Abstract
Codon use among the three domains of life is not confined to the universal genetic code. With only 22 tRNA genes in mammalian mitochondria, exceptions from the universal code are necessary for proper translation. A particularly interesting deviation is the decoding of the isoleucine AUA codon as methionine by the one mitochondrial-encoded tRNAMet. This tRNA decodes AUA and AUG in both the A- and P-sites of the metazoan mitochondrial ribosome. Enrichment of posttranscriptional modifications is a commonly appropriated mechanism for modulating decoding rules, enabling some tRNA functions while restraining others. In this case, a modification of cytidine, 5-formylcytidine (f5C), at the wobble position-34 of human mitochondrial  (
(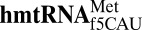 ) enables expanded decoding of AUA, resulting in a deviation in the genetic code. Visualization of the codon•anticodon interaction by X-ray crystallography revealed that recognition of both A and G at the third position of the codon occurs in the canonical Watson–Crick geometry. A modification-dependent shift in the tautomeric equilibrium toward the rare imino-oxo tautomer of cytidine stabilizes the f5C34•A base pair geometry with two hydrogen bonds.
) enables expanded decoding of AUA, resulting in a deviation in the genetic code. Visualization of the codon•anticodon interaction by X-ray crystallography revealed that recognition of both A and G at the third position of the codon occurs in the canonical Watson–Crick geometry. A modification-dependent shift in the tautomeric equilibrium toward the rare imino-oxo tautomer of cytidine stabilizes the f5C34•A base pair geometry with two hydrogen bonds.
Keywords: modified nucleosides, ribosome crystallography, tautomerism
The genetic code was initially deemed to be universal and frozen in time (1). However, deviations in sense and nonsense codon use are found in bacteria, archaea, and both nuclear and organellar eukaryotic genomes (2, 3). Use of genetic codes that deviate from the universal code provides insight into its evolution (4) and possibilities for investigator-initiated manipulation (synthetic biology) (5). In many cases, however, the translation of the deviant sense codons is facilitated by posttranscriptional modification chemistries that are enzymatically added to nucleosides at the first anticodon position. The modification chemistries and their impact on anticodon conformation alter the decoding capacity of the modified tRNA (6). When first proposed, modification-dependent wobble decoding was limited to inosine as the first modified anticodon residue. Inosine, a deaminated adenosine residue, expands the ability of a single isoacceptor tRNA to read three codons by base pairing with either U, C, or A at the third position of the codon (7). Many other wobble position–modified residues, mostly pyrimidines, are now known to modulate use of specific codons (8). Although modified uridines constitute a great majority of these modifications, modified cytidines are prevalent in controlling a switch between the universal genetic code and a deviant code in the shared isoleucine/methionine codon box (9, 10). The modification, 4-acetylcytidine (ac4C), prevents 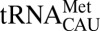 from reading AUA through wobble geometry (11), and lysidine (k2C) (12) and agmatidine (agm2C) (13, 14) prevent
from reading AUA through wobble geometry (11), and lysidine (k2C) (12) and agmatidine (agm2C) (13, 14) prevent 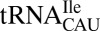 from reading AUG in bacteria and archaea. Interestingly, a fourth cytidine modification, 5-formylcytidine (f5C), facilitates the reading of AUA and AUG as methionine by a single
from reading AUG in bacteria and archaea. Interestingly, a fourth cytidine modification, 5-formylcytidine (f5C), facilitates the reading of AUA and AUG as methionine by a single 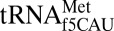 responding to both initiator and elongator codons in yeast and many metazoan mitochondrial genomes (6). Therefore, ac4C, k2C and agm2C are restrictive modifications that alter the physicochemical properties of the Watson–Crick edge, whereas f5C expands decoding using a modification on the C-H edge at the C5 position. It is evident that the many posttranscriptional modifications of the Watson–Crick edge alter base pairing abilities, but a clear mechanism of decoding expansion by C5 modifications remains poorly understood, especially modifications of cytidine.
responding to both initiator and elongator codons in yeast and many metazoan mitochondrial genomes (6). Therefore, ac4C, k2C and agm2C are restrictive modifications that alter the physicochemical properties of the Watson–Crick edge, whereas f5C expands decoding using a modification on the C-H edge at the C5 position. It is evident that the many posttranscriptional modifications of the Watson–Crick edge alter base pairing abilities, but a clear mechanism of decoding expansion by C5 modifications remains poorly understood, especially modifications of cytidine.
The mitochondrion’s decoding of AUA as methionine is important for proper translation. In humans, this codon constitutes 20% of mRNA initiator methionines and 80% of internal methionines (15, 16). Using chemical synthesis and incorporation of the f5C34 modification into the heptadecamer anticodon stem and loop domain (ASL) of human mitochondrial 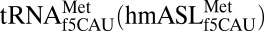 (17), we determined that f5C34 destabilized the
(17), we determined that f5C34 destabilized the 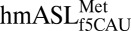 by increasing the motional dynamics of the loop residues (17). A further study detailing the codon-binding characteristics and solution structure of
by increasing the motional dynamics of the loop residues (17). A further study detailing the codon-binding characteristics and solution structure of 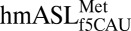 agreed with the f5C-dependent increase in residue dynamics (18). Proposed mechanisms for the decoding of AUA by
agreed with the f5C-dependent increase in residue dynamics (18). Proposed mechanisms for the decoding of AUA by 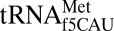 depend on the f5C34•A3 base pair forming a specific geometry (18–20). Based on molecular dynamics simulations, we suggested that this base pair could be in a sheared geometry that is supported by a bridging water molecule (18). To test this hypothesis, the geometry of the f5C34•A3 base pair in the decoding center of the ribosomal A-site was observed in crystal structures of natively modified
depend on the f5C34•A3 base pair forming a specific geometry (18–20). Based on molecular dynamics simulations, we suggested that this base pair could be in a sheared geometry that is supported by a bridging water molecule (18). To test this hypothesis, the geometry of the f5C34•A3 base pair in the decoding center of the ribosomal A-site was observed in crystal structures of natively modified 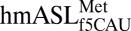 bound to AUA. Here, we show for the first time the modification-dependent f5C34•A3 base pair within the codon•anticodon interaction during ribosomal A-site decoding. Surprisingly, f5C34 forms a canonical Watson–Crick base pair with both the G of AUG and the A of AUA, refuting the conformation predicted from molecular dynamics simulations (18). This geometry of the f5C34•A3 base pair requires a novel amino-imino tautomerism in f5C34 similar to the keto-enol tautomerism seen for the two wobble position uridines cmo5U34 and mcm5s2U34 in Escherichia coli
bound to AUA. Here, we show for the first time the modification-dependent f5C34•A3 base pair within the codon•anticodon interaction during ribosomal A-site decoding. Surprisingly, f5C34 forms a canonical Watson–Crick base pair with both the G of AUG and the A of AUA, refuting the conformation predicted from molecular dynamics simulations (18). This geometry of the f5C34•A3 base pair requires a novel amino-imino tautomerism in f5C34 similar to the keto-enol tautomerism seen for the two wobble position uridines cmo5U34 and mcm5s2U34 in Escherichia coli
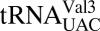 (21) and human
(21) and human 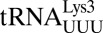 (22), respectively.
(22), respectively.
Results
Codon Bound 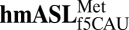 Structure.
Structure.
The ASL domain of 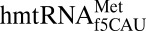 was chemically synthesized with the wobble modification f5C34, the native C33, and a pseudouridine, Ψ27, at the 5′ terminus (Fig. 1A). C33 is quite rare. The uridine at position 33 in tRNAs is considered invariant and recognized for its contribution to the “U-turn” structural motif. There are only 21 known instances of C33, 13 of which are found in initiator tRNAs (23). The
was chemically synthesized with the wobble modification f5C34, the native C33, and a pseudouridine, Ψ27, at the 5′ terminus (Fig. 1A). C33 is quite rare. The uridine at position 33 in tRNAs is considered invariant and recognized for its contribution to the “U-turn” structural motif. There are only 21 known instances of C33, 13 of which are found in initiator tRNAs (23). The 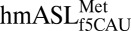 bound both AUG and AUA codons with significant affinity in the A-site of the bacterial ribosome (17) and within the A-site of the bovine mitochondrial 55S ribosome (18). In determining the crystallographic structure of the modified
bound both AUG and AUA codons with significant affinity in the A-site of the bacterial ribosome (17) and within the A-site of the bovine mitochondrial 55S ribosome (18). In determining the crystallographic structure of the modified 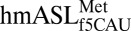 bound to the AUG or the AUA codons in the ribosomal A-site, native Thermus thermophilus 30S ribosomal subunit crystals were soaked with the
bound to the AUG or the AUA codons in the ribosomal A-site, native Thermus thermophilus 30S ribosomal subunit crystals were soaked with the 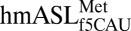 and hexameric oligonucleotides, each containing either AUG or AUA codons. Under the conditions used, both crystals diffracted to 3.3-Å resolution (Table 1). Unbiased difference Fourier electron density maps were generated and used for building the
and hexameric oligonucleotides, each containing either AUG or AUA codons. Under the conditions used, both crystals diffracted to 3.3-Å resolution (Table 1). Unbiased difference Fourier electron density maps were generated and used for building the 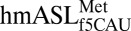 and mRNA residues into the X-ray crystal structures. The structure of the 30S ribosomal subunit (including all RNA and protein) was nearly identical to those reported previously (21, 22). The
and mRNA residues into the X-ray crystal structures. The structure of the 30S ribosomal subunit (including all RNA and protein) was nearly identical to those reported previously (21, 22). The 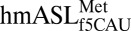 took a conformation nearly identical to that of the ASL of a ribosome-bound tRNA-EF-Tu complex (24, 25), demonstrating the biological relevance of the present structures. More importantly, the conserved A-site residues G530, A1492, and A1493 were in the correct orientation to constrain the codon•anticodon pair residues into the proper geometry for recognition (Fig. 1B) (26, 27). As such, differences in the characteristics of the codon•anticodon interaction can be attributed to the specific conformation of
took a conformation nearly identical to that of the ASL of a ribosome-bound tRNA-EF-Tu complex (24, 25), demonstrating the biological relevance of the present structures. More importantly, the conserved A-site residues G530, A1492, and A1493 were in the correct orientation to constrain the codon•anticodon pair residues into the proper geometry for recognition (Fig. 1B) (26, 27). As such, differences in the characteristics of the codon•anticodon interaction can be attributed to the specific conformation of 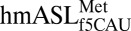 and properties of the f5C34 modification.
and properties of the f5C34 modification.
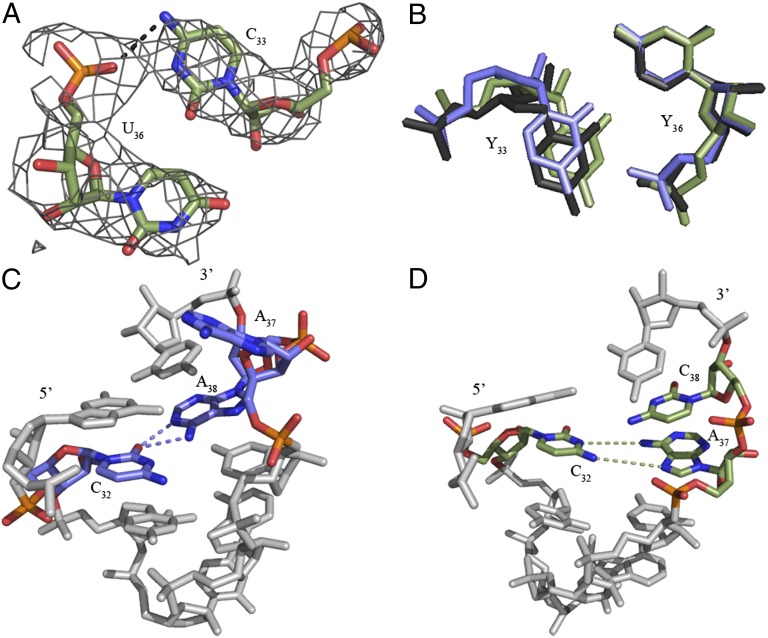
Fig. 1.
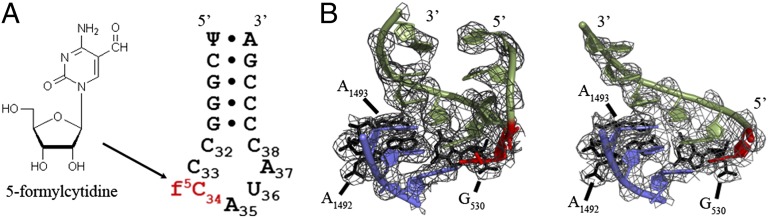
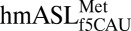 secondary structure and A-site codon•anticodon interaction. (A) The ASL was synthesized as a heptadecamer containing two modifications: pseudouridine at position 27 and 5-formylcytidine at position 34. (B) The structure of
secondary structure and A-site codon•anticodon interaction. (A) The ASL was synthesized as a heptadecamer containing two modifications: pseudouridine at position 27 and 5-formylcytidine at position 34. (B) The structure of 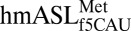 bound to AUG (Left) showed strong electron density for ASL residues 31–39, whereas when bound to AUA (Right), electron density was strong for only residues 34–39. In both structures, the ASL is in green, the mRNA codon is in blue, and the A-site interacting residues (G530, A1492, and A1493) are in black (2mFO-dFC contoured at 1.5 σ). The f5C34 modification is colored red.
bound to AUG (Left) showed strong electron density for ASL residues 31–39, whereas when bound to AUA (Right), electron density was strong for only residues 34–39. In both structures, the ASL is in green, the mRNA codon is in blue, and the A-site interacting residues (G530, A1492, and A1493) are in black (2mFO-dFC contoured at 1.5 σ). The f5C34 modification is colored red.
Table 1.
Data collection and refinement statistics
| Dataset |
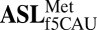 ;Ψ27-AUG ;Ψ27-AUG
|
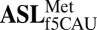 ;Ψ27-AUA ;Ψ27-AUA
|
| Data collection | ||
| Space group | P41212 | P41212 |
| Cell dimensions | ||
| a, b, c (Å) | 400.6, 400.6, 176.2 | 402.4, 402.4, 175.6 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| Resolution (Å) | 80.1–3.3 (3.38–3.30) | 97.6–3.3 (3.38–3.30) |
| Rmerge | 22.5 (133.7) | 19.8 (113.3) |
| I/σI | 6.35 (1.19)* | 5.50 (1.25)* |
| CC1/2 | 0.99 (0.41) | 0.99 (0.49) |
| Completeness (%) | 97.4 (95.0) | 94.7 (93.5) |
| Redundancy | 3.3 (3.2) | 2.6 (2.5) |
| Refinement | ||
| No. reflections | 207,954 | 230,910 |
| Rwork/Rfree | 18.8/22.2 | 19.5/23.6 |
| No. atoms | 52,227 | 52,187 |
| RNA | 32,766 | 32,726 |
| Protein | 19,231 | 19,231 |
| Ion | 188 | 188 |
| Paromomycin | 42 | 42 |
| B-factor | 80.1 | 87.4 |
| r.m.s. deviations | ||
| Bond lengths (Å) | 0.008 | 0.008 |
| Bond angles (°) | 1.36 | 1.35 |
Parentheses indicate highest-resolution shell.
I/σI = 2 at 3.48 Å for both structures.
Conformational Characteristics of 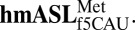
Nearly the entire 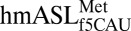 exhibited strong electron density when bound to the cognate AUG codon (Fig. 1B). In the structure of
exhibited strong electron density when bound to the cognate AUG codon (Fig. 1B). In the structure of 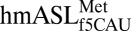 bound to the wobble AUA codon, the codon•anticodon interaction and the 3′ side are clearly resolved. However, there was poor electron density from the 5′-end to the anticodon similar to the poor electron density observed in crystal structures of the mitochondrial
bound to the wobble AUA codon, the codon•anticodon interaction and the 3′ side are clearly resolved. However, there was poor electron density from the 5′-end to the anticodon similar to the poor electron density observed in crystal structures of the mitochondrial 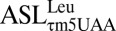 (28) and 8-nt loop containing ASLs (29), both in the T. thermophilus ribosomal A-site. Therefore, the overall conformational properties of the
(28) and 8-nt loop containing ASLs (29), both in the T. thermophilus ribosomal A-site. Therefore, the overall conformational properties of the 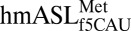 are best described from the perspective of the cognate codon-bound structure, whereas conclusions about the AUA-bound structure will be addressed from comparisons of its highly resolved codon•anticodon interaction and 3′-stacked nucleosides. In the canonical U-turn motif of tRNA’s anticodon loop, a sharp backbone curvature occurs between U33 and the wobble nucleoside at position 34 (30). Despite the presence of a C33, the
are best described from the perspective of the cognate codon-bound structure, whereas conclusions about the AUA-bound structure will be addressed from comparisons of its highly resolved codon•anticodon interaction and 3′-stacked nucleosides. In the canonical U-turn motif of tRNA’s anticodon loop, a sharp backbone curvature occurs between U33 and the wobble nucleoside at position 34 (30). Despite the presence of a C33, the 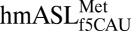 had a U-turn–like architecture. This result confirms NMR (18) and biophysical (17) characterizations. At physiological pH, protonation of U33N3 facilitates hydrogen bond formation between U33N3 and the phosphate of nucleoside 36, stabilizing the characteristic U-turn (30, 31). Although C33N3 is not protonated, the solution structure of
had a U-turn–like architecture. This result confirms NMR (18) and biophysical (17) characterizations. At physiological pH, protonation of U33N3 facilitates hydrogen bond formation between U33N3 and the phosphate of nucleoside 36, stabilizing the characteristic U-turn (30, 31). Although C33N3 is not protonated, the solution structure of 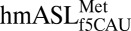 by NMR suggested a weak C33•U36 interaction (18). In the crystal structures, the backbone at C33 was found to be distorted in such a way that the position of C33 was shifted from that expected of a U33. A superimposition of our structure with that of the E. coli elongator
by NMR suggested a weak C33•U36 interaction (18). In the crystal structures, the backbone at C33 was found to be distorted in such a way that the position of C33 was shifted from that expected of a U33. A superimposition of our structure with that of the E. coli elongator 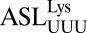 (32) and the human elongator
(32) and the human elongator 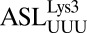 (22), both of which contain a U33 and a U36 (Fig. 2B), confirmed that the position of C33 was slightly altered from the geometry of a U33. This positioning allows for a hydrogen bond between C33N4 and the phosphate of U36 (Fig. 2A), thus stabilizing the backbone turn and the base pairing of the wobble position f5C34.
(22), both of which contain a U33 and a U36 (Fig. 2B), confirmed that the position of C33 was slightly altered from the geometry of a U33. This positioning allows for a hydrogen bond between C33N4 and the phosphate of U36 (Fig. 2A), thus stabilizing the backbone turn and the base pairing of the wobble position f5C34.
Fig. 2.
Unusual features of the 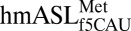 structure. (A) A hydrogen bond is able to form between C33N4 and the phosphate of U36. ASL carbons are colored green, and mRNA carbons are blue (m2FO-dFC contoured at 1.5 σ). (B)
structure. (A) A hydrogen bond is able to form between C33N4 and the phosphate of U36. ASL carbons are colored green, and mRNA carbons are blue (m2FO-dFC contoured at 1.5 σ). (B) 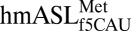 (green) is superimposed with E. coli elongator
(green) is superimposed with E. coli elongator 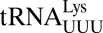 (blue) and human elongator
(blue) and human elongator 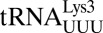 (black), showing a slightly distorted conformation. (C) In E. coli
(black), showing a slightly distorted conformation. (C) In E. coli
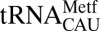 , the cross-loop interaction involves the familiar C32•A38 noncanonical base pair with A37 displaced from the loop, where it cannot participate in base stacking. (D) A cross-loop interaction in
, the cross-loop interaction involves the familiar C32•A38 noncanonical base pair with A37 displaced from the loop, where it cannot participate in base stacking. (D) A cross-loop interaction in 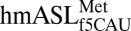 involves a unique interaction between C32 and A37 that consists of a base pair between the Watson–Crick edge of C32 and the Hoogsteen edge of A37.
involves a unique interaction between C32 and A37 that consists of a base pair between the Watson–Crick edge of C32 and the Hoogsteen edge of A37.
Two other properties of the canonical U-turn were investigated: the positioning of A37 over the first codon•anticodon base pair (22, 32, 33) and the formation of a noncanonical base pair between positions 32 and 38 (32–34). In ASLs responding to codons beginning with A, the conserved A37 is modified and positioned directly above the U36•A1 base pair (Fig. 1B) (22, 32, 33). In most organisms, the cytoplasmic methionyl-tRNAs contain an N6-threonylcarbamoyladenosine-37 (t6A37) (23) stacked with, and thus stabilizing, the U36•A1 base pair. However, A37 of 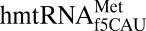 is unmodified, yet appears to satisfactorily promote a stable U36•A1 base pair. The geometry of the noncanonical base pair between residues 32 and 38 was also examined because of its importance in wobble decoding (34). Although the E. coli initiator tRNA is closed by a common C32•A38 noncanonical base pair (Fig. 2C), the primary sequence and secondary structure folding of
is unmodified, yet appears to satisfactorily promote a stable U36•A1 base pair. The geometry of the noncanonical base pair between residues 32 and 38 was also examined because of its importance in wobble decoding (34). Although the E. coli initiator tRNA is closed by a common C32•A38 noncanonical base pair (Fig. 2C), the primary sequence and secondary structure folding of 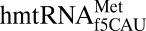 would allow for a C32•C38 cross-loop interaction. Interestingly, A37 and C38 adopt positions within the anticodon loop where C32 is planar with A37 rather than C38. C32 is base paired with A37 (Fig. 2D).
would allow for a C32•C38 cross-loop interaction. Interestingly, A37 and C38 adopt positions within the anticodon loop where C32 is planar with A37 rather than C38. C32 is base paired with A37 (Fig. 2D).
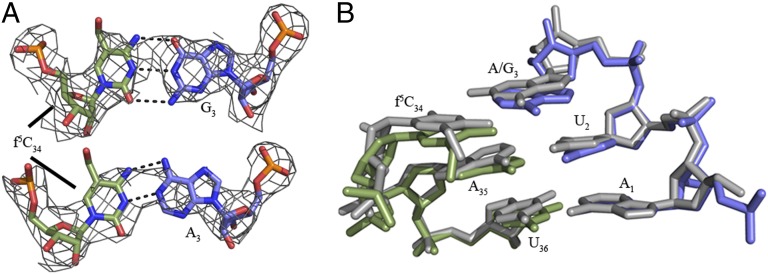
f5C34•A3 Base Pair Adopts a Watson–Crick-Like Geometry.
The f5C34•A3 and f5C34•A3 base pairs are clearly defined in our crystal structures, and both are in Watson–Crick geometry (Fig. 3A). A superposition of the two crystal structures revealed nearly identical codon•anticodon base pair conformations (Fig. 3B). More importantly, this geometry indicates that N4 of the f5C34 must be in the imino-oxo form rather than the common amino-oxo form (Fig. 4). If N4 of f5C34 was in the universal amino form, the close proximity of f5C34N4 and A3N6 (2.8 Å) would cause a very high electronic and steric repulsion. We hypothesized that the formyl group at the C5 position may reduce the energy difference between the tautomeric forms, thus shifting the equilibrium toward the imino-oxo tautomer. A quantum mechanical ground-state free energy calculation was performed on the amino-oxo and imino-oxo forms of the bases cytosine and 5-formylcytosine. A free energy difference of 6.3 kcal/mol between the two tautomeric forms of cytosine favored the amino-oxo form and confirmed previous estimations between 5.4 and 6.7 kcal/mol (35). The tautomers of 5-formylcytosine differed in free energy by 7.8 kcal/mol. Therefore, the f5C-dependent shift in tautomeric equilibrium is not caused by a simple reduction in the energetic properties of the free base.
Fig. 3.
Geometry of the wobble base pair and codon•anticodon interaction. (A) The electron density shows that both the f5C34•G3 and f5C34•A3 base pairs are in Watson–Crick geometry. ASL carbons are colored green, and mRNA carbons are blue (m2FO-dFC contoured at 1.5 σ). (B) Superposition of the 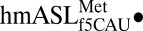 AUG complex with the
AUG complex with the 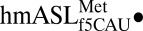 AUA structure aligned with respect to the mRNA residues. The overlay reveals a nearly identical orientation of the codon•anticodon interaction between the AUA-bound and AUG-bound structures. The ASL and mRNA residues for the AUA-bound structures are green and blue, respectively. The ASL and mRNA residues of the AUG-bound structure are both gray.
AUA structure aligned with respect to the mRNA residues. The overlay reveals a nearly identical orientation of the codon•anticodon interaction between the AUA-bound and AUG-bound structures. The ASL and mRNA residues for the AUA-bound structures are green and blue, respectively. The ASL and mRNA residues of the AUG-bound structure are both gray.
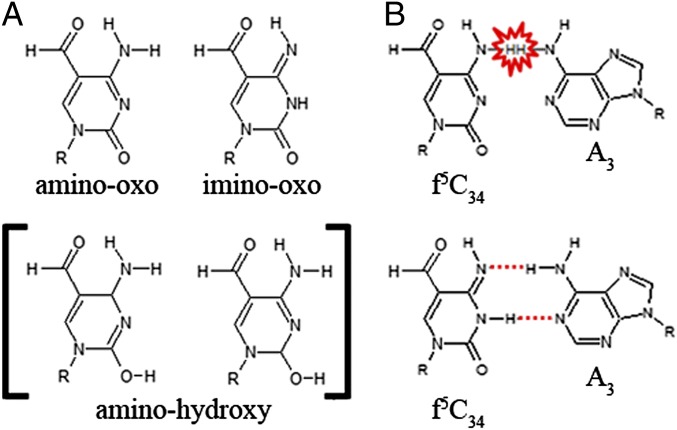
Fig. 4.
Prototropic tautomerism alleviates steric repulsion and allows a Watson–Crick f5C34•A3 base pair. (A) Three possible tautomeric forms of 5-formylcytidine are denoted by the chemical nature of the N4 and O2 positions. (B) In the common amino-oxo form (Upper) of f5C34, a Watson–Crick base pair with adenosine results in steric repulsion due to the proximity of f5C34N4H and A3N6H (marked in red); however, the imino-oxo form (Lower) allows for a favorable U•A-like hydrogen bonding.
Discussion
Cytoplasmic translation initiation at the AUG codon is distinct from elongation in which AUG is decoded in the A-site. There are two different cytoplasmic 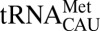 isoacceptors: one for initiating and one for elongating protein synthesis (17). In contrast in the human mitochondrion, a single
isoacceptors: one for initiating and one for elongating protein synthesis (17). In contrast in the human mitochondrion, a single 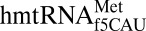 initiates and elongates at either AUG or the universal isoleucine codon AUA. Eighty percent of mitochondrial mRNAs initiate translation with AUG; whereas 80% of all methionine codons within the mRNA are AUA (15, 16). The f5C34-modified
initiates and elongates at either AUG or the universal isoleucine codon AUA. Eighty percent of mitochondrial mRNAs initiate translation with AUG; whereas 80% of all methionine codons within the mRNA are AUA (15, 16). The f5C34-modified 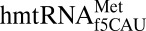 decodes the isoleucine AUA codon as methionine in vivo and in vitro (20). The crystal structures presented here offer a structural and chemical rationale for how
decodes the isoleucine AUA codon as methionine in vivo and in vitro (20). The crystal structures presented here offer a structural and chemical rationale for how 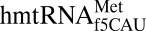 decodes both AUA and AUG and alters the anticodon domain architecture to act as both an initiator and elongator tRNA.
decodes both AUA and AUG and alters the anticodon domain architecture to act as both an initiator and elongator tRNA.
Three possible f5C34•A3 base pairing strategies have been proposed. The base pair could be in a sheared geometry in which A3N6 acts as a proton donor in a bifurcated hydrogen bond with f5C34N1 and O2. A bridging water molecule acts as a dual proton donor for hydrogen bonds with both f5C34O2 and A3N1 (18). In another possible geometry, a single hydrogen bond is formed between f5C34N4 and A3N1 (20). Finally, it has been proposed that A3 could become protonated and form an f5C34•A3+ base pair. The resulting noncononical pair could be in either of two different geometries: one in which f5C34 is shifted such that f5C34O2 and N3 act as hydrogen bond acceptors with A3N1 and N6, respectively (19), and one in which the bases are in the Watson–Crick orientation (20) with f5C34N3 bound to A3N1+.
Of the three possible base pairing schemes, only the Watson–Crick geometry with the protonated adenosine on the codon fits with our refined structure. The importance of Watson–Crick base pairing for codon recognition on the ribosome was first proposed by Crick (7), and more recently reiterated with the support of extensive crystallographic data (21, 22). However, a Watson–Crick geometry for an f5C34•A3+ base pair should be highly unstable, if not energetically impossible, due to the close proximity of the amine protons of f5C34N4 and A3N6 (Fig. 4B). The most likely scenario for resolving the clear geometric restraints observed in the electron density with the physicochemical properties of the two residues involves a novel shift in the tautomeric equilibrium of modified f5C34. Of the three possible prototropic tautomers of cytidine (Fig. 4A), equilibrium must exist between the most common amino-oxo form for base pairing with G3 and the less common imino-oxo tautomer for interacting with A3. Although theoretical calculations coupled with empirical photoemission data have identified a rarely populated existence of the imino-oxo tautomer (36), the C5-formyl modification may perturb the electronic structure of the heterocycle, reducing the energy of this tautomer. Prototropic tautomerism is not unprecedented at the wobble position. C5-modified uridines at position 34 in E. coli
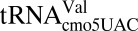 (21) and human
(21) and human 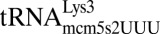 (22) also make use of tautomeric shifts to enable wobble decoding in the ribosomal A-site. Although the different tautomeric forms of uridine have been shown experimentally, this property has not been suggested for cytidine due to significant differences in the electronic structures of the two pyrimidines.
(22) also make use of tautomeric shifts to enable wobble decoding in the ribosomal A-site. Although the different tautomeric forms of uridine have been shown experimentally, this property has not been suggested for cytidine due to significant differences in the electronic structures of the two pyrimidines.
The apparent commonality of using prototropic tautomerization in tRNA’s decoding of bacterial, metazoan, and mitochondrial mRNA compelled us to explore how the C5 position modifications facilitate tautomeric shifts. It has been estimated that common tautomers of nucleobases outnumber their rarer counterparts by ∼104–105 (35), constituting an energy difference between the two states of a mere 5.4–6.7 kcal/mol. Such a relatively small energetic difference resulting in a large shift in the populations of the different tautomeric states indicates that these modifications must be finely tuned for their function in protein synthesis. We hypothesized that the addition of the formyl group at C5 may contribute to the preference for a proton shift from the N4 amine to N3. However, our computational investigation of the effects of the weak electron withdrawing formyl group shows that it increases the energy difference between the tautomers of the free base from 6.3 kcal/mol for cytosine to 7.8 kcal/mol for 5-formylcytosine. However, the tautomeric equilibrium may be affected by interactions of the modification with the environment. This proposition is justified by a study of the interaction between glycine and uridine using density functional theory and showing that the enol form of uridine can be stabilized by up to five orders of magnitude through intermolecular interactions (37). Also, the potential for a pseudobicyclic ring system resulting from a hydrogen bond between the formyl oxygen and the N4 imine proton may contribute energy to the system in the form of improved base stacking interactions and ring current effects.
In addition to providing clear evidence of mechanism by which 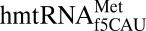 decodes AUA, the crystal structures provide insight into the ability of this single isoaccepting species to act as both an initiator and an elongator tRNA. Many other characteristics of the tRNA may be responsible for this dual role; however, architectural features of the ASL may also contribute. Because the mitochondrial translation system is more similar to prokaryotic translation (38, 39), we compared
decodes AUA, the crystal structures provide insight into the ability of this single isoaccepting species to act as both an initiator and an elongator tRNA. Many other characteristics of the tRNA may be responsible for this dual role; however, architectural features of the ASL may also contribute. Because the mitochondrial translation system is more similar to prokaryotic translation (38, 39), we compared 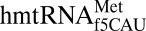 with prokaryotic initiator tRNAs. Although many examples of insect, plant, and vertebrate initiator tRNAs contain C33 (40–45), all known prokaryotic initiator tRNAs contain the universal U33. The ASL of the E. coli initiator
with prokaryotic initiator tRNAs. Although many examples of insect, plant, and vertebrate initiator tRNAs contain C33 (40–45), all known prokaryotic initiator tRNAs contain the universal U33. The ASL of the E. coli initiator 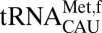 has a peculiar characteristic in which U33 is positioned outside of the anticodon loop rather than interacting with the phosphate of residue 36 in the canonical U-turn (46). Although this is at odds with a solution structure indicating that U33 is positioned in the canonical U-turn geometry (47), it raises the possibility that the positioning of residue 33 is important for discriminating A-site and P-site binding. In comparison, the E. coli
has a peculiar characteristic in which U33 is positioned outside of the anticodon loop rather than interacting with the phosphate of residue 36 in the canonical U-turn (46). Although this is at odds with a solution structure indicating that U33 is positioned in the canonical U-turn geometry (47), it raises the possibility that the positioning of residue 33 is important for discriminating A-site and P-site binding. In comparison, the E. coli
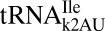 and archaeal
and archaeal 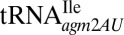 would decode AUG was it not for the restrictive recognition of A3 by the modifications lysidine and agmatidine, respectively (12, 13). The contrast with the
would decode AUG was it not for the restrictive recognition of A3 by the modifications lysidine and agmatidine, respectively (12, 13). The contrast with the 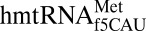 is dramatic. The
is dramatic. The 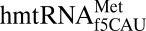 in participating in both A-site and P-site decoding should have the flexibility to adopt different conformations depending on the present function. Indeed, a solution structure of the modified
in participating in both A-site and P-site decoding should have the flexibility to adopt different conformations depending on the present function. Indeed, a solution structure of the modified 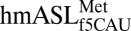 illustrates very little interaction between C33 and U36 (18), and the present crystal structure shows a clear hydrogen bonding between the two residues, albeit in an uncommon geometry. Therefore, our results suggest an induced-fit model that results in the A-site–bound conformation. The resulting conformation favors the canonical U-turn in which C33 interacts with U36 and may also fit into a slightly different conformation for the P-site. Additionally, the unique C32•A37 base pair may further highlight the dual character of this tRNA. In elongator tRNAs, especially where a U36•A1 base pair is present, A37 is typically modified to promote stacking that stabilizes this first base pair of the codon•anticodon interaction. Here the lack of an A37 modification may allow the
illustrates very little interaction between C33 and U36 (18), and the present crystal structure shows a clear hydrogen bonding between the two residues, albeit in an uncommon geometry. Therefore, our results suggest an induced-fit model that results in the A-site–bound conformation. The resulting conformation favors the canonical U-turn in which C33 interacts with U36 and may also fit into a slightly different conformation for the P-site. Additionally, the unique C32•A37 base pair may further highlight the dual character of this tRNA. In elongator tRNAs, especially where a U36•A1 base pair is present, A37 is typically modified to promote stacking that stabilizes this first base pair of the codon•anticodon interaction. Here the lack of an A37 modification may allow the 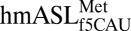 to adopt the more “initiator-like” conformation for P-site binding, whereas the unusual C32•A37 base pair promotes architecture more similar to the canonical elongator conformation for A-site codon recognition. The unusual C32•A37 base pairing does not preclude the possibility of a change in the C32 base-pairing partner between A37 and C38 (Fig. 2C). Such a change in base pairing could correspond to an architectural switch of the elongator
to adopt the more “initiator-like” conformation for P-site binding, whereas the unusual C32•A37 base pair promotes architecture more similar to the canonical elongator conformation for A-site codon recognition. The unusual C32•A37 base pairing does not preclude the possibility of a change in the C32 base-pairing partner between A37 and C38 (Fig. 2C). Such a change in base pairing could correspond to an architectural switch of the elongator 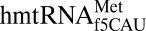 to that of the initiator function. Thus, we showed that tRNA’s codon recognition is expanded through a modification-dependent f5C34 amine-imine tautomerization of cytidine. Overall, the architecture afforded by the natural modifications and sequence change to C33 allow for a significantly altered mode of codon recognition, one in which a single tRNA acts as both an initiator and elongator. The single tRNAMet contributes to the mitochondrion’s challenge of maintaining a minimal genome.
to that of the initiator function. Thus, we showed that tRNA’s codon recognition is expanded through a modification-dependent f5C34 amine-imine tautomerization of cytidine. Overall, the architecture afforded by the natural modifications and sequence change to C33 allow for a significantly altered mode of codon recognition, one in which a single tRNA acts as both an initiator and elongator. The single tRNAMet contributes to the mitochondrion’s challenge of maintaining a minimal genome.
Materials and Methods
Crystallization.
T. thermophilus 30S ribosomal subunits were purified, crystallized, and cryoprotected in 26% (vol/vol) 2-methyl-2,4-pentanediol (MPD), 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.5), 200 mM KCl, 75 mM NH4Cl, and 15 mM MgCl2 (48). Hexameric mRNA oligonucleotides were chemically synthesized and gel-purified using preparative PAGE (Thermo Fisher) with the sequences 5′-AU(A/G)AAA-3′ (codons underlined). After cryoprotection, the empty 30S ribosome crystals were soaked in cryoprotection solution supplemented with 80 µM paromomycin, 300 µM ASL, and 300 µM mRNA (>48 h) (26, 27). Crystals were flash-frozen using liquid nitrogen and stored for data collection. Paromomycin was used for its ability to enhance ASL density and resolution (26) by inducing a closed form of the 30S ribosomal subunit without altering the ASL conformation (27). A recent study has shown that lack of covalent attachment between P- and A-site codons in 30S ribosome structures negates some restraint naturally imposed on the first codon•anticodon base pair (corresponding to U36•A1 in the current structures), allowing them to adopt a wobble base pairing geometry (49). However, currently known structures of cognate codon recognition in the 30S ribosomal subunit show this base pair in the canonical Watson–Crick geometry (21, 22), thereby imposing the same geometric restraints on the remaining codon residues as that seen in the 70S structures. Indeed, structures of cognate codon recognition on the 30S ribosomal subunit superimpose almost exactly with those of the 70S structures [all atom rmsd of codon•anticodon residues from Protein Data Bank entries 1IBL (30S) (26) and 2J00 (70S) (50) = 0.345 Å].
Data Collection and Refinement.
Data were collected at The Northeastern Collaborative Access Team (NE-CAT) beamlines 24-ID-C and 24-ID-E of the Advanced Photon Source. Processing was performed using XDS (51), PHENIX (52) for format manipulation and refinement, Coot (53) for visualization and model building, and PyMOL (54) for figure production. Geometry restraints and dictionary files for the nonstandard residues (paromomycin and 5-formylcytidine) were generated with the eLBOW (55) module within PHENIX using the semiempirical quantum mechanical AM1 method. The two different structures were generated from data sets that were collected at different times; therefore, resolution differences in the structures were likely caused by differences in the X-ray beam rather than in the crystals themselves. The CC1/2 values are calculated using the newest version of XDS (51).
Relative Ground-State Energy Calculations.
Structures corresponding to the amino-oxo tautomers of cytidine and 5-formylcytidine base were prepared using GaussView03 (56) by replacing the ribose moiety with a methyl group. All subsequent calculations were performed using Gaussian-03 (57). Additionally, the imino-oxo tautomers of each were built with the imino proton in the trans-orientation (facing away from the Watson–Crick face) to account for the geometry that would allow for base pairing without steric repulsion. The ground-state geometries were first optimized at the semiempirical level using the AM1 method. Further geometry optimization was achieved at the HF/6–31G(d,p) level. Single point energy calculations were then performed at the B3LYP/6–311++G(d,p) level. All HF and B3LYP calculations were performed with a Polarizable Continuum Model using the integral equation formalism variant (IEFPCM) to simulate water solvation (58).
Acknowledgments
We thank R. Kaiser and M. O. Delaney for synthesis of the 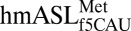 (17); W. D. Graham for purification of the ASL; and L. L. Spremulli, J. L. Spears, and C. J. Stark for critical manuscript reading. This work was supported by National Science Foundation Grant MCB1101859 (to P.F.A.). H.D. was supported by Grants GM019756 and GM094157 from the US National Institutes of Health. This work is based on research conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines supported by grants from the National Center for Research Resources (5P41RR015301-10) and the National Institute of General Medical Sciences (8 P41 GM103403-10). Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by US DOE Contract DE-AC02-06CH11357.
(17); W. D. Graham for purification of the ASL; and L. L. Spremulli, J. L. Spears, and C. J. Stark for critical manuscript reading. This work was supported by National Science Foundation Grant MCB1101859 (to P.F.A.). H.D. was supported by Grants GM019756 and GM094157 from the US National Institutes of Health. This work is based on research conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines supported by grants from the National Center for Research Resources (5P41RR015301-10) and the National Institute of General Medical Sciences (8 P41 GM103403-10). Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by US DOE Contract DE-AC02-06CH11357.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates for hmASLMetf5CAU-AUG and hmASLMetf5CAU-AUA have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4GKJ and 4GKK, respectively).
References
- 1.Crick FH. The origin of the genetic code. J Mol Biol. 1968;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- 2.Koonin EV, Novozhilov AS. Origin and evolution of the genetic code: The universal enigma. IUBMB Life. 2009;61(2):99–111. doi: 10.1002/iub.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengupta S, Yang X, Higgs PG. The mechanisms of codon reassignments in mitochondrial genetic codes. J Mol Evol. 2007;64(6):662–688. doi: 10.1007/s00239-006-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cedergren RJ. An evaluation of mitochondrial tRNA gene evolution and its relation to the genetic code. Can J Biochem. 1982;60(4):475–479. doi: 10.1139/o82-056. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs FJ, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science. 2011;333(6040):348–353. doi: 10.1126/science.1205822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe K, Yokobori S. tRNA modification and genetic code variations in animal mitochondria. J Nucleic Acids. 2011;2011:623095. doi: 10.4061/2011/623095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick FH. Codon—anticodon pairing: The wobble hypothesis. J Mol Biol. 1966;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 8.Agris PF. Wobble position modified nucleosides evolved to select transfer RNA codon recognition: A modified-wobble hypothesis. Biochimie. 1991;73(11):1345–1349. doi: 10.1016/0300-9084(91)90163-u. [DOI] [PubMed] [Google Scholar]
- 9.Cantara WA, et al. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res. 2011;39(Database issue):D195–D201. doi: 10.1093/nar/gkq1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yokoyama S, Nishimura S (1995) Modified nucleosides and codon recognition. tRNA: Structure, Biosynthesis and Function, eds Schimmel PR, Söll D, RajBhandary UL (ASM Press, Washington, DC), pp 207–223.
- 11.Oashi Z, et al. Characterization of C + located in the first position of the anticodon of Escherichia coli tRNA Met as N 4 -acetylcytidine. Biochim Biophys Acta. 1972;262(2):209–213. [PubMed] [Google Scholar]
- 12.Muramatsu T, et al. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J Biol Chem. 1988;263(19):9261–9267. doi: 10.1351/pac198961030573. [DOI] [PubMed] [Google Scholar]
- 13.Mandal D, et al. Agmatidine, a modified cytidine in the anticodon of archaeal tRNA(Ile), base pairs with adenosine but not with guanosine. Proc Natl Acad Sci USA. 2010;107(7):2872–2877. doi: 10.1073/pnas.0914869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voorhees RM, et al. The structural basis for specific decoding of AUA by isoleucine tRNA on the ribosome. Nat Struct Mol Biol. 2013;20(5):641–643. doi: 10.1038/nsmb.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CN, Wilkinson KA, Hung KT, Weeks KM, Spremulli LL. Lack of secondary structure characterizes the 5′ ends of mammalian mitochondrial mRNAs. RNA. 2008;14(5):862–871. doi: 10.1261/rna.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Gojobori T, Ikemura T. Codon usage tabulated from international DNA sequence databases: Status for the year 2000. Nucleic Acids Res. 2000;28(1):292. doi: 10.1093/nar/28.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lusic H, et al. Synthesis and investigation of the 5-formylcytidine modified, anticodon stem and loop of the human mitochondrial tRNAMet. Nucleic Acids Res. 2008;36(20):6548–6557. doi: 10.1093/nar/gkn703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbille Y, et al. The human mitochondrial tRNAMet: Structure/function relationship of a unique modification in the decoding of unconventional codons. J Mol Biol. 2011;406(2):257–274. doi: 10.1016/j.jmb.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leontis NB, Stombaugh J, Westhof E. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 2002;30(16):3497–3531. doi: 10.1093/nar/gkf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takemoto C, et al. Unconventional decoding of the AUA codon as methionine by mitochondrial tRNAMet with the anticodon f5CAU as revealed with a mitochondrial in vitro translation system. Nucleic Acids Res. 2009;37(5):1616–1627. doi: 10.1093/nar/gkp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weixlbaumer A, et al. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat Struct Mol Biol. 2007;14(6):498–502. doi: 10.1038/nsmb1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vendeix FA, et al. Human tRNA(Lys3)(UUU) is pre-structured by natural modifications for cognate and wobble codon binding through keto-enol tautomerism. J Mol Biol. 2012;416(4):467–485. doi: 10.1016/j.jmb.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jühling F, et al. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37(Database issue):D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeing TM, et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326(5953):688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmeing TM, Voorhees RM, Kelley AC, Ramakrishnan V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat Struct Mol Biol. 2011;18(4):432–436. doi: 10.1038/nsmb.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogle JM, et al. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292(5518):897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 27.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell. 2002;111(5):721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 28.Kurata S, et al. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J Biol Chem. 2008;283(27):18801–18811. doi: 10.1074/jbc.M800233200. [DOI] [PubMed] [Google Scholar]
- 29.Dunham CM, et al. Structures of tRNAs with an expanded anticodon loop in the decoding center of the 30S ribosomal subunit. RNA. 2007;13(6):817–823. doi: 10.1261/rna.367307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley GJ, Rich A. Structural domains of transfer RNA molecules. Science. 1976;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- 31. Dirheimer G, Keith G, Dumas P, Westhof E (1995) Primary, secondary, and tertiary structures of tRNAs. tRNA: Structure, Biosynthesis, and Function, eds Söll D, RajBhandary U (ASM Press, Washington, DC), pp 93–126.
- 32.Murphy FV, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Biol. 2004;11(12):1186–1191. doi: 10.1038/nsmb861. [DOI] [PubMed] [Google Scholar]
- 33.Murphy FV, 4th, Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol. 2004;11(12):1251–1252. doi: 10.1038/nsmb866. [DOI] [PubMed] [Google Scholar]
- 34.Olejniczak M, Uhlenbeck OC. tRNA residues that have coevolved with their anticodon to ensure uniform and accurate codon recognition. Biochimie. 2006;88(8):943–950. doi: 10.1016/j.biochi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Lippert B, Gupta D. Promotion of rare nucleobase tautomers by metal binding. Dalton Trans. 2009;(24):4619–4634. doi: 10.1039/b823087k. [DOI] [PubMed] [Google Scholar]
- 36.Feyer V, et al. Tautomerism in cytosine and uracil: An experimental and theoretical core level spectroscopic study. J Phys Chem A. 2009;113(19):5736–5742. doi: 10.1021/jp900998a. [DOI] [PubMed] [Google Scholar]
- 37.Dabkowska I, Gutowski M, Rak J. Interaction with glycine increases stability of a mutagenic tautomer of uracil. A density functional theory study. J Am Chem Soc. 2005;127(7):2238–2248. doi: 10.1021/ja048730k. [DOI] [PubMed] [Google Scholar]
- 38.Smits P, Smeitink J, van den Heuvel L. Mitochondrial translation and beyond: Processes implicated in combined oxidative phosphorylation deficiencies. J Biomed Biotechnol. 2010;2010:737385. doi: 10.1155/2010/737385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barraud P, Schmitt E, Mechulam Y, Dardel F, Tisné C. A unique conformation of the anticodon stem-loop is associated with the capacity of tRNAfMet to initiate protein synthesis. Nucleic Acids Res. 2008;36(15):4894–4901. doi: 10.1093/nar/gkn462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh HP, Ghosh K, Simsek M, RajBhandary UL. Nucleotide sequence of wheat germ cytoplasmic initiator methionine transfer ribonucleic acid. Nucleic Acids Res. 1982;10(10):3241–3247. doi: 10.1093/nar/10.10.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillum AM, Roe BA, Anandaraj MP, RajBhandary UL. Nucleotide sequence of human placenta cytoplasmic initiator tRNA. Cell. 1975;6(3):407–413. doi: 10.1016/0092-8674(75)90190-7. [DOI] [PubMed] [Google Scholar]
- 42.Gillum AM, Urquhart N, Smith M, RajBhandary UL. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- 43.Piper PW, Clark BF. Primary structure of a mouse myeloma cell initiator transfer RNA. Nature. 1974;247(442):516–518. doi: 10.1038/247516a0. [DOI] [PubMed] [Google Scholar]
- 44.Simsek M, RajBhandary UL. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- 45.Simsek M, RajBhandary UL, Boisnard M, Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974;247(442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- 46.Woo NH, Roe BA, Rich A. Three-dimensional structure of Escherichia coli initiator tRNAfMet. Nature. 1980;286(5771):346–351. doi: 10.1038/286346a0. [DOI] [PubMed] [Google Scholar]
- 47.Wakao H, et al. The solution structure of the Escherichia coli initiator tRNA and its interactions with initiation factor 2 and the ribosomal 30 S subunit. J Biol Chem. 1989;264(34):20363–20371. [PubMed] [Google Scholar]
- 48.Clemons WM, Jr, et al. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: Purification, crystallization and structure determination. J Mol Biol. 2001;310(4):827–843. doi: 10.1006/jmbi.2001.4778. [DOI] [PubMed] [Google Scholar]
- 49.Demeshkina N, Jenner L, Westhof E, Yusupov M, Yusupova G. A new understanding of the decoding principle on the ribosome. Nature. 2012;484(7393):256–259. doi: 10.1038/nature10913. [DOI] [PubMed] [Google Scholar]
- 50.Selmer M, et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313(5795):1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 51.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst. 1993;26(6):795–800. [Google Scholar]
- 52.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Emsley P, Cowtan K (2004) Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60(Pt 12 Pt 1):2126–2132. [DOI] [PubMed]
- 54.DeLano WL. Pymol. San Carlos, CA: Delano Scientific; 2006. [Google Scholar]
- 55.Moriarty NW, Grosse-Kunstleve RW, Adams PD. Electronic Ligand Builder and Optimization Workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 10):1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frisch AE, Dennington RD, Keith TA, Neilsen AB, Holder AJ. GaussView Version 3.0. Pittsburgh, PA: Gaussian; 2003. [Google Scholar]
- 57. Frisch MJ, et al. (2003) Gaussian 03, Revision C.01 (Gaussian, Pittsburgh, PA)
- 58.Cossi M, Rega N, Scalmani G, Barone V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem. 2003;24(6):669–681. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]