Abstract
Muscarinic acetylcholine receptors (mAChRs) are known to modulate synaptic plasticity in various brain areas. A signaling pathway triggered by mAChR activation is the production and release of endocannabinoids that bind to type 1 cannabinoid receptors (CB1R) located on synaptic terminals. Using whole-cell patch-clamp recordings from rat cerebellar slices, we have demonstrated that the muscarinic agonist oxotremorine-m (oxo-m) blocks the induction of presynaptic long-term potentiation (LTP) at parallel fiber (PF)–Purkinje cell synapses in a CB1R-dependent manner. Under control conditions, LTP was induced by delivering 120 PF stimuli at 8 Hz. In contrast, no LTP was observed when oxo-m was present during tetanization. PF-LTP was restored when the CB1R antagonist N-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide (AM251) was coapplied with oxo-m. Furthermore, the suppressive effect of oxo-m on PF-LTP was abrogated by the GDP analog GDP-β-S (applied intracellularly), the phospholipase C inhibitor U-73122, and the diacylglycerol lipase inhibitor tetrahydrolipstatin (THL), suggesting that cannabinoid synthesis results from the activation of Gq-coupled mAChRs present on Purkinje cells. The oxo-m–mediated suppression of LTP was also prevented in the presence of the M3 receptor antagonist DAU 5884, and was absent in M1/M3 receptor double-KO mice, identifying M3 receptors as primary oxo-m targets. Our findings allow for the possibility that cholinergic signaling in the cerebellum—which may result from long-term depression (LTD)-related disinhibition of cholinergic neurons in the vestibular nuclei—suppresses presynaptic LTP to prevent an up-regulation of transmitter release that opposes the reduction of postsynaptic responsiveness. This modulatory capacity of mAChR signaling could promote the functional penetrance of LTD.
Keywords: vestibulocerebellum, learning, retrograde signaling
Long-lasting changes in synaptic efficacy at parallel fiber (PF)–Purkinje cell synapses are widely considered to be a requisite for cerebellar motor learning (1–3). Neuromodulators that can shift the induction probabilities for various types of plasticity at this synapse may thus fine-tune the conditions under which motor learning can occur. One neuromodulator that may function in this role is acetylcholine, which has been shown to affect synaptic plasticity in the hippocampus, visual cortex, and dorsal cochlear nucleus (DCN; a cerebellum-like structure) through the activation of muscarinic acetylcholine receptors (mAChRs) (4–8). There are five known isoforms of mAChRs (designated M1–5), each of which exhibits a heterogeneous distribution pattern across different brain areas (9–11). All five isoforms are expressed in the cerebellar cortex (12) and are found predominantly in lobules IX and X (13), which form the bulk of the vestibulocerebellum. Cholinergic cerebellar afferents to this area are believed to originate from the vestibular nuclei of the brainstem (14, 15), and within these lobules the action of acetylcholine on Purkinje cells has been described as primarily muscarinic (16). Although previous work has characterized how cholinergic agonists (and antagonists) affect action potential firing rates (17–19) and synaptic transmission (16, 20, 21) in the vestibulocerebellum, how mAChR activation affects cerebellar synaptic plasticity has not yet been studied.
mAChRs have been shown to modulate synaptic plasticity by interacting with cannabinoid signaling pathways. For example, mAChR activation was found to enhance cannabinoid-mediated short-term plasticity in the hippocampus (22, 23) and striatum (24). Moreover, mAChR activation can alter the polarity of long-term plasticity by triggering retrograde endocannabinoid signaling in the DCN (8). Cannabinoids are released from the postsynaptic cells and bind to cannabinoid 1 receptors (CB1Rs) on presynaptic terminals, where they diminish neurotransmitter release probability through a Gi/o-coupled pathway (25). In addition to transiently reducing synaptic strength (26, 27), cannabinoid release has been shown to inhibit a presynaptic form of long-term potentiation (LTP) at PF–Purkinje cell synapses (PF-LTP) by suppressing the activation of adenylyl cyclase (28). Here we demonstrate that cholinergic signaling and the activation of mAChRs on Purkinje cells blocks the induction of presynaptic PF-LTP. This effect requires M3 mAChR activity, synthesis and release of endocannabinoids from Purkinje cell dendrites, and activation of CB1Rs. Postsynaptic LTP, but not LTD, is also suppressed by mAChR activation.
Results
Muscarinic Activation Blocks PF-LTP in a Cannabinoid-Dependent Manner.
To assess the impact of mAChR activation on PF-LTP, we performed whole-cell patch-clamp recordings from the soma of Purkinje cells in cerebellar slices obtained from rats at postnatal day (P) 22–31. Because mAChRs are not uniformly expressed throughout the cerebellar cortex, we limited our recordings to Purkinje cells located in lobules IX and X, where mAChR expression levels are highest (12, 13). In control recordings from Purkinje cells in lobules V–VIII, we observed no responses to mAChR activation using oxotremorine-m (oxo-m; 7 µM), a nonspecific muscarinic agonist [PF-excitatory postsynaptic current (EPSCs): +3.9 ± 2.7% of baseline; n = 13; t = 6–10 min; P = 0.17550, paired Student t test] (Fig. S1; compare with the responses in lobules IX and X shown in Fig. 1C).
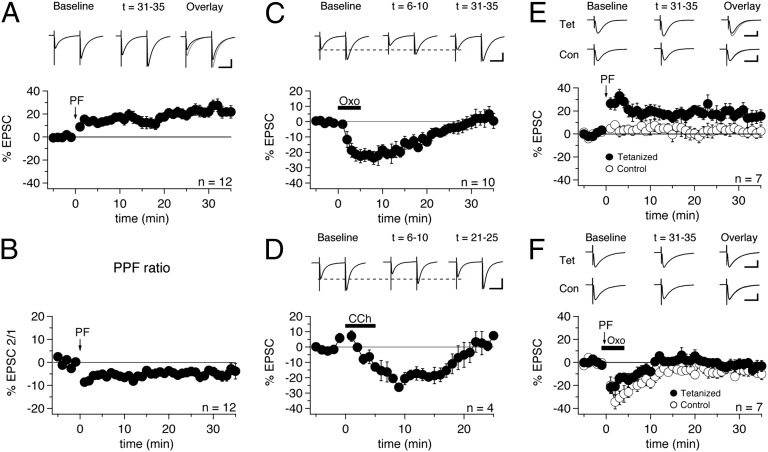
Fig. 1.
Oxo-m blocks presynaptically expressed PF-LTP. (A) Delivery of 120 PF stimuli at 8 Hz induced PF-LTP (n = 12). Each data point represents an average of four successive test responses delivered at 0.067 Hz. The arrow indicates the time point at which the tetanization protocol was administered. At the top of this and the other panels in this figure are representative PF-EPSCs produced by averaging 20 traces from the indicated time periods. (Scale bars: 50 ms, 100 pA.) (B) PPF ratio (EPSC2/EPSC1) of the data shown in A (n = 12). (C) Application of oxo-m (7 µM) for 5 min (period of bath application indicated by black bar) caused transient depression of evoked PF-EPSCs (n = 10). (Scale bars: 50 ms, 100 pA.) (D) Application of CCh (5 µM) also depressed PF-EPSCs (n = 4). (Scale bars: 50 ms, 200 pA.) (E) Synapse-specific PF-LTP was induced by applying the tetanization protocol to one PF input while a control pathway was left unstimulated during the induction period (n = 7). (Scale bars: 20 ms, 100 pA.) (F) Application of oxo-m during the induction period blocked potentiation in the tetanized pathway (n = 7). (Scale bars: 20 ms, 100 pA.) Error bars indicate SEM.
PF-EPSCs were recorded in voltage-clamp mode for at least 5 min to obtain a baseline measurement of PF-EPSC amplitude, after which the recording configuration was switched to current-clamp mode for tetanization, followed by a switch back to voltage-clamp mode to assess the effect on PF-EPSCs. PF-LTP was induced by stimulating the PF input 120 times at 8 Hz, as described previously (29). After tetanization, we observed a significant increase in PF-EPSC amplitudes that lasted at least 35 min (+24.0 ± 3.6%; n = 12; t = 31–35 min; P = 0.00115) (Fig. 1A).
To test whether this potentiation was expressed presynaptically, we measured the paired-pulse ratio (PPR) of PF-EPSCs during the test periods before and after tetanization and found that a significant reduction in PPR accompanied the increase in EPSC amplitude (−4.8 ± 1.8%; n = 12; P = 0.04616) (Fig. 1B). A reduction in PPR is characteristic of an increase in release probability (30), suggesting a presynaptic locus of expression.
Before examining the effect of mAChRs on PF-LTP, we investigated whether their activation had any effect on PF-EPSCs. After a stable baseline was acquired, oxo-m (7 µM) was bath-applied, and slices were exposed to the drug for 5 min. After wash-in, we observed a significant, yet transient, reduction in PF-EPSC amplitude (−21.1 ± 3.5%; n = 10; t = 6–10 min; P = 0.00029) (Fig. 1C). A similar reduction in synaptic responses after application of oxo-m has been reported in the DCN (8). The oxo-m–induced EPSC reduction was associated with an increase in PPR values (+8.3 ± 1.5%; n = 10; t = 6–10 min; P = 0.00233) (Fig. S2), suggesting that this transient depression of EPSCs is a presynaptic effect. We then performed similar experiments with carbachol (CCh; 5 µM), a nonspecific muscarinic and nicotinic agonist, and again observed a PF-EPSC reduction after wash-in comparable to that seen with oxo-m (−20.3 ± 4.8%; n = 4; t = 6–10 min; P = 0.00002; PPR: +10.5 ± 2.6%; n = 2; t = 6–10 min; P = 0.15243) (Fig. 1D), suggesting that acetylcholine receptors are indeed mediating this effect.
We next tested whether mAChR activation affects presynaptic LTP. In these experiments, we stimulated two independent sets of PF synapses (a tetanized input and a control input located on opposite sides of the primary dendrite, with ≥80 µm between the stimulus locations) to differentiate between the effects of oxo-m on basic synaptic transmission on the one hand and the induction of plasticity on the other hand. The independence of these two inputs was confirmed by paired-pulse stimulation, in which the first pulse was applied to input 1 and the second pulse was applied to input 2. Recordings were accepted only if no paired-pulse facilitation was seen. Under control conditions, LTP was observed in the tetanized pathway (+15.8 ± 6.2%; n = 7; t = 31–35 min; P = 0.04253) (Fig. 1E), but not in the control pathway (+1.7 ± 3.4%; n = 7; t = 31–35 min; P = 0.64367) (Fig. 1E). Next, oxo-m was applied during administration of the LTP protocol. Slices were exposed to oxo-m for 1 min before tetanization and for a total of 5 min. In the control pathway, we again observed a transient reduction in EPSCs on oxo-m wash-in, but no potentiation (−8.3 ± 3.2%; n = 7; t = 31–35 min; P = 0.04214) (Fig. 1F). In the tetanized pathway, we found a similar transient effect, and LTP was blocked (−3.1 ± 4.2%; n = 7; t = 31–35 min; P = 0.49200) (Fig. 1F). EPSC amplitude changes were not significantly different in the two pathways (t = 31–35 min; P = 0.23869; unpaired Student t test). The transient EPSC reduction observed was shorter in these experiments than in recordings in which no tetanization was applied (Fig. 1C). Considering that this difference was especially pronounced for the tetanized pathway, it is possible that a remaining transient potentiation counteracted the reduction triggered by oxo-m. Taken together, these experiments show that activation of mAChRs prevents the induction of presynaptic LTP (with no LTP seen in the presence of oxo-m), and that changes in EPSC amplitudes were no larger in tetanized input 1 than in control input 2.
When we repeated this experiment in the presence of the CB1R antagonist N-1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide (AM251; 3 µM) in the bath (with only one PF input stimulated), the potentiation was restored (+17.6 ± 5.7%; n = 7; t = 31–35 min; P = 0.02136) (Fig. 2A), indicating that mAChR activation suppresses PF-LTP by triggering retrograde cannabinoid signaling. In the presence of AM251, we still observed a brief period of PF-EPSC reduction before the resurgence of potentiation (Fig. 2A), suggesting that mAChRs can reduce EPSC amplitudes through mechanisms other than cannabinoid production (Discussion).
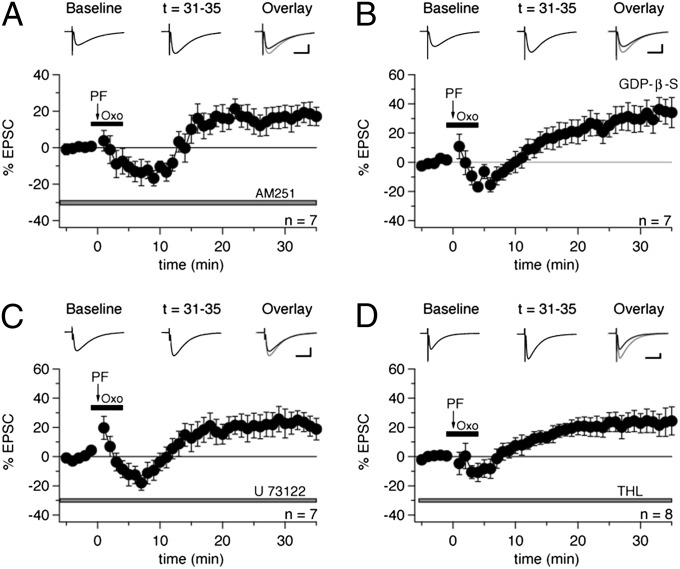
Fig. 2.
Oxo-m–mediated suppression of PF-LTP is CB1R-dependent and results from the activation of Gq-coupled muscarinic receptors present on Purkinje cells. (A) Bath application of the CB1R antagonist AM251 (3 µM; present in the bath throughout the recording; gray bar) blocked the effect of oxo-m and rescued PF-LTP (n = 7). (B) Addition of GDP-β-S (2 mM) to the pipette solution abrogated the effect of oxo-m on PF-LTP (n = 7). (C) Bath application of U-73122 (5 µM; gray bar) rescued PF-LTP (n = 7). (D) Bath application of THL (5 µM; gray bar) also restored PF-LTP (n = 8). (Scale bars: 20 ms, 100 pA.) Error bars indicate SEM.
Cannabinoid Production Results from Activation of Gq-Coupled mAChRs Present on Purkinje Cells.
Having shown that mAChR activation can block the induction of PF-LTP, we next attempted to further characterize the location and identity of the receptors responsible for this effect. To determine whether the mAChR-triggered pathway is located in Purkinje cells, we added the nonhydrolyzable GDP analog GDP-β-S (2 mM), which disrupts G protein-coupled pathways such as those required for cannabinoid production (25), to the recording pipette, and found that oxo-m did not block PF-LTP under these conditions (+33.5 ± 8.9%; n = 7; t = 31–35 min; P = 0.00909) (Fig. 2B). This finding suggests that cannabinoid synthesis follows the activation of mAChRs present on Purkinje cells. In each of these recordings, we also observed a ≥20% decrease in input resistance over time (Methods), which was presumably a side effect of GDP-β-S and has been reported previously (8). Of the five known mAChR subtypes (M1–5), M1, M3, and M5 are Gq-coupled receptors linked to the activation of phospholipase C (PLC) β isoforms (31, 32). PLCβ is a key element in the cannabinoid synthesis pathway (25) and is also activated by metabotropic glutamate receptor type 1 (mGluR1) (33), the metabotropic glutamate receptor required for synaptically evoked cannabinoid signaling in Purkinje cells (27), raising the possibility that mAChRs and mGluR1 trigger cannabinoid signaling through a common mechanism. To test this possibility, we assessed the effect of oxo-m on PF-LTP with the PLC inhibitor U-73122 (5 μM) present in the bath throughout the recording, and found that the muscarinic agonist did not suppress PF-LTP under these conditions (+22.6 ± 6.7%; n = 7; t = 31–35min; P = 0.01471) (Fig. 2C). This finding suggests that mAChR-dependent cannabinoid production relies on PLC as well.
Finally, we performed experiments in which tetrahydrolipstatin (THL; 5 μM), an inhibitor of diacylglycerol (DAG) lipase (DAGL), which is required for synthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG) from DAG, was bath-applied throughout the recordings. In the presence of THL, oxo-m did not block LTP (+23.3 ± 8.0%; n = 8; t = 31–35 min; P = 0.02225) (Fig. 2D), supporting the idea that endocannabinoid signaling mediates the suppression of presynaptic PF-LTP. Taken together, these findings indicate that the disruption of PF-LTP by oxo-m results from the activation of Gq-coupled mAChRs, most likely M1, M3, M5, or some combination thereof, on Purkinje cells and the subsequent synthesis and release of endocannabinoids and activation of CB1Rs.
Pharmacologic Blockade of mAChRs Prevents Oxo-m Effects.
In the DCN, oxo-m has been shown to modulate synaptic plasticity by activating M1/M3 mAChRs (8). To examine whether those same isoforms were responsible for mediating the effects of oxo-m described herein, we washed-in oxo-m (7 µM) after the slices were preincubated with selective inhibitors of M1 and M3 receptors. In the presence of the selective M1 receptor antagonist pirenzepine (10 µM), oxo-m still caused a transient reduction in EPSC amplitude (−13.5 ± 4.9%; n = 7; t = 6–10 min; P = 0.03335) (Fig. 3A). This reduction was less pronounced than that seen after oxo-m application alone (Fig. 1C), but the difference did not reach statistical significance (P = 0.21480). In contrast, the oxo-m–mediated suppression of EPSCs was absent in the presence of the selective M3 receptor antagonist DAU 5884 (1 µM; +2.0 ± 4.5%; n = 9; t = 6–10 min; P = 0.66217) (Fig. 3B). These data suggest that oxo-m activates predominantly M3 receptors on Purkinje cells, and that M1 receptors are not (or at least less significantly) involved.
Fig. 3.
Oxo-m activates predominantly M3 mAChRs on Purkinje cells. (A) Wash-in of oxo-m caused a moderate depression of EPSC amplitudes in the presence of the M1 receptor antagonist pirenzepine (10 µM; gray bar; n = 7). (B) In contrast, the suppressive effect of oxo-m was prevented in the presence of the M3 receptor antagonist DAU 5884 (1 µM; gray bar; n = 9). (C) Bath application of DAU 5884 (1 µM; gray bar) rescued LTP when oxo-m was present during tetanization (n = 6). (Scale bars: 20 ms, 100 pA.) Error bars indicate SEM.
To test whether M3 receptor blockade rescues LTP, we preincubated slices with DAU 5884 (1 µM) and applied the PF tetanization protocol after wash-in of oxo-m (7 µM). Under these conditions, LTP was intact (+22.9 ± 7.0%; n = 6; t = 31–35 min; P = 0.02227) (Fig. 3C), suggesting that it is primarily the activation of M3 receptors that causes the blockade of LTP.
Muscarinic Activation Does Not Block PF-LTP in M1/M3 Double-KO Mice.
To confirm the involvement of M1/M3 receptors in the suppression of LTP in a manner that does not rely on the use of antagonist drugs, we performed experiments in M1/M3 double-KO mice and WT mice with the same genetic background (Methods). We first induced PF-LTP in WT mice via an identical protocol as that used in rats (+34.4 ± 7.7%; n = 5; t = 26–30 min; P = 0.01117) (Fig. 4A). We then tested the effect of oxo-m on PF-LTP and found, as in rats, that the potentiation was blocked (+1.7 ± 6.8%; n = 8; P = 0.80541) (Fig. 4C). However, unlike in rats, we did not observe a temporary decrease in PF-EPSC amplitudes after the application of oxo-m during tetanization, with amplitudes simply returning to baseline after several minutes (compare Fig. 4C with Fig. 1F).
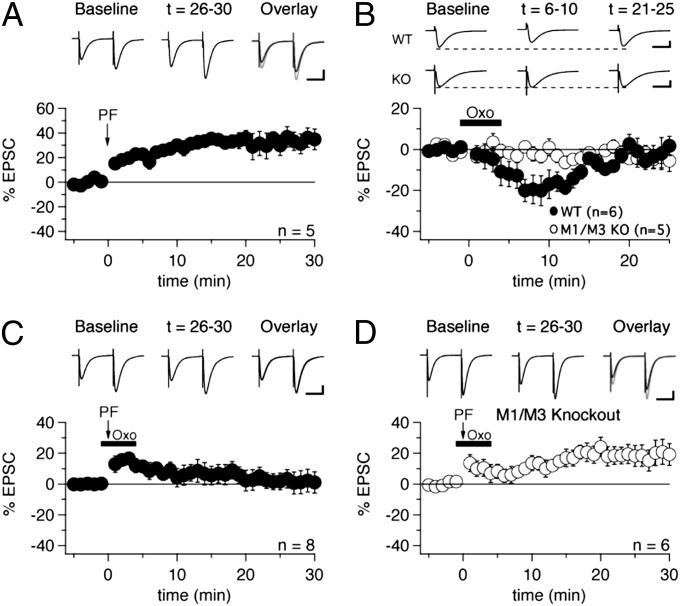
Fig. 4.
Suppressive effect of oxo-m on PF-LTP is absent in M1/M3 double-KO mice. (A) 120 PF stimuli delivered at 8 Hz induced PF-LTP in WT mice (n = 5). (Scale bars: 50 ms, 100 pA.) (B) Application of oxo-m caused transient depression of PF-EPSCs in WT mice (n = 6), but not in M1/M3 double-KO mice (n = 5). (Scale bars: 20 ms, 100 pA.) (C) Oxo-m blocked the induction of PF-LTP in WT mice (n = 8). (Scale bars: 20 ms, 100 pA.) (D) PF-LTP in M1/M3 double-KO mice was unaffected by oxo-m (n = 6). (Scale bars: 50 ms, 100 pA.) Error bars indicate SEM.
To verify that oxo-m affects basic PF transmission similarly in mice as in rats, we exposed WT slices to oxo-m without inducing PF-LTP, and found significantly diminished PF-EPSCs in mice as well (−17.9 ± 5.9%; n = 6; t = 6–10 min; P = 0.02835) (Fig. 4B). This transient EPSC reduction was not observed in recordings from M1/M3 receptor double-KO mice (−1.3 ± 2.2%; n = 5; t = 6–10 min; P = 0.56512) (Fig. 4B), demonstrating that activation of these mAChR isoforms initiates this effect.
Finally, we examined whether oxo-m suppresses PF-LTP in M1/M3 double-KO mice. In contrast to its effect in WT mice, oxo-m had no effect on PF-LTP in the double-KO mice (+18.8 ± 7.2%; n = 6; t = 26–30 min; P = 0.04763; not significantly different from PF-LTP under control conditions, P = 0.17505, unpaired Student t test) (Fig. 4D). This finding suggests that the activation of M1/M3 receptors occurs upstream of cannabinoid production in Purkinje cells. These results confirm the observations obtained using selective M1 and M3 receptor antagonists that implicate M3 receptors, and possibly to a lesser extent M1 receptors, as responsible for the consequences of oxo-m application (Fig. 3).
mAChR Activation Disrupts Postsynaptically Expressed LTP.
To compare the effects of mAChR activation on presynaptic PF-LTP and postsynaptically expressed LTP, we applied oxo-m while stimulating the PF input at 1 Hz for 5 min, an established protocol for the induction of postsynaptic LTP (34, 35). Under control conditions, this tetanization protocol resulted in a significant increase in PF-EPSC amplitude (+27.7 ± 9.4%; n = 5; t = 26–30 min; P = 0.04138) (Fig. S3A). LTP induction was prevented by the presence of oxo-m (7 µM) during tetanization (+4.8 ± 5.6%; n = 9; t = 26–30 min; P = 0.40386) (Fig. S3B).
We also examined the effects of oxo-m on LTD, which was induced by paired PF and climbing fiber (CF) stimulation at 1 Hz for 5 min (−20.5 ± 7.5%; n = 10; t = 31–35 min; P = 0.02262) (Fig. S3C). The presence of oxo-m (7 µM) during tetanization did not prevent the induction of LTD (−17.5 ± 3.8%; n = 9; t = 31–35 min; P = 0.00163) (Fig. S3D), and the levels of depression seen with and without oxo-m were not statistically significantly different (P = 0.73367, unpaired Student t test). To verify that the suppression of postsynaptic LTP by oxo-m did not result from a simultaneous promotion of LTD, we applied the LTP protocol (i.e., PF stimulation for 5 min at 1 Hz) in the presence of oxo-m with the PKC inhibitor chelerythrine (1.5 µM), which blocks LTD induction (36), applied to the bath throughout the recording. Under these experimental conditions, LTP was still blocked (−11.2 ± 7.2%; n = 6; t = 26–30 min; P = 0.17760) (Fig. S3B), indicating that the suppression of LTP is not related to any effect of oxo-m on LTD induction that might occur in parallel.
Discussion
In the present study, we examined the effect of mAChR activation on plasticity at the PF-Purkinje cell synapse and showed a link between mAChR activation and cannabinoid signaling in the cerebellar cortex. We have shown that the muscarinic agonist oxo-m blocks induction of PF-LTP in a CB1R-dependent manner, as demonstrated by the rescue of PF-LTP by AM251. In addition, PF-LTP is restored by the intracellular application of GDP-β-S, bath-application of the PLC inhibitor U-73122, and bath-application of the DAGL inhibitor THL, suggesting that cannabinoid production is triggered by the activation of Gq-coupled mAChRs present on Purkinje cells (Fig. 5). To identify the specific mAChR subtypes activated by oxo-m, we performed experiments using selective M1 and M3 receptor antagonists, as well as M1/M3 double-KO mice. Our data suggest that the effects of oxo-m are mediated by M1/M3 receptor activation, and that M3 receptors are predominantly involved.
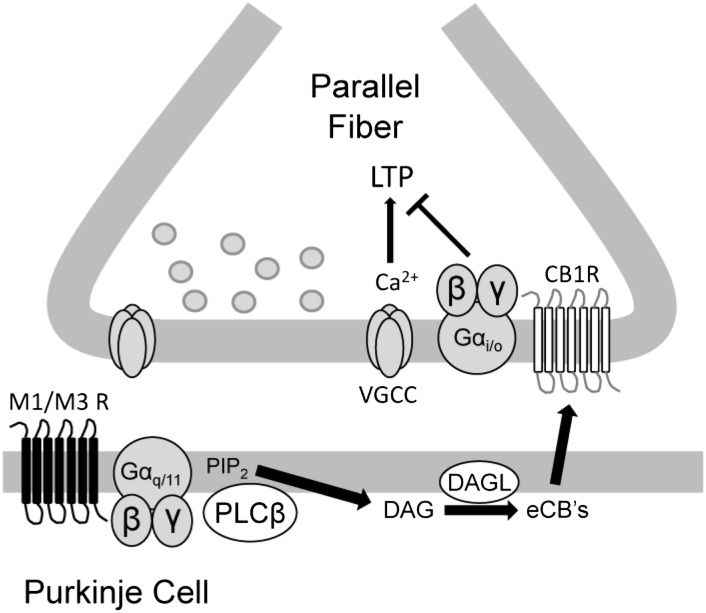
Fig. 5.
Suggested signaling pathway of M1/M3 muscarinic receptors. Activation of M1/M3 receptors on Purkinje cells linked to the activation of PLCβ triggers cannabinoid production and release that is sufficient to block presynaptically expressed LTP. eCBs, endocannabinoids; PIP2, phosphatidylinositol 4,5-bisphosphate; VGCC, voltage-gated calcium channel.
mAChRs on Purkinje Cells.
mAChR expression (12, 13), along with choline acetyltransferase immunoreactivity (14, 15, 37), have been found in both Purkinje cells and molecular layers, the location of Purkinje cell dendrites. Moreover, it has been suggested that acetylcholine exerts a direct effect on Purkinje cells (14, 19). Those previous studies suggest the possibility that Purkinje cells express mAChRs. The results reported here, specifically the lack of LTP suppression on intracellular application of GDP-β-S, provide further support for this idea.
Our experiments in M1/M3 double-KO mice suggest that mAChR subtype M1, subtype M3, or both are responsible for inhibiting LTP induction. In addition, given that the oxo-m–mediated depression of EPSCs and the inhibitory effect on LTP were completely blocked in the presence of the M3 receptor antagonist DAU 5884, whereas oxo-m still caused a reduction (although less pronounced) in the presence of the M1 receptor antagonist pirenzepine, our results suggest that the activation of M3 receptors is primarily responsible for the suppression of LTP. However, considering that M1 and M3 receptors are coupled to the same downstream effectors (31, 32), and that the effect of oxo-m on PF-EPSCs was still somewhat reduced by pirenzepine, we cannot entirely rule out the possibility that M1 receptors partially mediate the effects reported here.
The transient reduction in PF-EPSC amplitudes on bath-application of oxo-m seems to be largely presynaptic (PPR increase) and depends on activation of M3 receptors and, possibly to a lesser degree, M1 receptors (Figs. 3 and 4). Nonetheless, we still observed a significant EPSC reduction in the presence of the CB1R antagonist AM251 (Fig. 2A). These data suggest that this effect is presynaptic, but its predominant component does not depend on CB1R activation. In contrast, the oxo-m–mediated suppression of LTP requires activation of postsynaptic mAChRs, endocannabinoid signaling, and activation of CB1Rs. PF stimulation using double pulses (60-ms interval) has been reported to activate NMDA receptors located on PF terminals (38). Note, however, that the single-pulse stimulation used here is unlikely to engage these NMDA receptors, and thus whether cholinergic signaling affects NMDA receptors and their control of PF plasticity remains to be determined.
Cholinergic Signaling Modulates Synaptic Plasticity in the Cerebellar Cortex.
Our results indicate that mAChR activation has a suppressive effect on LTP induction at PF–Purkinje cell synapses. The blockade of presynaptic LTP via retrograde cannabinoid signaling and CB1R activation is reminiscent of our previous description of a cannabinoid-mediated suppression of LTP resulting from application of the PF-LTP induction protocol (8-Hz, 15-s PF tetanization) combined with CF stimulation at 1–4 Hz (28). Because more prolonged pairing of PF and CF activity can lead to PF-LTD (39), the putative cellular correlate of cerebellar learning (40, 41; but see ref. 42), and because cannabinoids are required for this process (43), it was hypothesized that the role of cannabinoid signaling in LTD induction is to prevent a simultaneous potentiation of transmitter release from diluting the consequences of postsynaptic depression (28). It is possible that in the vestibulocerebellum muscarinic input functions in a similar capacity as that of the CF; that is, it is delivered in a context-dependent fashion and decreases the likelihood of induction for different forms of LTP, potentially enhancing the overall penetrance of LTD. Our data show that the LTD mechanism itself is not affected by mAChR activation, which had seemed possible considering that mAChRs share downstream effectors with mGluR1 (PLC/DAG signaling cascade) in a signaling pathway that is essential for cerebellar LTD (44, 45). In addition, blockade of the LTD pathway by the PKC inhibitor chelerythrine (36) did not interfere with the oxo-m–mediated suppression of postsynaptic LTP. These results suggest that mAChR activation selectively targets presynaptic and postsynaptic LTP mechanisms, but does not directly strengthen the LTD process. Rather, LTD penetrance could be enhanced indirectly by the prevention of coincident potentiation.
Retrograde cannabinoid signaling may play a role in cerebellar motor learning, as evidenced by a study showing deficits in eyeblink conditioning, a cerebellum-dependent process, in CB1R KO mice (46). Notably, the authors did not observe gross ataxia in these mice. We previously argued that cerebellar synaptic plasticity might not be strictly required for the development of basic motor coordination skills, but rather may be needed for motor learning, particularly in the context of fast and complex motor adaptation processes (47). We believe that cholinergic activation of retrograde endocannabinoid signaling may be essential to motor adaptation processes controlled by the vestibulocerebellum that fall into this second category of motor function. Behavioral evidence for such a role of mAChR signaling in motor gain control comes from a study showing that the microinjection of cholinergic agonists into the vestibulocerebellum adjusts the gain of both the vestibulo-ocular reflex and the optokinetic reflex (48). In multiple studies of cerebellar motor learning in genetically altered mice, increases in vestibulo-ocular reflex gain were linked to LTD (1, 2; but see ref. 42). Preynaptic and postsynaptic LTP may play equally important roles and enable—in concert with LTD—bidirectional changes in movement gain (49, 50).
It seems likely that in various motor learning contexts, diverse plasticity mechanisms in the cerebellar cortex and beyond cooperate to adjust motor gains in a situation-appropriate manner. Thus, suppression of presynaptic LTP under conditions that promote postsynaptic LTD (CF coactivity) or when LTD expression is already in progress (see below) may provide a useful mechanism for preventing the simultaneous occurrence of conflicting forms of plasticity, such as potentiation of transmitter release (LTP) on one hand and a reduction in postsynaptic responsiveness (LTD) on the other hand. A conceivable scenario is that LTD expression enhances the likelihood of suppression of LTP, because LTD would promote disinhibition of the vestibular nuclei, which give rise to the cholinergic fibers that innervate the vestibulocerebellum (14, 15). Enhanced activity of this modulatory pathway may suppress the induction of presynaptic and postsynaptic LTP at PF synapses and prevent dilution of the effects of LTD. It is in this context that the potential relevance of cholinergic signaling in the cerebellum becomes obvious; mAChR activation prevents LTP under conditions that require a pronounced depression of PF synaptic strength.
Materials and Methods
Animals.
All procedures were performed in accordance with guidelines of the University of Chicago’s Animal Care and Use Committee. Experiments were performed using Sprague–Dawley rats (P22–31) or M1/M3 double-KO and WT mice (P20–28) with the same genetic background (129SvEv × CF1) (51), generously provided by Dr. Jürgen Wess (National Institute of Diabetes and Digestive and Kidney Diseases).
Slice Preparation.
Animals were anesthetized with halothane and promptly decapitated. The cerebellar vermis from each was then removed and cooled to 4 °C in artificial cerebrospinal fluid (ASCF) containing 124 mM NaCl, 5 mM KCl, 1.25 mM Na2HPO4, 2 mM CaCl2, 2 mM MgSO4, 26 mM NaHCO3, and 10 mM d-glucose, bubbled with 95% O2 and 5% CO2. Parasagittal slices of the cerebellar vermis (220 µm in mice, 250 µm in rats) were prepared with a Leica VT-1000S vibratome, then incubated for at least 1 h at room temperature in oxygenated ACSF.
Somatic Whole-Cell Patch-Clamp Recordings.
Slices were held at room temperature and continuously perfused with ACSF throughout the recordings. Patch-clamp recordings from Purkinje cells were performed with a HEKA EPC-10 amplifier. Currents were filtered at 3 kHz, sampled at 20 kHz, and acquired using HEKA Patchmaster software. Patch pipettes (2–4 MΩ) were filled with a solution containing 120 mM K gluconate, 9 mM KCl, 10 mM KOH, 3.48 mM MgCl2, 10 mM Hepes, 4 mM NaCl, 4 mM Na2ATP, 0.4 mM Na3GTP, and 17.5 mM sucrose (pH 7.25–7.35). PFs were stimulated at 0.067 Hz with a pulse duration of 0.25 ms (0.5 ms for experiments examining postsynaptic LTP) by placing a glass electrode filled with ACSF in the upper one-third of the molecular layer. In experiments with a tetanized pathway and a control pathway, electrodes were placed on different sides of the primary dendrite, at least 80 μm apart, and PF inputs were tested for independence via a paired-pulse assay. Picrotoxin (200 µM) was added to the ACSF throughout all recordings.
The input and series resistance were monitored throughout the experiments by applying hyperpolarizing voltage steps (−10 mV) at the end of each sweep. Recordings were excluded if series or input resistance varied by ≥15% over the course of the experiments (≤20% changes were allowed when EPSCs changed by ≥40%), except for recordings with GDP-β-S, in which all cells exhibited a ≥20% increase in input resistance as the recording progressed (8). All drugs were purchased from Sigma-Aldrich, except for U-73122 and DAU 5884, which were purchased from Tocris Bioscience.
Data Analysis.
Data were analyzed using Excel (Microsoft) and Igor (Wavemetrics). All data are expressed as mean ± SEM. Statistical analyses were performed using paired and unpaired Student t tests as appropriate.
Supplementary Material
Acknowledgments
We thank A. Fox and members of the C.H. laboratory for helpful discussions, and Dr. Jürgen Wess (National Institute of Diabetes and Digestive and Kidney Diseases) for providing the M1/M3 double-KO mice. This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS-062771, to C.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221803110/-/DCSupplemental.
References
- 1.De Zeeuw CI, et al. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20(3):495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- 2.Hansel C, et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006;51(6):835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Schonewille M, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67(4):618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19(5):1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Origlia N, et al. Muscarinic acetylcholine receptor knockout mice show distinct synaptic plasticity impairments in the visual cortex. J Physiol. 2006;577(Pt 3):829–840. doi: 10.1113/jphysiol.2006.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo J, et al. Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci. 2010;13(10):1216–1224. doi: 10.1038/nn.2636. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci. 2011;31(9):3158–3168. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortés R, Probst A, Tobler HJ, Palacios JM. Muscarinic cholinergic receptor subtypes in the human brain, II: Quantitative autoradiographic studies. Brain Res. 1986;362(2):239–253. doi: 10.1016/0006-8993(86)90449-x. [DOI] [PubMed] [Google Scholar]
- 10.Tice MA, Hashemi T, Taylor LA, McQuade RD. Distribution of muscarinic receptor subtypes in rat brain from postnatal to old age. Brain Res Dev Brain Res. 1996;92(1):70–76. doi: 10.1016/0165-3806(95)01515-9. [DOI] [PubMed] [Google Scholar]
- 11.Wevers A. Localisation of pre- and postsynaptic cholinergic markers in the human brain. Behav Brain Res. 2011;221(2):341–355. doi: 10.1016/j.bbr.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Tayebati SK, Vitali D, Scordella S, Amenta F. Muscarinic cholinergic receptors subtypes in rat cerebellar cortex: Light microscope autoradiography of age-related changes. Brain Res. 2001;889(1-2):256–259. doi: 10.1016/s0006-8993(00)03146-2. [DOI] [PubMed] [Google Scholar]
- 13.Neustadt A, Frostholm A, Rotter A. Topographical distribution of muscarinic cholinergic receptors in the cerebellar cortex of the mouse, rat, guinea pig, and rabbit: A species comparison. J Comp Neurol. 1988;272(3):317–330. doi: 10.1002/cne.902720303. [DOI] [PubMed] [Google Scholar]
- 14.Jaarsma D, et al. Cholinergic innervation and receptors in the cerebellum. Prog Brain Res. 1997;114:67–96. doi: 10.1016/s0079-6123(08)63359-2. [DOI] [PubMed] [Google Scholar]
- 15.Barmack NH, Baughman RW, Eckenstein FP. Cholinergic innervation of the cerebellum of rat, rabbit, cat, and monkey as revealed by choline acetyltransferase activity and immunohistochemistry. J Comp Neurol. 1992;317(3):233–249. doi: 10.1002/cne.903170303. [DOI] [PubMed] [Google Scholar]
- 16.Crepel F, Dhanjal SS. Cholinergic mechanisms and neurotransmission in the cerebellum of the rat: An in vitro study. Brain Res. 1982;244(1):59–68. doi: 10.1016/0006-8993(82)90904-0. [DOI] [PubMed] [Google Scholar]
- 17.McCance I, Phillis JW. Discharge patterns of elements in cat cerebellar cortex, and their responses to iontophoretically applied drugs. Nature. 1964;204:844–846. doi: 10.1038/204844a0. [DOI] [PubMed] [Google Scholar]
- 18.McCance I, Phillis JW. Cholinergic mechanisms in the cerebellar cortex. Int J Neuropharmacol. 1968;7(5):447–462. doi: 10.1016/0028-3908(68)90044-0. [DOI] [PubMed] [Google Scholar]
- 19.Crawford JM, Curtis DR, Voorhoeve PE, Wilson VJ. Acetylcholine sensitivity of cerebellar neurones in the cat. J Physiol. 1966;186(1):139–165. doi: 10.1113/jphysiol.1966.sp008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andre P, Pompeiano O, White SR. Activation of muscarinic receptors induces a long-lasting enhancement of Purkinje cell responses to glutamate. Brain Res. 1993;617(1):28–36. doi: 10.1016/0006-8993(93)90608-p. [DOI] [PubMed] [Google Scholar]
- 21.Takayasu Y, Iino M, Furuya N, Ozawa S. Muscarine-induced increase in frequency of spontaneous EPSCs in Purkinje cells in the vestibulo-cerebellum of the rat. J Neurosci. 2003;23(15):6200–6208. doi: 10.1523/JNEUROSCI.23-15-06200.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22(23):10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno-Shosaku T, et al. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18(1):109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- 24.Narushima M, et al. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27(3):496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63(2):154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6(10):1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- 27.Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45(3):419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 28.van Beugen BJ, Nagaraja RY, Hansel C. Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. J Neurosci. 2006;26(32):8289–8294. doi: 10.1523/JNEUROSCI.0805-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16(4):797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- 30.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 31.Felder CC. Muscarinic acetylcholine receptors: Signal transduction through multiple effectors. FASEB J. 1995;9(8):619–625. [PubMed] [Google Scholar]
- 32.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10(1):69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka J, et al. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12(3):781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 34.Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA. 2002;99(12):8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44(4):691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 36.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25(46):10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojima H, Kawajiri S, Yamasaki T. Cholinergic innervation of the rat cerebellum: Qualitative and quantitative analyses of elements immunoreactive to a monoclonal antibody against choline acetyltransferase. J Comp Neurol. 1989;290(1):41–52. doi: 10.1002/cne.902900104. [DOI] [PubMed] [Google Scholar]
- 38.Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33(1):123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Sakurai M, Tongroach P. Climbing fibre-induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4(5):467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 41.Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001;81(3):1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 42.Schonewille M, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70(1):43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48(4):647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Aiba A, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79(2):377–388. [PubMed] [Google Scholar]
- 45.Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12(6):1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 46.Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26(34):8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinaldo L, Hansel C. Ataxias and cerebellar dysfunction: Involvement of synaptic plasticity deficits? Funct Neurol. 2010;25(3):135–139. [PMC free article] [PubMed] [Google Scholar]
- 48.Tan HS, Collewijn H. Cholinergic modulation of optokinetic and vestibulo-ocular responses: A study with microinjections in the flocculus of the rabbit. Exp Brain Res. 1991;85(3):475–481. doi: 10.1007/BF00231730. [DOI] [PubMed] [Google Scholar]
- 49.Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: The role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- 50.Boyden ES, et al. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006;51(6):823–834. doi: 10.1016/j.neuron.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Gautam D, et al. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66(2):260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.