Abstract
Mitochondrial Ca2+ homeostasis is fundamental to regulation of mitochondrial membrane potential, ATP production, and cellular Ca2+ homeostasis. It has been known for decades that isolated mitochondria can take up Ca2+ from the extramitochondrial solution, but the molecular identity of the Ca2+ channels involved in this action is largely unknown. Here, we show that a fraction of canonical transient receptor potential 3 (TRPC3) channels is localized to mitochondria, a significant fraction of mitochondrial Ca2+ uptake that relies on extramitochondrial Ca2+ concentration is TRPC3-dependent, and the up- and down-regulation of TRPC3 expression in the cell influences the mitochondrial membrane potential. Our findings suggest that TRPC3 channels contribute to mitochondrial Ca2+ uptake. We anticipate our observations may provide insights into the mechanisms of mitochondrial Ca2+ uptake and advance understanding of the physiological role of TRPC3.
Mitochondrial Ca2+ uptake is critical for regulation of numerous cellular processes, including energy metabolism and cytosolic Ca2+ homeostasis. Mitochondria undergo rapid changes in matrix Ca2+ concentration ([Ca2+]mito) on cell stimulation to affect aerobic metabolism and cell survival (1–4). Mitochondrial Ca2+ buffering also shapes the amplitude and spatiotemporal patterns of cytosolic Ca2+ concentration ([Ca2+]cyt) changes (5, 6). Mitochondrial calcium uniporter (MCU) has been shown to affect mitochondrial Ca2+ uptake (7, 8). However, mitochondrial Ca2+ uptake remains evident when MCU is down-regulated (7, 8), suggesting that other routes responsible for its Ca2+ uptake might exist. Transient receptor potential (TRP) channels have emerged as important cellular sensors, and although many TRP channels are expressed on the plasma membrane, some members of the TRP channel proteins are also found in the intracellular organelles such as endoplasmic reticulum (ER), secretory vesicles, granules, endosomes, and lysosomes (9–14). Recently, a proteomics study showed that canonical TRP 3 (TRPC3) interacts with a large number of mitochondrial proteins (15); we thus studied whether the TRPC3 protein localizes to mitochondria and plays a role in maintaining mitochondrial Ca2+ homeostasis.
Results and Discussion
TRPC3 Is Also Localized to Mitochondria.
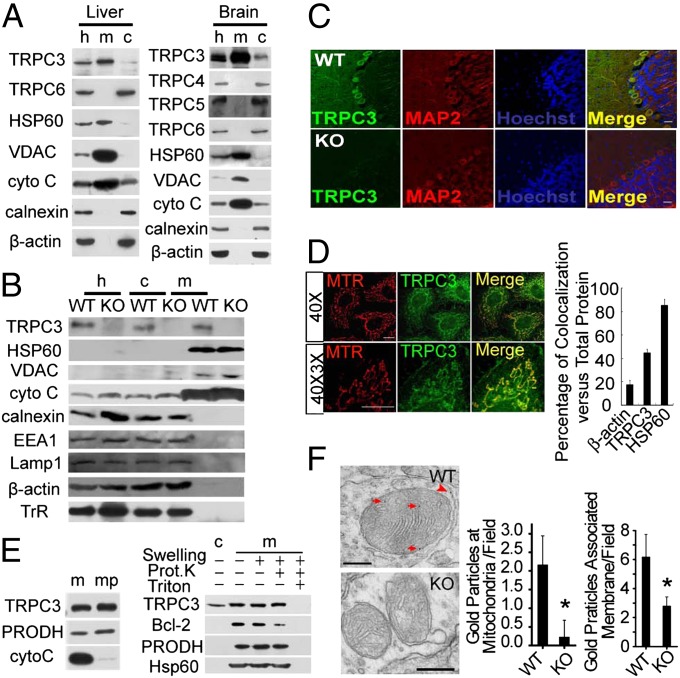
First, we examined the presence of TRPC3 in purified mitochondria prepared from rat liver and brain using the Percoll density gradient centrifugation, a method well established to obtain high purity mitochondria (16). In both tissues, Western blotting showed that TRPC3, but not other members of the TRPC subfamily, including TRPC4, TRPC5, and TRPC6, were enriched in the mitochondrial fraction (Fig. 1A). We used four anti-TRPC3 antibodies from different sources (Fig. S1A), including a monoclonal anti-TRPC3 antibody generated in our laboratory (Fig. 1B); all of them detected TRPC3 in the mitochondrial fraction. The purity of mitochondria was confirmed by a series of markers for cell organelles, including three well-established mitochondria-targeted proteins [heat shock protein 60 (HSP60); voltage-dependent anion-selective channel protein (VDAC); and cytochrome C], an ER marker (calnexin), an endosome marker [early endosome autoantigen 1 (EEA1)], a lysosome marker [lysosome associated membrane glycoprotein 1 (Lamp1)], and a plasma membrane-targeted protein [transferrin receptor (TrR); Fig. 1B]. The specificity of the anti-TRPC3 antibodies was verified and are shown in Fig. S1 B–D. The immunoreactivity of the TRPC3 protein was absent in the mitochondrial fraction and the whole homogenates isolated from the cerebellum of Trpc3−/− mice (Fig. 1B). Further, TRPC3 immunoreactivity was also lost in cerebellum neurons from Trpc3−/− mice (Fig. 1C). The expression of other members of the TRPC family in Trpc3−/− and WT mice was similar (Fig. S1 E and F).
Fig. 1.
Localization of TRPC3 in mitochondria. (A) TRPC3 was found in the homogenate (h), mitochondrial (m), or cytosolic (c) fractions isolated from rat liver (Left) and brain (Right). HSP60, VDAC protein, and cytochrome (cyto) C served as mitochondrial markers, calnexin as an ER marker and β-actin as a loading control. Polyclonal anti-TRPC antibodies were used. (B) TRPC3 immunoreactivity detected by the monoclonal anti-TRPC3 antibody in homogenates (h), cytosolic (c), and mitochondrial (m) fractions of the cerebellum from the WT but not Trpc3−/− (KO) mice. HSP60, VDAC, and cyto C served as mitochondrial markers, calnexin as an ER marker, EEA1 as an endosome marker, Lamp1 as a lysosome marker, TrR as plasma membrane marker, and β-actin as a loading control. For Western blotting, all isolated fractions from WT and Trpc3−/− tissues were run on the same gel and blotted on the same membrane. (C) Immunocytochemical staining of cerebella from WT and Trpc3−/− mice using the polyclonal anti-TRPC3 antibody (green; Alomone. Cat. No. ACC016, Lot No. AN0702) and monoclonal anti-MAP2 antibody (red). Hochest33258 was used to label nuclei (blue). (Scale bars, 20 µm.) (D) Immunofluorescent labeling of TRPC3 in mitochondria. Representative images of HeLa cells stained with the polyclonal anti-TRPC3 antibody (green; from C. Montell) and loaded with 10 nM MitoTraker red (red). (Scale bars, 5 µm.) 40×, 40× objective; 40× 3×, 40× objective with 3× zoom. (Right) Summary of percent fluorescence intensity of actin, TRPC3, or HSP60 colocalized with MitoTraker red over the total fluorescence intensity of the corresponding protein, n = 10 for actin and TRPC3, respectively, n = 3 for HSP60. Representative images for actin and HSP60 are shown in Fig. S2A. (E) (Left) Immunobloting of TRPC3 in the intact mitochondria and mitoplast of rat liver cells. (Right) Immunobloting of TRPC3 in the swelled mitochondria incubated with or without proteinase K in 0.5% TritonX-100 or not. Bcl-2 served as an outer membrane marker, cyto C a mitochondrial intermembrane space marker, PRODH a mitochondrial inner membrane marker, and Hsp60 a matrix marker. Cytosolic (c), mitochondrial (m), mitoplasts (mp), and proteinase K (Prot. K) (F) Electron microscopic images (Left) of immunogold labeling by the polyclonal anti-TRPC3 antibody (Alomone Cat. No. ACC016, Lot No. AN0702) of mitochondrial inner membranes (red arrows) of the cerebellum from WT, but not Trpc3−/− (KO), mice. Labeling to other membrane structures, presumably plasma membrane, is indicated (red arrowhead). (Scale bar, 200 nm.) Statistics of gold particles associated with mitochondrial inner membranes per field (Center) and that associated with all membrane structures per field (Right) are shown as means ± SEM of 50 EM fields from three mice for each genotype.
To confirm the mitochondrial localization of the TRPC3 protein in intact cells, we examined the subcellular localization of TRPC3 by immunocytochemistry in HeLa cells using the anti-TRPC3 antibody. As shown in Fig. 1D, the endogenous TRPC3 protein was found in both the plasma membrane and the mitochondria. Double staining using the Mitotraker and the anti-TRPC3 antibody further revealed that in HeLa cells, about 44.4% of the total TRPC3 is localized to mitochondria. Similarly, 84.9% of HSP60 is localized to mitochondria. In contrast, only 17.6% of the immunostaining for β-actin, a cytoskeletal protein, is associated with mitochondria (Fig. 1D; Fig. S2A). The fraction of mitochondrial TRPC3 staining ranged from 28.4% to 56.4% of the total TRPC3 labels in different cells, including human embryonic kidney 293 (HEK 293) cells, Chang-liver cells, mouse embryonic fibroblast (MEF) cells, and hippocampal neurons (Fig. S2 B and C). Mitochondrial fractionation and proteinase K protection assay further showed that in rat liver cells, TRPC3 mainly localized to the inner membrane of mitochondria (IMM) (Fig. 1E). We then performed immunoelectron microscopy using the antibody against TRPC3 on mouse cerebella. Gold particles were found on the inner mitochondrial membrane of WT, but not Trpc3−/−, cerebella (Fig. 1F). The number of gold particles per field on IMM in WT or Trpc3−/− mice was 2.15 ± 0.79 or 0.13 ± 0.46, respectively. Similarly, the number of gold particles per field on all membrane structures in WT or Trpc3−/− mice was 4.77 ± 0.68 or 1.71 ± 0.44, respectively. With heterologous expression, although most of the C-terminal myc-tagged TRPC3 (TRPC3myc) was localized to the plasma membrane (Fig. S2D), a substantial level of the TRPC3myc proteins was also evident in the mitochondria. At similar total expression levels, the immunoreactivity of TRPC3myc associated with the mitochondrial fraction in HeLa cells was 13.0 ± 3.9-fold higher (n = 4) than that of TRPC4myc (Fig. S2E). Together, these results suggest that, in addition to the plasma membrane, TRPC3 is also localized to mitochondria.
TRPC3 Regulates Mitochondrial Ca2+ Homeostasis.
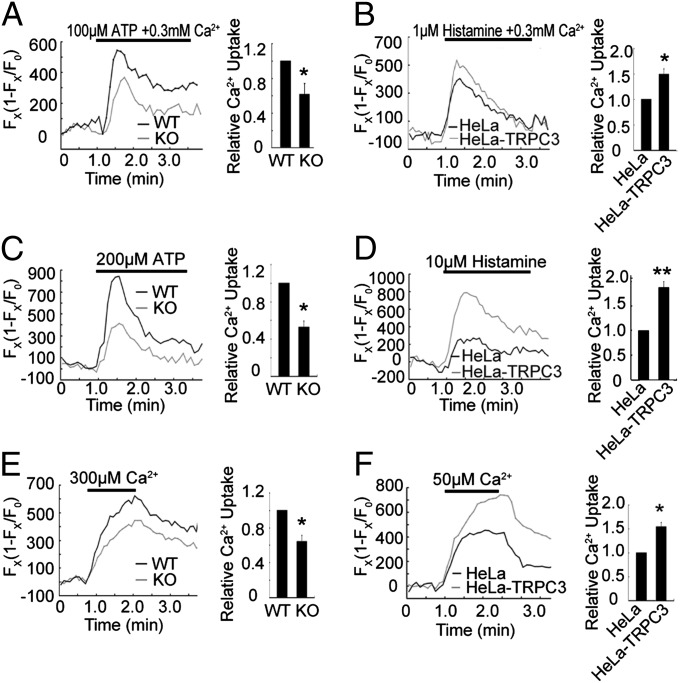
We next tested whether TRPC3 regulates mitochondrial Ca2+ homeostasis. For the loss-of-function experiments, we examined the mitochondrial Ca2+ signals in MEF cells isolated from WT and Trpc3−/− mice. For the gain-of-function experiments, we used HeLa cells that stably overexpressed human TRPC3 (HeLa-TRPC3). The MEF cells derived from Trpc3−/− mice appeared normal, and their mitochondrial morphology was not different from that of the WT cells (Fig. S2F). The expression of mitochondrial fusion and fission proteins in Trpc3−/− mice was not different from that in WT mice (Fig. S2G). Mitochondrial Ca2+ elevation was assessed with Pericam, a mitochondria-targeted Ca2+-sensitive fluorescent protein (17) (Fig. S2H), and Rhod 5N. Treatment of MEF cells from WT mice with ATP elevated mitochondrial Ca2+ concentration, and this elevation was greatly reduced in the MEF cells from Trpc3−/− mice (Fig. 2A; Fig. S3A). By contrast, histamine-induced mitochondrial Ca2+ elevation in HeLa-TRPC3 was markedly enhanced (Fig. 2B; Fig. S3B).
Fig. 2.
TRPC3 regulates mitochondrial Ca2+ uptake. (A) Mitochondrial Ca2+ dynamics ([Ca2+]mito) in WT or Trpc3−/− MEF cells treated with 100 μM ATP in 0.3 mM extracellular Ca2+. [Ca2+]mito was monitored by changes in Pericam fluorescence. (B) Similar measurements in HeLa cells or HeLa cells stably transfected with human TRPC3 (HeLa-TRPC3) treated with 1 μM histamine in 0.3 mM extracellular Ca2+. (C and D) Same experiments as A and B, but cells treated with 200 μM ATP or 10 μM histamine in a Ca2+-free extracellular solution. (E) [Ca2+]mito changes in digitonin-permeabilized WT and KO MEF cells challenged with cytosolic loading of 300 μM Ca2+. (F) [Ca2+]mito changes in digitonin-permeabilized HeLa and HeLa-TRPC3 cells challenged with cytosolic loading of 50 μM Ca2+. (B–F) (Left) Representative traces from single experiments. (Right) Means ± SEM of peak [Ca2+]mito changes normalized to that of control (WT, MEF, or HeLa) of three independent experiments with the total number of MEF cells, n = 30, control HeLa and HeLa-TRPC3 cells, n = 186. *P < 0.05, **P < 0.01.
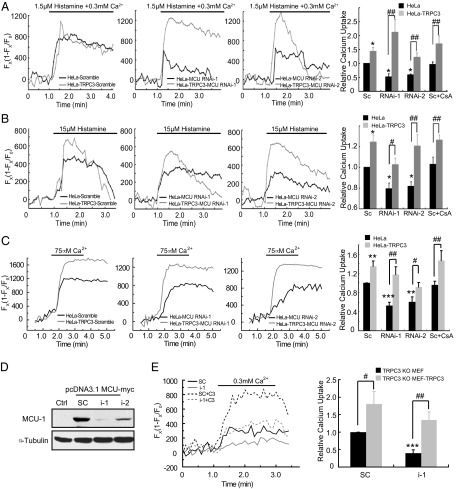
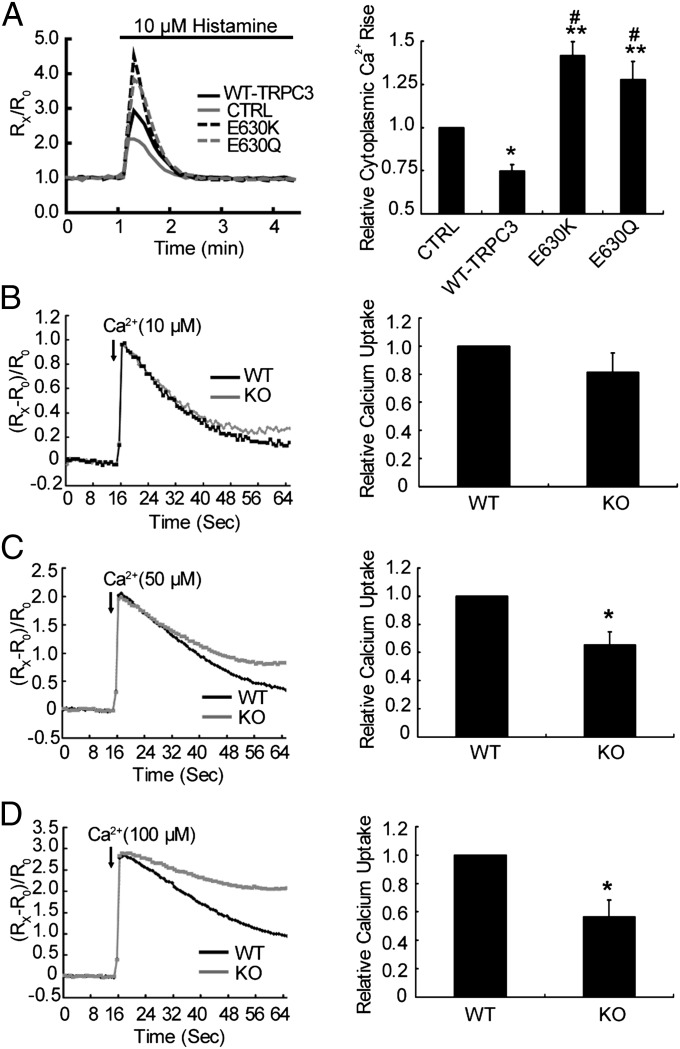
The Ca2+ influx through plasma membrane TRPC3 may contribute to cytosolic Ca2+ elevation and subsequent mitochondrial Ca2+ uptake. To distinguish the effect of plasma membrane TRPC3 and mitochondrial TRPC3 on mitochondrial Ca2+ uptake, we stimulated MEF and HeLa cells in the absence of extracellular Ca2+. As shown in Fig. 2C and Fig. S3C, under this condition, the mitochondrial Ca2+ uptake remained evident in WT MEF cells, whereas it was greatly suppressed in Trpc3−/− MEF cells. Mirroring the results of Trpc3−/− MEF cells, the mitochondrial Ca2+ uptake in HeLa-TRPC3 showed a marked increase compared with control cells (Fig. 2D; Fig. S3D). To further demonstrate the contribution of the mitochondrial TRPC3 to its Ca2+ uptake, we permeabilized the plasma membrane with digitonin (10 μM) and then applied Ca2+ to the permeabilized cells. As shown in Fig. 2E and Fig. S3E, the mitochondrial Ca2+ signals in the Trpc3−/− MEF cells were substantially reduced compared with the WTs. The mitochondrial Ca2+ signals in the HeLa-TRPC3 cells were significantly higher than those in the control cells (Fig. 2F; Fig. S3F). Further, in the HeLa-TRPC3 cells or the permeabilized HeLa-TRPC3 cells, in which MCU was down-regulated by siRNA and incubated with cyclosporine A to inhibit mPTP opening, the enhancement in mitochondrial Ca2+ signals after histamine or Ca2+ treatment remained evident (Fig. 3 A–C). Moreover, in the permeabilized Trpc3−/− MEF cells, in which MCU was down-regulated (Fig. 3D), expressing TRPC3 greatly increased the mitochondrial Ca2+ uptake (Fig. 3E). Together, these results suggest that in addition to MCU, TRPC3 also contributes to mitochondrial Ca2+ uptake. As mitochondria play a critical role in shaping cytosolic Ca2+ dynamics (1), we examined agonist-evoked changes in cytosolic Ca2+ transient in the absence of extracellular Ca2+. As shown in Fig. 4A, overexpression of TRPC3 in HeLa cells led to a substantial reduction in the cytosolic Ca2+ transient evoked by histamine. By contrast, the Ca2+ transient was enhanced in the cells that expressed the dominant-negative nonpermeant mutant (E630K) or the Ca2+-impermeable mutant of TRPC3 (E630Q) (18). Together, these results suggest that TRPC3 is indeed involved in mitochondrial Ca2+ uptake.
Fig. 3.
In the absence of MCU, mitochondrial Ca2+ uptake remains regulated by TRPC3. [Ca2+]mito changes in HeLa cells or HeLa-TRPC3 cells transfected with scrambled (sc) or indicated siRNAs in the absence or presence of 4 µM cyclosporine A (CsA) and evoked by 1.5 µM histamine in the presence of 300 µM Ca2+ (A), by 15 µM histamine in a Ca2+-free extracellular solution (B), or by 75 µM Ca2+ in digitonin-permeabilized HeLa cells (C). (A–C) (Right) Means ± SEM of peak [Ca2+]mito changes normalized to that of sc HeLa of four independent experiments with the total number of HeLa and HeLa-TRPC3 cells, n = 103. **P < 0.01, ***P < 0.001 vs. sc HeLa. #P < 0.05, ##P < 0.01. (D) Down-regulation of MCU by its RNAi-1 or -2 (i-1 or 2) in HEK 293 cells, in which MCU-myc was overexpressed. (E) [Ca2+]mito changes in Trpc3−/− MEF cells or Trpc3−/− MEF-TRPC3 cells transfected with scramble (sc) or i-1, treated with 10 µM digitonin, and evoked by 300 µM Ca2+. (Right) Means ± SEM of peak [Ca2+]mito changes normalized to that of sc of four independent experiments with the total number of Trpc3−/− MEF and Trpc3−/− MEF-TRPC3 cells, n = 69. ***P < 0.001, vs. sc Trpc3−/− MEF. #P < 0.05, ##P < 0.01.
Fig. 4.
Reduction in extramitochondrial Ca2+ by TRPC3 in HeLa cells or in isolated liver mitochondria from Trpc3−/− mice. (A) Cytosolic Ca2+ dynamics ([Ca2+]cyto) in HeLa cells transfected with control vector (CTRL), WT-TRPC3, and TRPC3 mutants (E630Q or E630K) treated with 10 μM histamine in the absence of extracellular Ca2+ were monitored by changes in Fura 2 fluorescence. Representative traces from a single experiment (Left) and means ± SEM of peak [Ca2+]cyto changes normalized to CTRL of four independent experiments (Right) with the total number: CTRL, n = 58; WT-TRPC3, n = 50; E630K, n = 58; E630Q, n = 27. *P < 0.05, **P < 0.01 vs. CTRL, #P < 0.001 vs. WT-TRPC3. (B–D) Reduction of medium Ca2+ by isolated liver mitochondria from WT and Trpc3−/− mice. Purified liver mitochondria were incubated in a Ca2+-free buffer in the presence of Fluo-5N (1 µM). Ca2+ was added at a final concentration of 10 (B), 50 (C), or 100 μM (D) and changes in extramitochondrial [Ca2+] monitored by Fluo-5N fluorescence for 65 s. Shown are representative traces of time courses of relative fluorescence changes (Left) and means ± SEM (n = 4) of overall Ca2+ uptake during the entire experimental period, normalized to that of WT. *P < 0.05 vs. WT.
To further support the notion that mitochondrial TRPC3 channels contribute to mitochondrial Ca2+ uptake, we performed Ca2+ uptake experiments in isolated liver mitochondria. The maximal quantity of Ca2+ that can be sequestered by mitochondria was determined by monitoring the changes of free Ca2+ in extramitochondrial media using Fluo-5N (19). Liver mitochondria isolated from the WT littermates exhibited a higher ability to take up Ca2+ from the medium than that from Trpc3−/− mice. Moreover, the TRPC3-dependent mitochondrial Ca2+ uptake was dependent on extramitochondrial Ca2+ concentrations. With 10 μM Ca2+ added to isolated liver mitochondria, Ca2+ sequestration was similar between Trpc3−/− and WT preparations (Fig. 4B). However, with 50 and 100 μM Ca2+ in the medium, Ca2+ sequestration by mitochondria was substantially reduced for samples from Trpc3−/− mice compared with the WT (Fig. 4 C and D). These results suggest that TRPC3 is particularly relevant in mitochondrial Ca2+ uptake when extramitochondrial Ca2+ concentration is relatively high (≥50 μM).
TRPC3 Channels Affect Mitochondrial Membrane Potential.
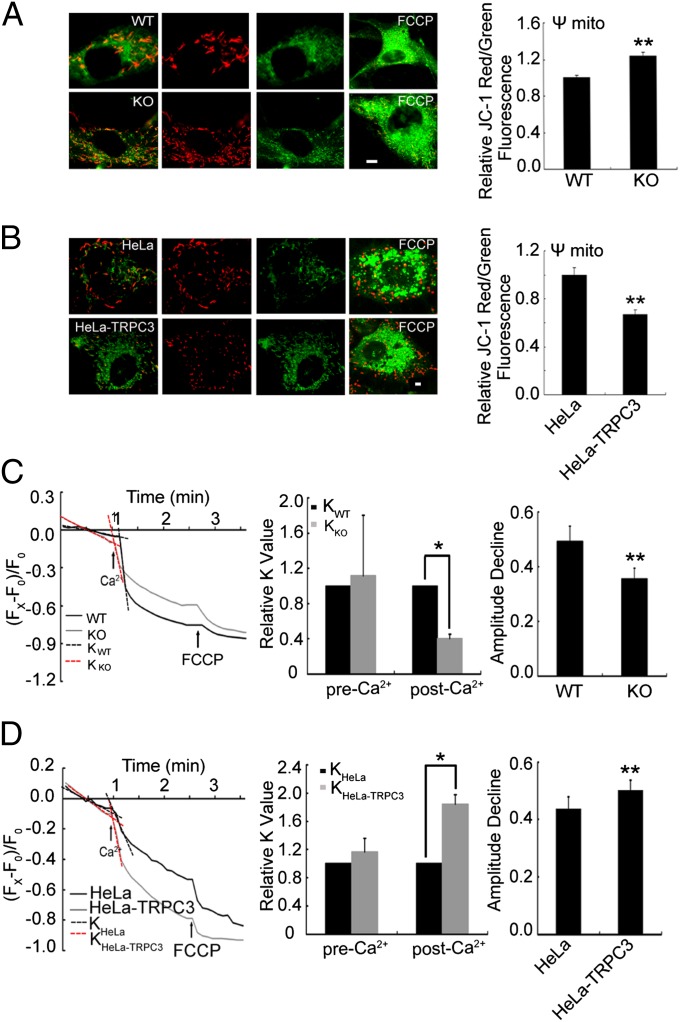
A large negative membrane potential (ΔΨm) across the inner mitochondrial membrane generated by H+ gradient and cations, such as Ca2+ and K+ (20), is important for mitochondrial functions. Mitochondrial depolarization induces Ca2+ release from Ca2+-loaded mitochondria (21, 22) and prevents mitochondrial Ca2+ uptake (5). In contrast, Ca2+ uptake may collapse the ΔΨm (23, 24). To test whether TRPC3 channels affect mitochondrial membrane potential, we measured ΔΨm using 5,5′,6,6'-tetrachloro-1,1',3,3′-tetrethyl benzimidalyl carbocyanine iodide (JC-1) and tetramethylrhodamine ethyl ester (TMRE), known as sensitive probes of changes in the ΔΨm. As shown in Fig. 5 A and B by JC-1 staining, the resting ΔΨm in Trpc3−/− MEF cells was increased compared with that in WT MEF cells. Consistently, HeLa-TRPC3 cell mitochondria showed more depolarized ΔΨm than those of control cells. Then, we measured ΔΨm by TMRE in response to Ca2+ stimulation. As shown in Fig. 5C, the reduction in ΔΨm in Trpc3−/− MEF cells, in response to Ca2+ stimulation, was slower and to a lesser extent than that in WT MEF cells. The ΔΨm of HeLa-TRPC3 mitochondria decreased faster and to a greater extent than that of control cells (Fig. 5D). Together, these results suggest that TRPC3 channels play an important role in regulating the mitochondrial membrane potential in response to changes in extramitochondrial Ca2+ levels.
Fig. 5.
TRPC3 contributes to the mitochondrial membrane potential (ΔΨm). Representative images of WT or Trpc3−/− MEF cells (A) and of control HeLa or HeLa-TRPC3 cells (B) loaded with JC-1 (Left). Red fluorescence indicates mitochondria with formation of J-aggregates at high transmembrane potentials; green fluorescence shows mitochondria with formation of JC-1 monomers at low transmembrane potentials. FCCP treatment (Rightmost) was used as a positive control. Statistics of the JC-1 ratio of red/green fluorescence intensity (Right). Data are means ± SEM of four independent experiments. Changes in TMRE fluorescence in WT and Trpc3−/−MEF cells treated with 500 μM Ca2+ (C) or in HeLa and HeLa-TRPC3 cells treated with 300 μM Ca2+ (D). The fluorescence values were normalized to those of the baseline. The dotted lines represent the slope (K) of the curve before and after Ca2+ treatment. The K values of the baseline were calculated by the linear fitting. After Ca2+ treatment, the K values were calculated by fitting the normalized TMRE fluorescence during the first 20 s immediately following Ca2+ addition with a linear function. FCCP was used as a positive control. Shown are representative traces from single experiments (Left), statistics of the changes in K values normalized to those of WT or HeLa cells (Center), and statistics of maximal TMRE fluorescence decreases induced by Ca2+ (Right). Data in bar graphs are means ± SEM of three independent experiments with 128 WT cells, 122 Trpc3−/−cells, 352 control HeLa cells, and 337 HeLa-TRPC3 cells. *P < 0.05, **P < 0.01.
The results presented here reveal TRPC3 channels as a unique mitochondrial Ca2+ uptake pathway. A fraction of the TRPC3 protein is localized to mitochondria and mediates mitochondrial Ca2+ uptake when extramitochondrial Ca2+ concentrations are relatively high. TRPC3 channels appear to be important in regulating the mitochondrial membrane potential. It has been reported that the MCU regulates mitochondrial Ca2+ uptake (7). However, silencing MCU did not abolish the mitochondrial Ca2+ uptake (7), indicating that other routes are also responsible for its Ca2+ uptake. We found that application of Ruthenium red, known as a putative MCU inhibitor, to the isolated liver mitochondria derived from the WT and Trpc3−/− mice blocked all of the mitochondrial Ca2+ uptake in both WT and Trpc3−/− mitochondria. Indeed, Ruthenium red has been shown to inhibit TRP channels (25). The lost portion of the Ca2+ uptake by TRPC3 was Ruthenium Red sensitive, which suggested that TRPC3 might be another conduit besides MCU. The MCU regulates mitochondrial Ca2+ uptake with a high affinity to Ca2+ (26), and 2 μM [Ca2+] could initiate MCU Ca2+ uptake. Letm1, a mitochondrial Ca2+/H+ antiporter, contributes to mitochondrial Ca2+ influx when extramitochondrial Ca2+ concentrations are relatively low (<1 μM) (27). Therefore, although TRPC3, MCU, and Letm1 affect mitochondrial Ca2+ uptake in a Ca2+ concentration-dependent manner, TRPC3 likely plays an important role in mitochondrial Ca2+ uptake in some physiological conditions with high extramitochondrial Ca2+ concentrations. Indeed, TRPC3 exhibits a low permeability to Ca2+ over Na+. When cytoplasmic Ca2+ is low (<5 μM) and cytoplasmic Na+ is ∼10 mM, TRPC3 does not support much Ca2+ flux into the matrix (28). When high, local Ca2+ emanates from the InsP3 receptors (29), and TRPC3 in mitochondria located within 20–200 nm of ER can uptake Ca2+. A recent study demonstrated that, during IP3-mediated store release, [Ca2+] at the ER-mitochondrial interface often exceeds 10 μM (30). Cytosolic Ca2+ levels within the nanodomain of the hot spot region are transiently expected to be high (>50 μM) (31). Whether the mitochondrial TRPC3 channels are constitutively open or modulated by extramitochondrial Ca2+ concentration is unclear at present. The Ca2+ uptake studies in isolated mitochondria suggest that TRPC3 channels in mitochondria can be either directly activated by Ca2+, which could be similar to the activation of Ca2+ uniporter (32) or modulated by other Ca2+ sensing proteins. Although there is no evidence until now to show that Ca2+ itself can gate the plasma membrane TRPC3, several TRP channels (TRPA1, TRPM4, and TRPM5) can be directly gated by Ca2+ (33–35). Our results are consistent with previous findings showing that plasma membrane TRPC3 channels are stimulated in a Ca2+-dependent manner (36). Phosphitidylinositol-4,5-bisphosphate (PIP2) and phospholipase C (PLC) are located in mitochondria (37, 38). It is also possible that the activation of mitochondrial TRPC3 involves the PIP2-PLC pathway. Furthermore, investigations on targeting of TRPC3 channels to mitochondria and the activation mechanisms of mitochondrial TRPC3 channels will expand our understanding on the membrane trafficking mechanisms of plasma membrane channel proteins and their specific roles in different intracellular localizations in physiological and pathological conditions.
Materials and Methods
More extensive descriptions can be found in SI Materials and Methods.
Isolation of Mitochondria.
Mitochondria were isolated from adult rat or mouse liver and brain, respectively, by Percoll density gradient centrifugation following the method described previously (16). For details, see SI Materials and Methods.
Measurement of Mitochondrial and Intracellular Ca2+ Changes.
Mitochondrial Ca2+ uptake was measured with Pericam (gift from Atsudhi Miyawaki, Brain Science Institute, RIKEN, Wako City, Japan) or Rhod 5N-AM. Cytosolic Ca2+ changes were measured with fura-2. For details Ca2+ imaging experiments, see SI Materials and Methods.
Ca2+ Uptake of Isolated Liver Mitochondria.
Extramitochondrial Ca2+ reduction was measured with Fluo-5N according to the method described previously (19). See SI Materials and Methods for details.
Statistical Analysis.
All data were expressed as the means ± SEM. Data were analyzed using the SPSS 11.5 software. The means between two groups were analyzed by either paired or unpaired t tests. ANOVA followed by a post hoc least significant difference test was performed for statistical comparison of several groups. Significance was taken at P < 0.05.
Supplementary Material
Acknowledgments
We thank C. Montell and W. P. Schilling for TRPC3 antibodies, A. Miyawaki for Pericam, V. Flockerzi for comments, and Q. Hu and Z. J. Fan for technical assistance. This work was supported in part by a grant (81130081) from National Neural Science Foundation of China (NNSF), the 973 program (2011CBA00400), the Intramural Research Program of the National Institutes of Health (NIH; Z01-ES-101684; to L.B.), and NIH Grant R01 GM081658 (to M.X.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309531110/-/DCSupplemental.
References
- 1.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 2.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96(24):13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinton P, et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: Significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20(11):2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacher P, Hajnóczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 2001;20(15):4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145(4):795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinel H, et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca(2+) signals. EMBO J. 1999;18(18):4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem J. 2003;371(Pt 2):341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6(8):709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 11.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455(7215):992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange I, et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2(71):ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oancea E, et al. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal. 2009;2(70):ra21. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem. 2006;281(25):17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockwich T, et al. Analysis of TRPC3-interacting proteins by tandem mass spectrometry. J Proteome Res. 2008;7(3):979–989. doi: 10.1021/pr070496k. [DOI] [PubMed] [Google Scholar]
- 16.Sims NR, Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc. 2008;3(7):1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 17.Palty R, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107(1):436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poteser M, et al. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc Natl Acad Sci USA. 2011;108(26):10556–10561. doi: 10.1073/pnas.1106183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci USA. 1996;93(18):9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M, et al. Involvement of mitoKATP channel in protective mechanisms of cerebral ischemic tolerance. Brain Res. 2008;1238:199–207. doi: 10.1016/j.brainres.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Montero M, Alonso MT, Albillos A, García-Sancho J, Alvarez J. Mitochondrial Ca(2+)-induced Ca(2+) release mediated by the Ca(2+) uniporter. Mol Biol Cell. 2001;12(1):63–71. doi: 10.1091/mbc.12.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saotome M, et al. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;288(4):H1820–H1828. doi: 10.1152/ajpheart.00589.2004. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers S, McCarron JG. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J Cell Sci. 2008;121(Pt 1):75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- 24.Talbot J, Barrett JN, Barrett EF, David G. Stimulation-induced changes in NADH fluorescence and mitochondrial membrane potential in lizard motor nerve terminals. J Physiol. 2007;579(Pt 3):783–798. doi: 10.1113/jphysiol.2006.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clapham DE. SnapShot: Mammalian TRP channels. Cell. 2007;129(1):220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 27.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326(5949):144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 29.Moreau B, Parekh AB. 2008. Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr Biol 18(11):855–859.
- 30.Csordás G, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39(1):121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thul R, Falcke M. Release currents of IP(3) receptor channel clusters and concentration profiles. Biophys J. 2004;86(5):2660–2673. doi: 10.1016/S0006-3495(04)74322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 33.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282(18):13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 34.Launay P, et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109(3):397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann T, Chubanov V, Gudermann T, Montell C. 2003. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13(13):1153–1158. [DOI] [PubMed]
- 36.Zitt C, et al. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138(6):1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knox CD, et al. Novel role of phospholipase C-delta1: Regulation of liver mitochondrial Ca2+ uptake. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G533–G540. doi: 10.1152/ajpgi.00050.2004. [DOI] [PubMed] [Google Scholar]
- 38.Watt SA, Kular G, Fleming IN, Downes CP, Lucocq JM. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C delta1. Biochem J. 2002;363(Pt 3):657–666. doi: 10.1042/0264-6021:3630657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.