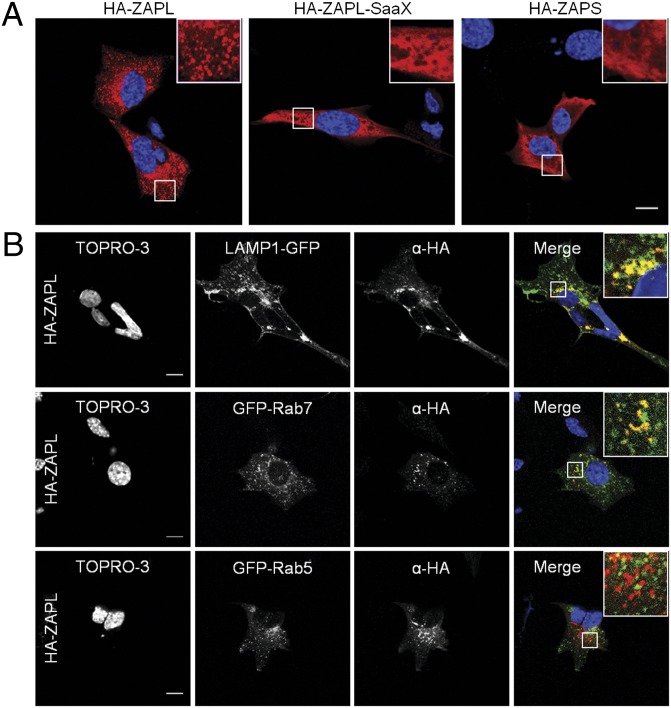
We next investigated whether S-farnesylation of ZAPL affected membrane partitioning and cellular localization. For these studies, mouse embryonic fibroblasts (MEFs) were transfected with HA-ZAPL, HA-ZAPL-SaaX, or HA-ZAPS and fractionated into cytoplasmic, membrane, and nuclear fractions. Each fraction was analyzed for ZAP distribution by anti-HA immunoblot along with histone H3, calnexin, and GAPDH as controls for nuclear, membrane, and cytoplasmic fractions, respectively (Fig. 2D). Although ZAPS was found in nuclear, membrane, and cytosolic fractions, ZAPL was markedly depleted from the cytosol (Fig. 2D). This cellular fractionation was attributed to S-farnesylation as ZAPL-SaaX was redistributed to the cytosol similar to ZAPS (Fig. 2D). MEFs transfected with the HA-ZAP constructs were also analyzed by immunofluorescence using confocal microscopy. Although ZAPL exhibited punctate clusters, ZAPL-SaaX showed a more diffuse staining, similar to ZAPS (Fig. 3A). Cotransfection of plasmids expressing HA-ZAP and cellular markers indicated that ZAPL localizes to lysosomes (LAMP1-GFP) and late endosomes (GFP-Rab7), but not early endosomes (GFP-Rab5) (Fig. 3B). This localization was not observed with ZAPL-SaaX or ZAPS (Figs. S5–S7). S-farnesylation is thus required for targeting ZAPL to late endosomal and lysosomal compartments.
Fig. 3.
S-Farnesylation–dependent clustering of ZAPL to endo/lysosomes. (A) MEFs grown on coverslips were transfected with pCMV-HA-ZAPL, pCMV-HA-ZAPL-SaaX, or pCMV-HA-ZAPS and stained with anti-HA (red) and TOPRO-3 (blue). Insets are enlargements of the white-squared regions. (Scale bar: 10 μm.) (B) MEFs were also cotransfected with pCMV-HA-ZAPL and plasmids expressing cellular markers LAMP1-GFP, GFP-Rab7, or GFP-Rab5 (green) as indicated. (Scale bars: 10 μm.)
S-Farnesylation Regulates ZAPL Antiviral Activity.
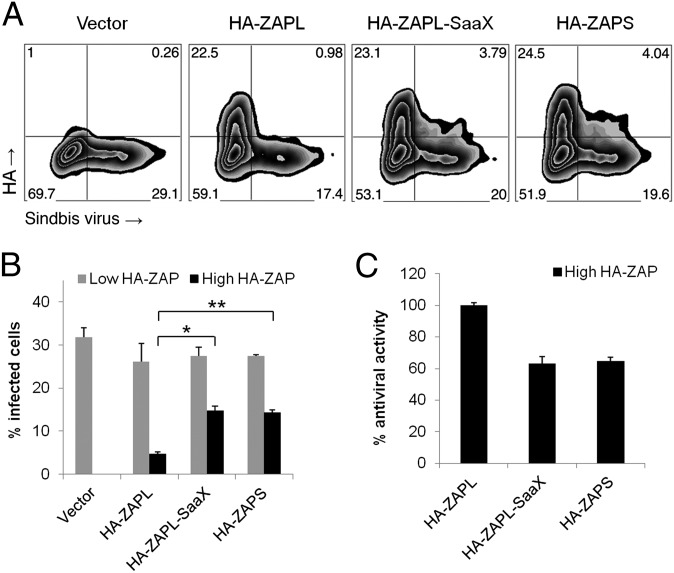
We then determined whether isoform-specific S-farnesylation was crucial for ZAPL antiviral activity using Sindbis virus (SINV) as a prototypic alphavirus for these infection assays. Because murine ZAP mRNA level is up-regulated by type I IFN (43) and ZAP exhibits homotypic interactions (44), we used Stat1-deficient (Stat1−/−) MEFs that are defective in IFN signaling (45) to mitigate the effects of IFN-induced endogenous ZAP. Stat1−/− MEFs transfected with HA-ZAP constructs were thus infected with a SINV encoding enhanced green fluorescent protein (TE/5′2J/GFP) (46) at a multiplicity of infection (MOI) of 10 for 24 h before immunostaining and flow cytometric analysis of the HA tag (ZAP) and EGFP (virus) expression. This analysis allowed direct comparison of virus infection in transfected and nontransfected cells in the same sample by gating on the cells with high and low HA staining, respectively. Transfected Stat1−/− MEFs with low HA staining were infected at comparable levels (26%–28%) to vector control samples (32%) (Fig. 4A, lower quadrants, and Fig. 4B, gray bars). In addition, transfected cells expressed similar levels of different HA-ZAP proteins, as determined by the percentage of cells with high HA staining and the mean fluorescence intensity (MFI) of those cells (Fig. 4A, upper quadrants). Only 5% of HA-ZAPL expressing Stat1−/− MEFs were infected whereas cells expressing the nonfarnesylated HA-ZAPL-SaaX and HA-ZAPS proteins had higher levels of infected cells (14%–15%; Student t test: P = 0.00016 and 0.00003, respectively) (Fig. 4B, black bars). ZAPL inhibited SINV to a significantly greater extent than ZAPS (Fig. 4C), with ZAPS demonstrating only 65% of the antiviral activity of ZAPL. These data are consistent with previous ZAP studies with MuLV (35). This increased antiviral activity is mostly attributed to ZAPL S-farnesylation because HA-ZAPL-SaaX expression resulted in infected cell levels similar to HA-ZAPS (Fig. 4 B and C). The S-farnesylation–dependent antiviral activity of ZAPL was also observed at a lower MOI (Fig. S8 A and B) and in HEK293T cells (Fig. S8 C–F). These results demonstrate that S-farnesylation significantly enhances the antiviral activity of ZAPL.
Fig. 4.
Antiviral activity of ZAPL is regulated by S-farnesylation. Stat1−/− MEFs were transfected with pCMV-HA (vector), pCMV-HA-ZAPL, pCMV-HA-ZAPL-SaaX, or pCMV-HA-ZAPS (shown in Fig. 2A) and infected with Sindbis virus encoding the enhanced green fluorescent protein (EGFP) from a duplicated subgenomic promoter (TE/5′2J/GFP) with multiplicity of infection (MOI) of 10 for 24 h. Virus replication and ZAP protein levels were examined by flow cytometry using GFP fluorescence and anti-HA staining, respectively. After gating (shown in A), nontransfected and transfected cells expressing ZAP from the same culture were analyzed for the percentage of these cells that were infected (shown in B). *P = 0.00016, **P = 0.00003 by Student t test; error represents SD, n = 3. (C) Percentages in B were normalized such that the difference in infection rates for vector control and HA-ZAPL–transfected cells was set at 100% antiviral activity.
Concluding Remarks.
Prenylation provides an essential membrane-targeting mechanism that controls the functions of many proteins in eukaryotic biology. The direct biochemical analysis of these lipidated proteins can therefore reveal important activities in cellular membranes not readily apparent by monitoring protein expression alone. The application of an alkyne-farnesol reporter and improved bioorthogonal proteomics described here has enabled large-scale proteomic analysis of known prenylated proteins, such as small GTPases, as well as unannotated substrates like ZAPL. Our discovery and characterization of ZAPL lipidation demonstrates that S-farnesylation enhances the membrane targeting and inhibitory activity of this antiviral protein against SINV (Figs. 2–4). As expression of ZAPS constructs has been shown to inhibit SINV infection by blocking translation of incoming viral RNA and thus amplification of newly synthesized plus strand genomic RNA (25), S-farnesylation may further enhance this antiviral activity by directing ZAPL to endocytic membranes to interact with incoming virus. Interestingly, SINV RNA replication has been observed to initiate in plasma membrane-associated spherules (47), which at later times can be internalized and form cytopathic vacuoles bearing markers of both endosomal and lysosomal membranes (48). ZAPL enriched on endo/lysosomal membranes could therefore also have an inhibitory effect during SINV replication. It will be interesting to evaluate whether S-farnesylation will also enhance the antiviral activity of ZAPL against other viruses such as MuLV (35) and HIV-1 (24, 27). The results presented here suggest that S-farnesylated ZAPL exhibits a unique antiviral activity on cellular membranes, which may be important for the development of new antiviral strategies. Overall, these studies highlight how bioorthogonal proteomics of protein S-prenylation can reveal insights into host–pathogen interactions that should be useful for exploring other biological pathways and human diseases.
Materials and Methods
Cell Culture, Tranfections, Virus Infections, and Flow Cytometry.
RAW264.7 macrophages, HEK293T, HeLa cells, wild-type and Stat1−/− MEFs were grown in DMEM with 10% (vol/vol) FBS. HEK293T cells were transfected using X-tremeGENE 9 DNA Transfection Reagent (Roche) whereas HeLa cells and MEFs were transfected using Lipofectamine 2000 (Invitrogen). Sindbis virus encoding the enhanced green fluorescent protein (EGFP) from a duplicated subgenomic promoter (TE/5′2J/GFP) has been previously reported (46). Stocks were prepared and titers determined on BHK-J cells with 10-fold serial dilutions of sample, and then plaques were visually enumerated after crystal violet staining, as previously described (25); multiplicities of infection (MOI) were calculated based on BHK-J–derived titers. For flow cytometry, cells were fixed with PBS plus 3.7% paraformaldehyde and then permeabilized and blocked with PBS plus 0.1% Triton X-100 plus 2% FBS. Cells were then incubated with mouse anti-HA antibody (1/1,000, H3663; Sigma), washed three times, and stained with goat anti-mouse antibody conjugated to Rhodamine Red-X (1/1,000, R6393; Invitrogen). Results were analyzed with FlowJo software.
Metabolic Labeling, Immunoprecipitations, and CuAAC.
Metabolic labeling of RAW264.7, HEK293T, and HeLa cells with alk-FOH (50 μM, 4 h) or DMSO control was performed in DMEM and 2% charcoal-filtered FBS. For coincubation with inhibitors, cells were pretreated for 1 h with FTI-277 (10 μM) or GGTI-2133 (10 μM) before alk-FOH metabolic labeling. Chemical syntheses of alk-FOH (22), az-rho (39), and az-azo-biotin (40) have been previously reported. Alk-FOH labeled cells were lysed [4% (wt/vol) SDS, 50 mM triethanolamine pH 7.4, 150 mM NaCl, EDTA-free Roche protease inhibitor mixture, 1 mM PMSF], and protein concentration was determined by the BCA assay (Pierce). Proteins (50 μg) were conjugated to az-rho in 50 μL with CuAAC reactants [az-rho (0.1 mM), Tris(2-carboxyethyl)phosphine hydrochloride (TCEP, 1 mM), Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA, 0.1 mM), and CuSO4·5H2O (1 mM)] for 1 h at room temperature, and methanol precipitated before SDS/PAGE. For immunoprecipitations, 4% SDS was replaced by 1% Brij 97 as the detergent, and 400 μg of proteins in 250 μL were rocked at 4 °C for 1 h with 15 μL of packed anti-HA agarose-antibody conjugate (A2095; Sigma). The beads were washed thrice (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM triethanolamine pH 7.4, 150 mM NaCl), and proteins were conjugated to az-rho in 50 μL of PBS with CuAAC reactants for 1 h at 4 °C and washed thrice again before SDS/PAGE. In-gel fluorescence scanning was performed using a Typhoon 9400 imager (Amersham Biosciences; 532 nm laser, 580 nm filter 30BP). Western blots for HA-tagged proteins were performed using a mouse anti-HA antibody (1/1,000, H3663; Sigma) followed by HRP-conjugated donkey anti-mouse antibody (1/15,000, 715–035-150; Jackson ImmunoResearch).
For proteomic experiments, RAW264.7 macrophages were pretreated with lovastatin (10 μM, 12 h); then alk-FOH was added (50 μM, 12 h) and cells were harvested, lysed in 1 mL of ice-cold hypotonic buffer (5 mM triethanolamine, pH 7.4, 5 mM MgCl2, EDTA-free Roche protease inhibitor mixture, 1 mM PMSF), and solubilized by dilution with 1 mL of 2× SDS buffer (8% SDS, 100 mM triethanolamine, pH 7.4, 300 mM NaCl) plus 2 μL of Benzonase nuclease (E1014; Sigma). Cell lysates (20 mg) were then reacted with az-azo-biotin (40) in 20 mL with CuAAC reactants (same as above) for 2 h at room temperature. Methanol-precipitated and washed protein pellets were resuspended in 2 mL of 1× SDS buffer plus 10 mM EDTA. Proteins (15 mg) were diluted with 8 mL of Brij buffer (1% Brij 97, 50 mM triethanolamine, pH 7.4, 150 mM NaCl) and incubated with 300 μL of prewashed streptavidin-agarose beads (20357; Thermo Scientific) for 1 h at room temperature. The beads were washed once with PBS plus 1% SDS, thrice with PBS, and twice with ABC buffer (50 mM ammonium bicarbonate). The beads were incubated in 500 μL of ABC buffer containing 8 M urea, 10 mM TCEP, and 20 mM iodoacetamide for 0.5 h at room temperature, and then washed twice with ABC buffer. Proteins were eluted by incubating the beads twice in 250 μL of ABC buffer containing 1% SDS and 25 mM Na2S2O4 for 1 h at room temperature. Proteins from the pooled supernatants were concentrated using an Amicon Ultracel-10K (UFC501096; Millipore). Samples were then subjected to SDS/PAGE and staining with Coomassie blue. DMSO and alk-FOH lanes of the gel were then cut for trypsin digestion and peptide extraction. Extracted peptides were dried and resuspended in 0.1% trifluoroacetic acid for mass spectrometry.
Cell Fractionation.
Qproteome Cell Compartment Kit (Qiagen; 37502) was used following the manufacturer’s procedure, and Western blots were performed using mouse anti-HA (1/1,000, H3663; Sigma), rabbit anti-histone H3 (1/2,000, 06–755; Millipore), rabbit anti-calnexin (1/1,500, ab22595; Abcam), or rabbit anti-GAPDH (1/5,000, ab70699; Abcam) antibodies, followed by HRP-conjugated donkey anti-mouse (1/15,000, 715–035-150; Jackson ImmunoResearch) or HRP-conjugated goat anti-rabbit (1/15,000, 12–348; Millipore) antibodies.
Microscopy.
For determination of ZAP localization, transfected HEK293T or MEF cells were fixed with PBS containing 3.7% paraformaldehyde, permeabilized with PBS containing 0.1% Triton X-100, and blocked with PBS containing 2% FBS. Cells were then incubated with anti-HA antibody (1/1,000, H3663; Sigma), washed thrice, and stained with goat anti-mouse antibody conjugated to Rhodamine Red-X (1/1,000, R6393; Invitrogen). Cells were incubated with TOPRO-3 (1/1,000; Invitrogen) as a final step.
Supplementary Material
Acknowledgments
G.C. acknowledges the Weill-Cornell/Rockefeller/Sloan-Kettering Tri-institutional Program in Chemical Biology. M.M.H.L. acknowledges support from the Northeast Biodefense Center. M.R.M. acknowledges support from National Institutes of Health (NIH) Grant AI057905, the Irma T. Hirschl/Monique Weill-Caulier Trust, the Greenberg Medical Research Institute, and the Starr Foundation. H.C.H. acknowledges support from the Ellison Medical Foundation and NIH/National Institute of General Medical Sciences Grant 1R01GM087544.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302564110/-/DCSupplemental.
References
- 1.Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65(65):241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 2.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: Post-translational modification of RAS. Nat Rev Mol Cell Biol. 2012;13(1):39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 4.Davies BS, Fong LG, Yang SH, Coffinier C, Young SG. The posttranslational processing of prelamin A and disease. Annu Rev Genomics Hum Genet. 2009;10:153–174. doi: 10.1146/annurev-genom-082908-150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011;11(11):775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meta M, Yang SH, Bergo MO, Fong LG, Young SG. Protein farnesyltransferase inhibitors and progeria. Trends Mol Med. 2006;12(10):480–487. doi: 10.1016/j.molmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Otto JC, Casey PJ. The hepatitis delta virus large antigen is farnesylated both in vitro and in animal cells. J Biol Chem. 1996;271(9):4569–4572. doi: 10.1074/jbc.271.9.4569. [DOI] [PubMed] [Google Scholar]
- 8.Lee CZ, Chen PJ, Lai MM, Chen DS. Isoprenylation of large hepatitis delta antigen is necessary but not sufficient for hepatitis delta virus assembly. Virology. 1994;199(1):169–175. doi: 10.1006/viro.1994.1109. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SB, Lai MM. A unique conformation at the carboxyl terminus of the small hepatitis delta antigen revealed by a specific monoclonal antibody. Virology. 1993;193(2):924–931. doi: 10.1006/viro.1993.1201. [DOI] [PubMed] [Google Scholar]
- 10.Glenn JS, Watson JA, Havel CM, White JM. Identification of a prenylation site in delta virus large antigen. Science. 1992;256(5061):1331–1333. doi: 10.1126/science.1598578. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov SS, Charron G, Hang HC, Roy CR. Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. J Biol Chem. 2010;285(45):34686–34698. doi: 10.1074/jbc.M110.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price CT, Al-Quadan T, Santic M, Jones SC, Abu Kwaik Y. Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J Exp Med. 2010;207(8):1713–1726. doi: 10.1084/jem.20100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price CT, Jones SC, Amundson KE, Kwaik YA. Host-mediated post-translational prenylation of novel dot/icm-translocated effectors of legionella pneumophila. Front Microbiol. 2010;1:131. doi: 10.3389/fmicb.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinicke AT, et al. A Salmonella typhimurium effector protein SifA is modified by host cell prenylation and S-acylation machinery. J Biol Chem. 2005;280(15):14620–14627. doi: 10.1074/jbc.M500076200. [DOI] [PubMed] [Google Scholar]
- 15.Maurer-Stroh S, Eisenhaber F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005;6(6):R55. doi: 10.1186/gb-2005-6-6-r55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock JF. Prenylation and palmitoylation analysis. Methods Enzymol. 1995;255:237–245. doi: 10.1016/s0076-6879(95)55026-7. [DOI] [PubMed] [Google Scholar]
- 17.Hang HC, Wilson JP, Charron G. Bioorthogonal chemical reporters for analyzing protein lipidation and lipid trafficking. Acc Chem Res. 2011;44(9):699–708. doi: 10.1021/ar200063v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannoush RN, Sun J. The chemical toolbox for monitoring protein fatty acylation and prenylation. Nat Chem Biol. 2010;6(7):498–506. doi: 10.1038/nchembio.388. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen UT, et al. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol. 2009;5(4):227–235. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- 20.Kho Y, et al. A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc Natl Acad Sci USA. 2004;101(34):12479–12484. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan LN, et al. A novel approach to tag and identify geranylgeranylated proteins. Electrophoresis. 2009;30(20):3598–3606. doi: 10.1002/elps.200900259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charron G, Tsou LK, Maguire W, Yount JS, Hang HC. Alkynyl-farnesol reporters for detection of protein S-prenylation in cells. Mol Biosyst. 2011;7(1):67–73. doi: 10.1039/c0mb00183j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeGraw AJ, et al. Evaluation of alkyne-modified isoprenoids as chemical reporters of protein prenylation. Chem Biol Drug Des. 2010;76(6):460–471. doi: 10.1111/j.1747-0285.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao G, Guo X, Goff SP. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science. 2002;297(5587):1703–1706. doi: 10.1126/science.1074276. [DOI] [PubMed] [Google Scholar]
- 25.Bick MJ, et al. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J Virol. 2003;77(21):11555–11562. doi: 10.1128/JVI.77.21.11555-11562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller S, et al. Inhibition of filovirus replication by the zinc finger antiviral protein. J Virol. 2007;81(5):2391–2400. doi: 10.1128/JVI.01601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc Natl Acad Sci USA. 2011;108(38):15834–15839. doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Chen G, Ji X, Gao G. ZAP is a CRM1-dependent nucleocytoplasmic shuttling protein. Biochem Biophys Res Commun. 2004;321(3):517–523. doi: 10.1016/j.bbrc.2004.06.174. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Carroll JW, Macdonald MR, Goff SP, Gao G. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J Virol. 2004;78(23):12781–12787. doi: 10.1128/JVI.78.23.12781-12787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, et al. Structure of N-terminal domain of ZAP indicates how a zinc-finger protein recognizes complex RNA. Nat Struct Mol Biol. 2012;19(4):430–435. doi: 10.1038/nsmb.2243. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Guo X, Lv F, Xu Y, Gao G. p72 DEAD box RNA helicase is required for optimal function of the zinc-finger antiviral protein. Proc Natl Acad Sci USA. 2008;105(11):4352–4357. doi: 10.1073/pnas.0712276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye P, Liu S, Zhu Y, Chen G, Gao G. DEXH-Box protein DHX30 is required for optimal function of the zinc-finger antiviral protein. Protein Cell. 2010;1(10):956–964. doi: 10.1007/s13238-010-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Ma J, Sun J, Gao G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc Natl Acad Sci USA. 2007;104(1):151–156. doi: 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aravind L. The WWE domain: A common interaction module in protein ubiquitination and ADP ribosylation. Trends Biochem Sci. 2001;26(5):273–275. doi: 10.1016/s0968-0004(01)01787-x. [DOI] [PubMed] [Google Scholar]
- 35.Kerns JA, Emerman M, Malik HS. Positive selection and increased antiviral activity associated with the PARP-containing isoform of human zinc-finger antiviral protein. PLoS Genet. 2008;4(1):e21. doi: 10.1371/journal.pgen.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayakawa S, et al. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat Immunol. 2011;12(1):37–44. doi: 10.1038/ni.1963. [DOI] [PubMed] [Google Scholar]
- 37.Leung AK, et al. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42(4):489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 39.Charron G, et al. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J Am Chem Soc. 2009;131(13):4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 40.Yang YY, Ascano JM, Hang HC. Bioorthogonal chemical reporters for monitoring protein acetylation. J Am Chem Soc. 2010;132(11):3640–3641. doi: 10.1021/ja908871t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silvera D, Gamarnik AV, Andino R. The N-terminal K homology domain of the poly(rC)-binding protein is a major determinant for binding to the poliovirus 5′-untranslated region and acts as an inhibitor of viral translation. J Biol Chem. 1999;274(53):38163–38170. doi: 10.1074/jbc.274.53.38163. [DOI] [PubMed] [Google Scholar]
- 42.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2(11):584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 43.Ryman KD, et al. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J Virol. 2005;79(3):1487–1499. doi: 10.1128/JVI.79.3.1487-1499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law LM, et al. Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions. J Virol. 2010;84(9):4504–4512. doi: 10.1128/JVI.02018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84(3):443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 46.Frolova EI, et al. Roles of nonstructural protein nsP2 and Alpha/Beta interferons in determining the outcome of Sindbis virus infection. J Virol. 2002;76(22):11254–11264. doi: 10.1128/JVI.76.22.11254-11264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frolova EI, Gorchakov R, Pereboeva L, Atasheva S, Frolov I. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J Virol. 2010;84(22):11679–11695. doi: 10.1128/JVI.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froshauer S, Kartenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988;107(6 Pt 1):2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.