Abstract
Smoking is a major risk factor for osteoporosis and fracture, but the mechanism through which smoke causes bone loss remains unclear. Here, we show that the smoke toxins benzo(a)pyrene (BaP) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) interact with the aryl hydrocarbon receptor (Ahr) to induce osteoclastic bone resorption through the activation of cytochrome P450 1a/1b (Cyp1) enzymes. BaP and TCDD enhanced osteoclast formation in bone marrow cell cultures and gavage with BaP stimulated bone resorption and osteoclastogenesis in vivo. The osteoclastogenesis triggered by BaP or RANK-L was reduced in Ahr−/− cells, consistent with the high bone mass noted in Ahr−/− male mice. The receptor activator of NF-κB ligand (RANK-L) also failed to induce the expression of Cyp1 enzymes in Ahr−/− cells. Furthermore, the osteoclastogenesis induced by TCDD was lower in Cyp1a1/1a2−/− and Cyp1a1/1a2/1b1−/− cultures, indicating that Ahr was upstream of the Cyp enzymes. Likewise, the pharmacological inhibition of the Cyp1 enzymes with tetramethylsilane or proadifen reduced osteoclastogenesis. Finally, deletion of the Cyp1a1, Cyp1a2, and Cyp1b1 in triple knockout mice resulted in reduced bone resorption and recapitulated the high bone mass phenotype of Ahr−/− mice. Overall, the data identify the Ahr and Cyp1 enzymes not only in the pathophysiology of smoke-induced osteoporosis, but also as potential targets for selective modulation by new therapeutics.
Keywords: skeletal remodeling, bone formation, toxicology, osteoblast
Smoking is the leading cause of preventable morbidity and mortality worldwide in the 21st century (1). Estimates from the World Health Organization suggest that ∼6 million people die each year from smoke-related causes. Osteoporosis is an inevitable consequence of long-term smoking and has become a critical determinant of the fracture risk assessment tool FRAX (2). Several metaanalyses have found up to a 40% increase in overall risk of fracture, and an 85% increase in risk of hip fracture in smokers (3, 4).
Smoking is known to impair bone formation by the osteoblast. However, new evidence suggests that smoking also increases osteoclastic bone resorption (5, 6). Notably, bone resorption markers, including total and free deoxypyridinoline and C-telopeptide levels, are higher in current male smokers than in former or never-smokers (5). Likewise, a significant increase of urinary N-telopeptide is notable in elderly women smokers compared with women who do not smoke (6).
Cigarettes contain more than 1,000 chemicals, including at least 150 known toxins. Besides nicotine, these environmental pollutants include polycyclic aryl hydrocarbons (PAHs), of which benzo[a]pyrene (BaP) is a prototype. Another family of smoke contaminants is polychlorinated dibenzodioxins (dioxins), such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Both chemicals bind the aryl hydrocarbon receptor (Ahr), a transcription factor expressed ubiquitously, including in osteoblasts and osteoclasts (7). TCDD has specifically been shown to reduce osteoblast function in a chicken periosteal osteogenesis model (8). TCDD, BaP, and/or cigarette smoke extracts (CSE) also impair chondrocyte differentiation and fracture healing (9).
Studies using RAW264.7 cells show that BaP decreases osteoclast formation by inhibiting receptor activator of NF-κB ligand (RANK-L)–induced NFκB activation (10). However, Ahr can modulate RelB and RelA to induce or inhibit gene expression, respectively (11–13). Furthermore, cell density is a critical determinant of PAH effects, as noted in rabbit osteoclast cultures (14). Although the effect of BaP is inhibitory at high cell densities, it is proosteoclastogenic at low densities. Likewise, another Ahr ligand, 3-methylcholanthrene (3-MC), also inhibits osteoclastogenesis, but by targeting RANK-L production from osteoblasts rather than RANK-L action (15). Additionally, PAHs are known to attenuate hematopoiesis through the Cyp1b1 pathway (16).
Perfect synchrony between the rates of bone formation and bone resorption is required for the maintenance of skeletal integrity (17). Osteoporosis occurs when resorption rates exceed bone formation rates either in absolute terms, or when the two processes become uncoupled. There is no doubt that PAHs reduce bone formation. However, the magnitude of bone loss seen in smokers seems to be consistent with the uncoupling of bone formation from resorption, with resorption rising in the face of reduced bone formation. Although certain studies suggest that bone resorption may be inhibited, this contradicts hyperresorption seen in chronic smokers (5, 6).
We therefore reexamined the mechanism through which smoke carcinogens affect osteoclastic bone resorption. We present genetic and pharmacological evidence that Ahr agonists act through the cytochrome P450 1a/1b (Cyp1) enzymes to stimulate, rather than inhibit, osteoclastogenesis. Thus, mice in which the Ahr or Cyp1a1, Cyp1a2, and Cyp1b1 genes are deleted display reduced resorption and high bone mass. In contrast, Ahr activation by administering BaP to wild-type mice increases osteoclastogenesis and bone resorption.
Results
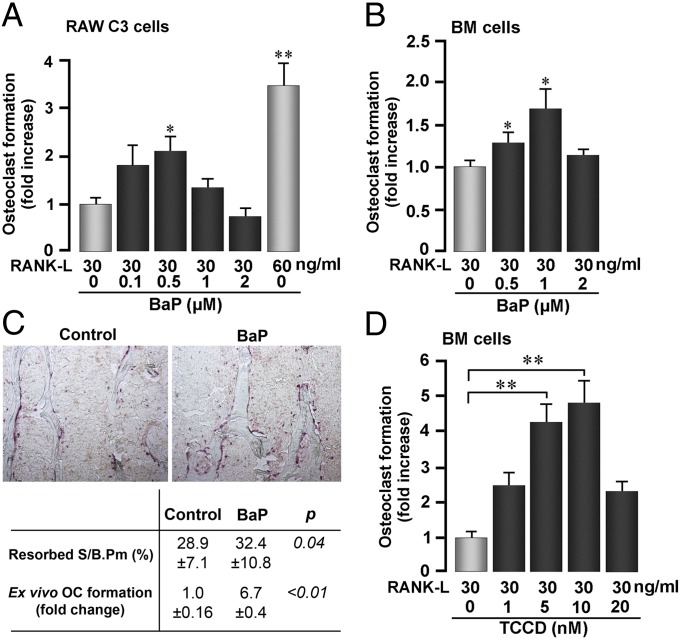
We first examined the ability of the Ahr agonist BaP to modulate osteoclast formation in a pure population of osteoclast precursors, namely RAW-C3 cells (18). We used RANK-L at a concentration (30 ng/mL) that would preserve osteoclast sensitivity to further stimulation by other agents. Indeed, a higher RANK-L concentration (60 ng/mL) stimulated osteoclastogenesis even further (Fig. 1A). BaP caused a significant stimulation of osteoclastogenesis at low concentrations, but no effect was noted at the highest dose (2 µM) (Fig. 1A). Our data contrast that of Voronov et al., who found that BaP reduces osteoclastogenesis in a related cell line RAW264.7 (14). However, they also find that BaP enhances Ahr/NF-κB interaction only in the absence of RANK-L (10).
Fig. 1.
Ahr ligands BaP and TCDD increase osteoclastic resorption. (A, B, and D) TRAP-positive osteoclasts formed from RAW-C3 precursor cells or in bone marrow (BM) cell cultures treated with RANKL (concentrations as noted) and with benzo[a]pyrene (BaP) (A and B) or 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (D). (C) Effect of BaP gastric gavage (120 mg/kg, six doses) or corn oil (control) on bone resorption quantified as Resorbed S/B.Pm (TRAP-stained sections shown, ×10) vertebral bone or osteoclast formation in ex vivo BM cell cultures (C). ANOVA; comparing with zero-dose BaP or TCDD; *P ≤ 0.05; **P ≤ 0.01; n = 4 or 6 per group for ex vivo and in vivo studies, respectively.
We confirmed these findings in cultures of bone marrow-derived osteoclast precursors, wherein low BaP concentrations enhanced osteoclastogenesis (Fig. 1B). Gavage with BaP likewise stimulated osteoclastogenesis and bone resorption (Fig. 1C). Another Ahr agonist, TCDD, similarly increased osteoclastogenesis in a concentration-dependent manner (Fig. 1D). Together, these data show that Ahr agonists, at low doses, augment, rather than decrease, osteoclastogenesis both in cell culture and in vivo.
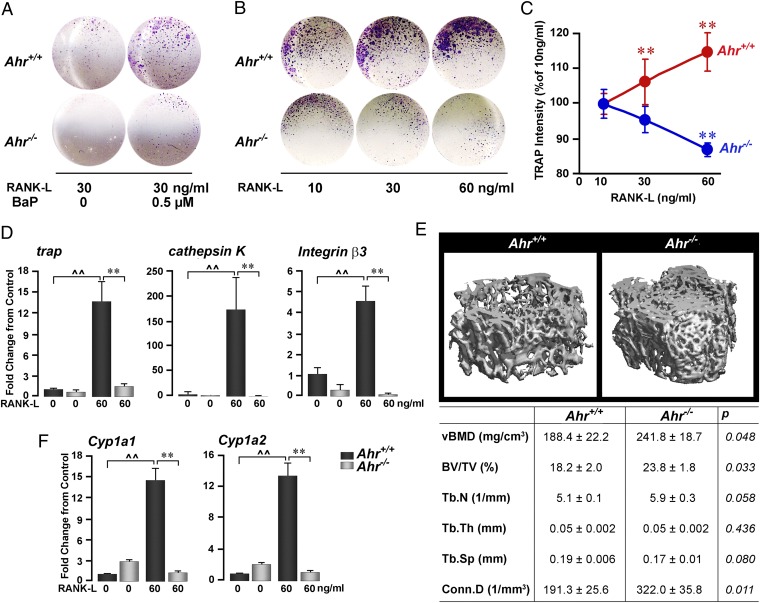
We further examined whether the effects of BaP were Ahr mediated. Notably, the stimulatory effect of BaP on osteoclastogenesis was abolished in Ahr−/− cultures (Fig. 2A), as was the effect of RANK-L (Fig. 2B). Furthermore, whereas RANK-L increased tartrate-resistant acid phosphatase (TRAP) levels in wild-type culture supernatants, it reduced supernatant TRAP in Ahr−/− cultures (Fig. 2C). Of note is that far fewer osteoclasts were formed in Ahr−/− cultures than in wild-type cultures, suggesting that Ahr may in fact be required for basal RANK-L–induced osteoclastogenesis (Fig. 2 A and B).
Fig. 2.
Ahr is required for BaP- and RANK-L–induced osteoclast formation. TRAP-positive osteoclast number (A and B), TRAP production (C), and expression (measured by quantitative PCR) of trap, cathepsin K, and integrin β3 (D) in bone marrow cell cultures from wild-type or Ahr−/− mice treated with BaP (A) or RANK-L (B–D) (concentrations as shown). (E) Micro-CT images and structural parameters in femur metaphyseal bone, including volumetric bone mineral density (vBMD), bone volume/trabecular volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and connectivity density (Conn.D) from wild-type ∼4 mo-old male Ahr+/+ and Ahr−/− mice. (F) Cyp1a1 and Cyp1a2 expression in bone marrow cell cultures from Ahr+/+ and Ahr−/− mice treated with RANK-L (60 ng/mL). ANOVA; comparisons: quantitative PCR, triplicate, wild-type zero-dose vs. RANK-L, ^^P ≤ 0.01; wild type vs. Ahr−/− both with RANK-L, **P ≤ 0.01; micro-CT, P values as shown, n = 5–7 mice per group; cell assays: 4 wells per group.
To confirm that Ahr was required for the effects of RANK-L, we studied the expression of osteoclast differentiation genes in wild-type and Ahr−/− cells. In line with diminished osteoclast formation, RANK-L–induced expression of cathepsin K, trap, and β3 integrin were profoundly suppressed in Ahr−/− cultures (Fig. 2D). Together, these data suggest that Ahr not only mediates the proosteoclastogenic action of BaP, but also contributes, at least in part, to RANK-L–induced osteoclast formation.
The effect of Ahr deletion on osteoclastogenesis per se (Fig. 2 A and B) suggested that Ahr−/− mice might have a basal bone phenotype. Analysis of femoral metaphyseal trabecular bone by micro-CT showed increases in volumetric bone mineral density (vBMD), bone volume/trabecular volume (BV/TV), trabecular number (Tb.N) (P = 0.058), and connectivity density (Conn.D) in Ahr−/− males compared with wild-type controls (Fig. 2E). These findings are opposite to what is seen in female Ahr−/− mice (19).
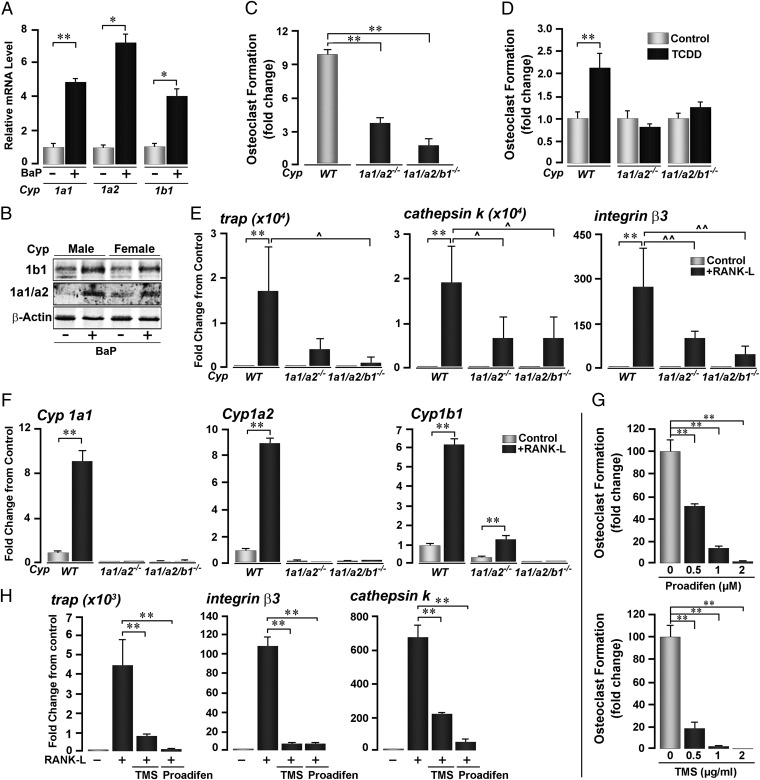
Ahr activation leads to the induction of a number of genes regulating metabolism, most specifically genes encoding the Cyp1 enzymes. We found that RANK-L dramatically increased Cyp1a1 and Cyp1a2 expression in wild-type cells, but not in Ahr−/− cells (Fig. 2F). Studies have suggested a role for Cyp1a1 and Cyp1b1 in BaP-mediated bone marrow toxicity (20–23). Consistent with these studies, gavaging wild-type mice with BaP caused the induction of Cyp1a1, Cyp1a2, and Cyp1b1 mRNAs (Fig. 3A). Furthermore, consistent with a report from Galvan et al. (20), basal bone marrow Cyp1b1 mRNA was 3.8- and 4.8-fold higher than Cyp1a1 and Cyp1a2, respectively. Furthermore, BaP induced Cyp1 protein expression (Fig. 3B). It is of note that these direct effects of BaP, noted in bone marrow cultures, might be confounded in vivo by potentially distinct actions of BaP on hepatic Cyp1 enzymes. Furthermore, BaP-induced Cyp induction was similar in male and female mice, although basal Cyp1a1/Cyp1a2 levels appeared to be lower in male mice (Fig. 3B).
Fig. 3.
Cyp1 induction is necessary for TCDD- and RANK-L–induced osteoclastogenesis. (A and B) Induction of Cyp1a1, Cyp1a2, and Cyp1b1 mRNA (A) and protein (B) expression in bone marrow cell cultures isolated from mice treated with BaP (+, oral gavage, 120 mg/kg, 6 doses). (C and D) TRAP-positive osteoclast formation in bone marrow cell cultures derived from Cyp1a1/1a2−/− double knockout, Cyp1a1/1a2/1b1−/− triple knockout and Cyp1+/+ wild-type mice either without (C) or after treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (D). (E and F) Effect of RANK-L on the expression (quantitative PCR) of the osteoclast genes trap, cathepsin K and β3 integrin (E) and the P450 genes Cyp1a1, Cyp1a2, and Cyp1b1 (F) in the three mouse genotypes. (G and H) Effect of the Cyp inhibitors proadifen and TMS (concentrations as shown) on RANK-L–induced TRAP-positive osteoclast formation (G), and expression of trap, β3 integrin, and cathepsin K mRNA (H). RANK-L was used at 30 ng/mL; with 1 µM proadifen and 1 µg/mL TMS in H. ANOVA; comparisons either with wild-type littermates (C) or zero-dose controls (D–H); **P ≤ 0.01; comparisons between treatments as shown, ^P ≤ 0.05; ^^P ≤ 0.01; for cell cultures, four wells per group; quantitative PCR in triplicate.
We next used Cyp1a1/1a2−/− double knockout and Cyp1a1/1a2/1b1−/− triple knockout mice. Bone marrow cell cultures from these two genotypes showed a marked attenuation in osteoclast formation, albeit not as dramatic as that noted in Ahr−/− mice (Fig. 3C, compare with Fig. 2A). Furthermore, whereas cultures from wild-type mice displayed enhanced osteoclastogenesis in response to TCDD, cultures from Cyp1a1/1a2−/− and Cyp1a1/1a2/1b1−/− mice did not (Fig. 3D). Parallel studies with Cyp1a1/1a2−/− and Cyp1a1/1a2/1b1−/− bone marrow cultures showed decreased expression of several osteoclast differentiation genes, namely cathepsin K, trap, and integrin β3 (Fig. 3E). As expected, Cyp1 gene expression was absent in the triple knockout mice, whereas a small amount of Cyp1b1 induction was noted in Cyp1a1/1a2−/− cultures (Fig. 3F).
The data suggest that Ahr-induced Cyp1 expression mediates, at least in part, the proosteoclastogenic actions of both BaP and RANK-L. If these enzymes are essential, their pharmacological inhibition should likewise diminish osteoclast formation. To test this possibility, we used two well characterized Cyp1 inhibitors, proadifen and tetramethylsilane (TMS). Both compounds suppressed osteoclastogenesis in a concentration-dependent manner (Fig. 3G). Consistent with this, expression of the osteoclast differentiation genes trap, integrin β3, and cathepsin K was markedly attenuated by treatment with either inhibitor (Fig. 3H). Together, these data indicate that Cyp1 inhibition, genetic or pharmacological, potently suppresses osteoclast formation.
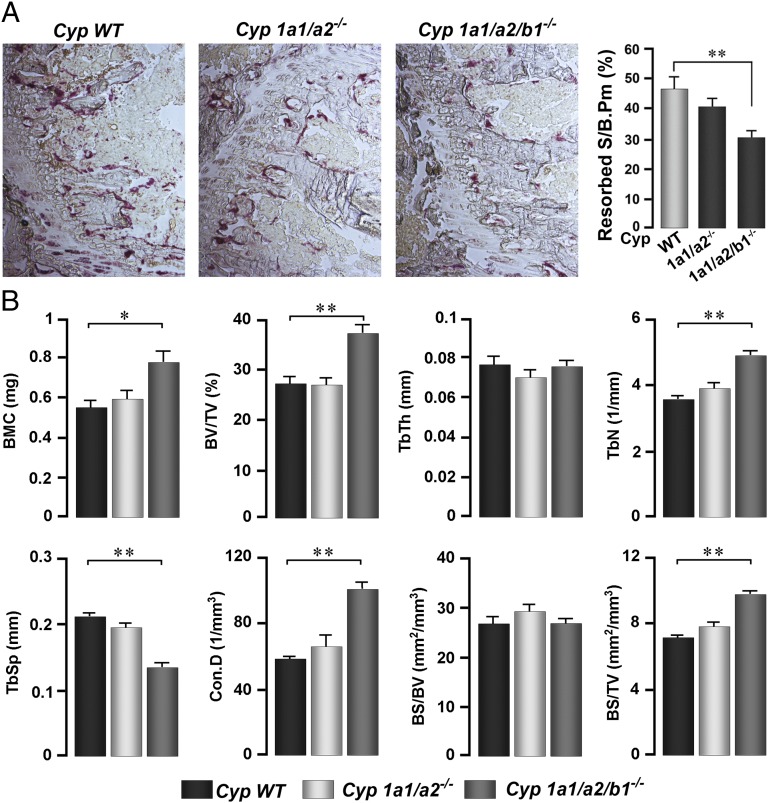
We studied the skeletal phenotype of Cyp1a1/1a2−/− double knockout and Cyp1a1/1a2/1b1−/− triple knockout mice. A significant reduction in Resorbed S/B.Pm was noted in Cyp1a1/1a2/1b1−/− mice (Fig. 4A). These mice further displayed elevated bone mineral content (BMC), BV/TV, Tb.N, Conn.D, and bone surface to total volume (BS/TV) (Fig. 4B). There were minimal or no changes in resorbed surface or bone mass in Cyp1a1/1a2−/− double knockout mice, suggesting the increases in Cyp1b1, noted in Fig. 3F, most likely compensated for the loss of both Cyp1a1 and Cyp1a2 in vivo.
Fig. 4.
Genetic deletion of Cyp1 enzymes reduces bone resorption and increases bone mass in vivo. (A) Bone resorption quantified as Resorbed S/B.Pm (TRAP-stained sections shown, ×10) in proximal tibial metaphyseal trabecular bone of ∼4 mo-old male Cyp1+/+ wild-type (WT), and Cyp1a1/1a2−/− and Cyp1a1/1a2/1b1−/− knockout mice. (B) Structural parameters measured by micro-CT on vertebral bone, including volumetric bone mineral content (BMC), bone volume/trabecular volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.Sp), connectivity density (Conn.D), bone surface/bone volume (BS/BV), and bone surface/trabecular volume (BS/TV) from the three genotypes. ANOVA; comparisons between WT and respective knockout lines; *P ≤ 0.05; **P ≤ 0.01; n = 5–6 mice per group.
Discussion
Both male and female smokers are known to lose bone mass. This smoke-induced osteoporosis has traditionally been attributed to impaired osteoblastic bone formation, although reports of smoke carcinogens on bone resorption remain conflicting (5, 6, 10, 15). For example, BaP has been shown to reduce, rather than increase, osteoclast formation, suggesting that resorption may be suppressed in smokers (10). This notion, however, is inconsistent with the hyperresorptive state documented in women who smoke (5, 6). Here, we show that low concentrations of the Ahr agonists BaP and TCDD stimulate osteoclastogenesis and bone resorption. In contrast, osteoclastogenesis is low in Ahr-null mice. These findings not only explain the hyperresorption seen in smokers (5, 6), but are also consistent with the ∼60% increase in bone resorption noted in mice overexpressing the constitutively activated Ahr (24).
We argue that both Ahr agonists BaP and TCDD directly stimulate osteoclast precursors to form mature osteoclasts via Ahr-mediated Cyp1 activation. We find that RANK-L– and TCDD-induced osteoclastogenesis is inhibited in cells lacking Cyp1a1 and Cyp1a2, or lacking all three Cyp1 enzymes. Furthermore, the Cyp1 antagonists proadifen and TMS inhibit RANK-L–induced osteoclast formation. Importantly, mice lacking all three Cyp1 enzymes display impaired bone resorption resulting in a high bone mass phenotype. Overall, we provide compelling genetic and pharmacologic evidence for a direct role of the Cyp1 pathway in skeletal regulation.
It is known that smokers have decreased baseline estrogen levels, with premenopausal women showing lower luteal phase estrogen. Postmenopausal women smokers likewise display lower estrogen levels, even while on similar hormone replacement regimens as nonsmoking women. Cyp1b1 modulates estrogen levels by hydroxylation of estrogen at 2, 4, and 16 positions (25–28). Thus, by inducing macrophages to express Cyp1b1, BaP may decrease estrogen availability in vivo (25, 26). This antiestrogenic action may, in part, explain the increased bone resorption seen upon BaP gavage. However, that isolated cells show increased osteoclastogenesis in ex vivo cultures suggests that the proresorptive action of BaP is largely estrogen-independent.
Other interactions of BaP in vivo may confound the consequences of its direct action on the Ahr. For example, the Ahr is known to heterodimerize with the estrogen receptor-α (ER-α) to modulate the expression of estrogen-responsive genes (29). Likewise, Cyp1s are involved in the synthesis and degradation of eicosanoids, which are known to affect both osteoclasts and osteoblasts (17, 23, 30). Furthermore, although BaP and TCDD are converted to quinones and dihydrodiol epoxides, respectively, their similar actions on osteoclastogenesis argues for a direct effect of these molecules on the AhR. With that said, other smoke-related dioxins and heterocyclic PAHs, which potentiate the activation of xenobiotic response elements (XRE), may also confound the effects of Ahr agonists on bone in vivo (31).
It is obvious from our data that bone formation, which is known to decrease with PAHs, is uncoupled from the increases we note in osteoclastic bone resorption. This finding could explain the unexpectedly profound osteoporosis and high fracture risk seen in chronic smokers. We would expect that smoke-associated bone loss would be less pronounced if resorption-formation coupling were to remain intact as a result of parallel decreases in bone resorption. Our contradiction of previous findings with PAHs may arise from dose and cell density effects, as noted by others (14); a low cell density and submaximal doses of PAHs may be required to elicit stimulatory actions and thus, recapitulate the hyper-resorption seen in chronic smokers (5, 6).
Although establishing that smoke carcinogens induce, rather than inhibit resorption, our data also reveal a hitherto unexpected physiologic role for Ahr and Cyp1 enzymes in the regulation of osteoclastogenesis, bone remodeling and bone mass. Nonetheless, even deletion of all three Cyp1 enzymes does not completely abolish osteoclast differentiation. This finding indicates that Cyp1 enzymes are necessary, but are not sufficient for osteoclast formation.
Finally, the study implicates AHR as a potential drug target to inhibit bone loss in smokers or those exposed to environmental PAHs, particularly in light of new AHR modulators (AHRMs) (32). Whereas certain AHRMs could potentially diminish osteoclast formation to a therapeutic advantage in smokers, issues regarding specificity are likely to arise. For example, AHR activation is involved in stem cell expansion, and its inhibition may affect other hematopoietic cell lineages (33). However, as with selective estrogen receptor modulators (34), AHRMs might act in a tissue-specific manner, so that the effects of modulation of the same AHR in bone-derived cells by a specific small molecule may yield a different outcome from that triggered in another cell type. The challenge arising from our studies is to develop an AHRM that would inhibit bone resorption while sparing other organs.
Methods
Male and female C57BL/6J inbred mice from The Jackson Laboratory, Cyp1a1/1a2−/− double knockout (22) and Cyp1a1/1a2/1b1−/− triple knockout mice (35) were used in all studies. The knockout lines carry >99.8% C57BL/6J background; we therefore used inbred C57BL/6J as Cyp1+/+ controls. For separate experiments, mice were gavaged orally with BaP (120 mg/kg) for 6 d (given in corn oil).
Mice were killed per protocols approved by University of Pennsylvania and Mount Sinai School of Medicine Institutional Animal Care and Use Committees. Both femurs and tibiae were surgically extracted and placed in medium for bone marrow flushing (α-MEM). Bone marrow cell cultures were performed and stained for TRAP, as described in SI Methods: Osteoclast Cultures from Bone (36). For PCR analysis, cells were stimulated, washed and shredded with a QiaShredder (Qiagen). Total RNA was prepared using TRIzol reagent per manufacturer’s instructions (Invitrogen). RNA (1 µg) was converted to cDNA using high-capacity cDNA synthesis kit (Applied Biosystems) or SuperScript II cDNA synthesis kit (Invitrogen). For quantitative PCR, Supermix with Rox (Bio-Rad) was used with gene-specific primers on an ABI 7900HT (36). TRAP levels in culture supernatants were determined using a Sigma kit (387A-1KT). RAW-C3 cells were cultured as described (18).
Immunoblots were carried out with total cell lysates of RBC-free bone marrow prepared in cell lysis buffer [50 mM Tris⋅HCl, pH 8.0/150 mM NaCl/1% (wt/vol) Nonidet P-40] supplemented with protease inhibitors. Proteins (50 µg) were separated on 12% SDS/PAGE and transferred onto nitrocellulose membrane. Membranes were probed with antibodies against Cyp1a1/1a2, Cyp 1b1 (Santa Cruz Biotechnology) and β-actin (Santa Cruz Biotechnology). Anti-rabbit or anti-goat secondary antibodies conjugated to IR-dyes were used and signals were detected on a Licor Odyssey system.
For the measurement of resorption surfaces (Resorbed S/B.Pm), femur or proximal tibial metaphyses or vertebral bodies were sectioned and TRAP stained using a kit (Sigma 387A-1KT). Images were acquired using a Zeiss Observer Z1 and quantitated with ImageJ software. For micro-CT measurements, trabecular bone was scanned nondestructively by using a Scanco µCT scanner (µCT-40; Scanco Medical) at 12-µm isotropic voxel size, with X-ray source power of 55 kV and 145 µA, and integration time of 300 ms. The scanned grayscale images were processed by using a low-pass Gaussian filter to remove noise, and a fixed threshold of 220 mg/cm3 was used to extract the mineralized bone from soft tissue and the marrow phase. The reconstruction and 3D quantitative analyses were performed using software provided by Scanco Medical; the same settings for scan and analysis were used for all samples. Trabecular bone parameters included vBMD, BMC, BS/TV, BS/BV, BV/TV, Tb.Th, Tb.N, Tb.Sp, and Conn.D.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK80459 (to M.Z. and L.S.), AG23176 (to M.Z.), AG40132 (to M.Z.), GM34883 (to N.G.A.), CA22763 (to N.G.A.), and ES08147, ES014403, and P30 ES06096 (to D.W.N.). J.C. is supported by the US Department of Agriculture Agricultural Research Service Current Research Information System Program (5450-51000-046-00D). N.G.A. is supported by an endowment from the Harriet Ellison Woodward Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.R.J. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220919110/-/DCSupplemental.
References
- 1.Wipfli H, Samet JM. Global economic and health benefits of tobacco control: Part 1. Clin Pharmacol Ther. 2009;86(3):263–271. doi: 10.1038/clpt.2009.93. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization Collaborating Centre for Metabolic Bone Disease. (2013) FRAX (University of Sheffield, Sheffield, UK). Available at www.shef.ac.uk/FRAX.
- 3.Kanis JA, et al. Smoking and fracture risk: A meta-analysis. Osteoporos Int. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 4.Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68(5):259–270. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szulc P, et al. Increased bone resorption in moderate smokers with low body weight: The Minos study. J Clin Endocrinol Metab. 2002;87(2):666–674. doi: 10.1210/jcem.87.2.8232. [DOI] [PubMed] [Google Scholar]
- 6.Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Smoking and bone metabolism in elderly women. Bone. 2000;27(3):429–436. doi: 10.1016/s8756-3282(00)00341-0. [DOI] [PubMed] [Google Scholar]
- 7.Ilvesaro J, Pohjanvirta R, Tuomisto J, Viluksela M, Tuukkanen J. Bone resorption by aryl hydrocarbon receptor-expressing osteoclasts is not disturbed by TCDD in short-term cultures. Life Sci. 2005;77(12):1351–1366. doi: 10.1016/j.lfs.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Singh SU, et al. Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. J Endocrinol. 2000;167(1):183–195. doi: 10.1677/joe.0.1670183. [DOI] [PubMed] [Google Scholar]
- 9.Kung MH, Yukata K, O’Keefe RJ, Zuscik MJ. Aryl hydrocarbon receptor-mediated impairment of chondrogenesis and fracture healing by cigarette smoke and benzo(a)pyrene. J Cell Physiol. 2012;227(3):1062–1070. doi: 10.1002/jcp.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voronov I, Li K, Tenenbaum HC, Manolson MF. Benzo[a]pyrene inhibits osteoclastogenesis by affecting RANKL-induced activation of NF-kappaB. Biochem Pharmacol. 2008;75(10):2034–2044. doi: 10.1016/j.bcp.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 11.DiNatale BC, Schroeder JC, Perdew GH. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol Carcinog. 2011;50(3):173–183. doi: 10.1002/mc.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77(4):734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel CF, et al. Interaction of aryl hydrocarbon receptor and NF-κB subunit RelB in breast cancer is associated with interleukin-8 overexpression. Arch Biochem Biophys. 2011;512(1):78–86. doi: 10.1016/j.abb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voronov I, Heersche JN, Casper RF, Tenenbaum HC, Manolson MF. Inhibition of osteoclast differentiation by polycyclic aryl hydrocarbons is dependent on cell density and RANKL concentration. Biochem Pharmacol. 2005;70(2):300–307. doi: 10.1016/j.bcp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Naruse M, et al. Inhibition of osteoclast formation by 3-methylcholanthrene, a ligand for arylhydrocarbon receptor: Suppression of osteoclast differentiation factor in osteogenic cells. Biochem Pharmacol. 2004;67(1):119–127. doi: 10.1016/j.bcp.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 16.N’jai AU, Larsen MC, Bushkofsky JR, Czuprynski CJ, Jefcoate CR. Acute disruption of bone marrow hematopoiesis by benzo(a)pyrene is selectively reversed by aryl hydrocarbon receptor-mediated processes. Mol Pharmacol. 2011;79(4):724–734. doi: 10.1124/mol.110.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 18.Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 19.Wejheden C, et al. Transgenic mice with a constitutively active aryl hydrocarbon receptor display a gender-specific bone phenotype. Toxicol Sci. 2010;114(1):48–58. doi: 10.1093/toxsci/kfp284. [DOI] [PubMed] [Google Scholar]
- 20.Galván N, Jaskula-Sztul R, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Bone marrow cytotoxicity of benzo[a]pyrene is dependent on CYP1B1 but is diminished by Ah receptor-mediated induction of CYP1A1 in liver. Toxicol Appl Pharmacol. 2003;193(1):84–96. doi: 10.1016/s0041-008x(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 21.Galván N, et al. Induction of CYP1A1 and CYP1B1 in liver and lung by benzo(a)pyrene and 7,12-d imethylbenz(a)anthracene do not affect distribution of polycyclic hydrocarbons to target tissue: Role of AhR and CYP1B1 in bone marrow cytotoxicity. Toxicol Appl Pharmacol. 2005;202(3):244–257. doi: 10.1016/j.taap.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Uno S, et al. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69(4):1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 23.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368(1612):20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lees RL, Sabharwal VK, Heersche JN. Resorptive state and cell size influence intracellular pH regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone. 2001;28(2):187–194. doi: 10.1016/s8756-3282(00)00433-6. [DOI] [PubMed] [Google Scholar]
- 25.Bruno RD, Njar VC. Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg Med Chem. 2007;15(15):5047–5060. doi: 10.1016/j.bmc.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227(2):115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Jefcoate CR, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000;(27):95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 28.Sissung TM, Price DK, Sparreboom A, Figg WD. Pharmacogenetics and regulation of human cytochrome P450 1B1: Implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res. 2006;4(3):135–150. doi: 10.1158/1541-7786.MCR-05-0101. [DOI] [PubMed] [Google Scholar]
- 29.Labrecque MP, et al. Distinct roles for aryl hydrocarbon receptor nuclear translocator and ah receptor in estrogen-mediated signaling in human cancer cell lines. PLoS ONE. 2012;7(1):e29545. doi: 10.1371/journal.pone.0029545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nebert DW, Karp CL. Endogenous functions of the aryl hydrocarbon receptor (AHR): Intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J Biol Chem. 2008;283(52):36061–36065. doi: 10.1074/jbc.R800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasai A, et al. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006;66(14):7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- 32.Yin XF, Chen J, Mao W, Wang YH, Chen MH. A selective aryl hydrocarbon receptor modulator 3,3′-Diindolylmethane inhibits gastric cancer cell growth. J Exp Clin Cancer Res. 2012;31:46. doi: 10.1186/1756-9966-31-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem Cell Rev. 2012;8(4):1223–1235. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagan R. The tissue selective estrogen complex: A novel approach to the treatment of menopausal symptoms. J Womens Health (Larchmt) 2012;21(9):975–981. doi: 10.1089/jwh.2011.3448. [DOI] [PubMed] [Google Scholar]
- 35.Dragin N, et al. Phenotype of the Cyp1a1/1a2/1b1-/- triple-knockout mouse. Mol Pharmacol. 2008;73(6):1844–1856. doi: 10.1124/mol.108.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.