Abstract
Using a propagating cell culture system of adipocyte precursors from 70-400-g rats, we explored the possibility that regional variations in properties of adipose tissue may reflect site-specific characteristics intrinsic to the cells, rather than extracellular influences. Initially, studies were made of the nature of the fibroblastlike cells from perirenal adipose tissue stroma. Using colony-forming techniques, it was shown that these cells were adipocyte precursors; each confluent colony that was derived from a single cell displayed differentiated adipocytes. This characteristic was evident in cells from rats of all ages and persisted during secondary culture. At all ages of rats studied, perirenal cells replicated more rapidly than epididymal precursors, e.g., for 179-g rats the population-doubling times were 19.3 +/- 0.7 vs. 25.5 +/- 1.2 h (means +/- SEM, P less than 0.03). With aging of the rats, the replication rate of their perirenal cells decreased progressively. Under clonal conditions, the colony size distribution of both perirenal and epididymal precursors revealed heterogeneity in their capacity for replication, perirenal cells showing greater proliferation. These also differentiated more extensively by morphologic and enzymatic criteria. Age and site had effects that persisted through many cell generations; however, high-fat feeding had no perpetuating influence. The dissimilar properties of perirenal as compared with epididymal precursors may reflect differences in regulation of gene expression. The data are also compatible with a later development in embryological life of perirenal tissue. We suggest that the composition of the adipocyte precursor pool is an important determinant of the growth of adipose tissue that occurs in response to a nutrient load. Interregional or interindividual variation in composition may explain regional and individual differences in fat accumulation.
Full text
PDF


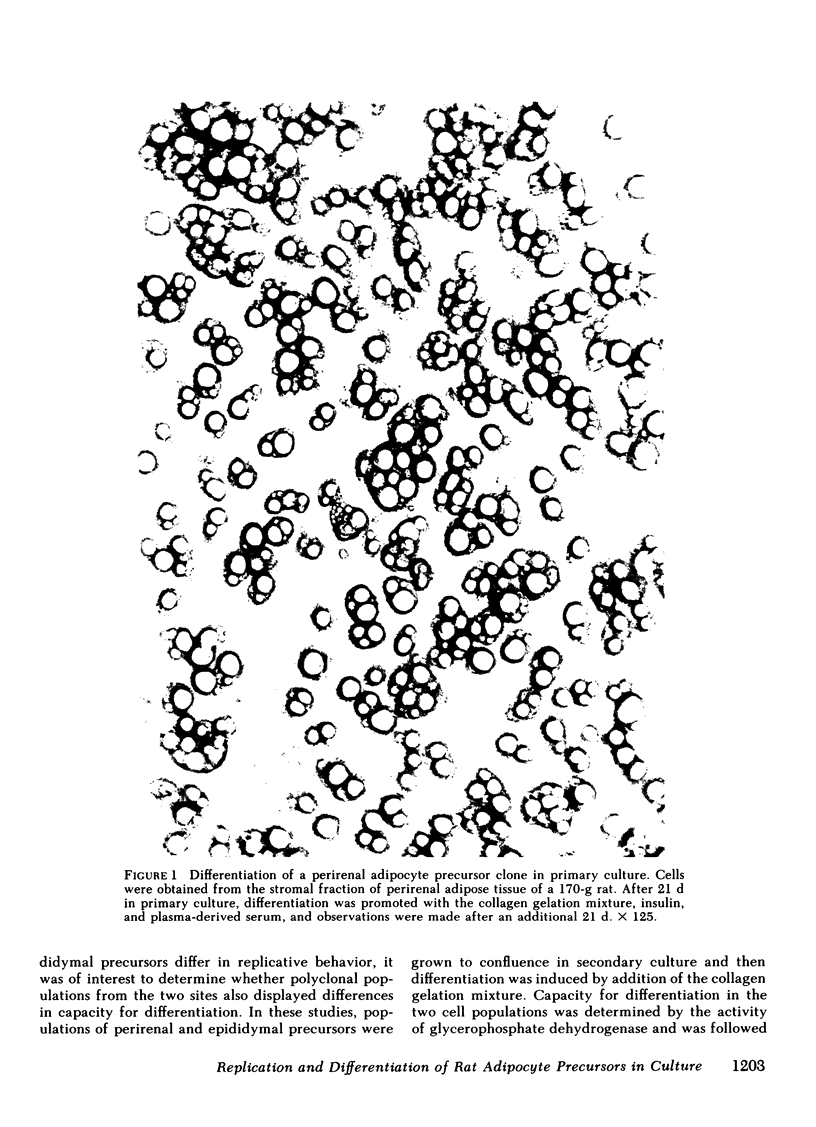
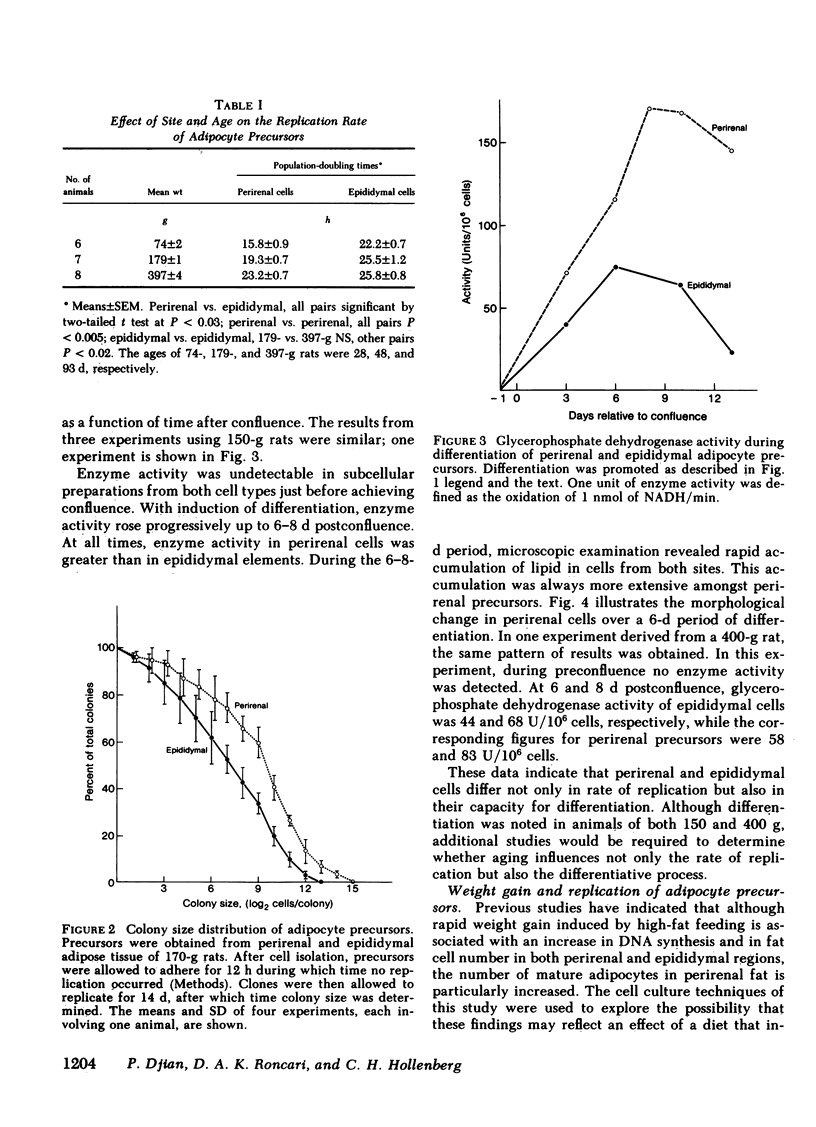



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björntorp P., Karlsson M., Gustafsson L., Smith U., Sjöström L., Cigolini M., Storck G., Pettersson P. Quantitation of different cells in the epididymal fat pad of the rat. J Lipid Res. 1979 Jan;20(1):97–106. [PubMed] [Google Scholar]
- Burns T. W., Langley P. E., Terry B. E., Bylund D. B., Hoffman B. B., Tharp M. D., Lefkowitz R. J., García-Saínz J. A., Fain J. N. Pharmacological characterizations of adrenergic receptors in human adipocytes. J Clin Invest. 1981 Feb;67(2):467–475. doi: 10.1172/JCI110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Hirsch J. Adipose tissue regeneration following lipectomy. Science. 1977 Jul 22;197(4301):391–393. doi: 10.1126/science.877563. [DOI] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Allman D. R., Durrant J. L., Wilson J. D. Variation in steroid 5 alpha-reductase activity in cloned human skin fibroblasts. Shift in phenotypic expression from high to low activity upon subcloning. J Biol Chem. 1981 Apr 25;256(8):3662–3666. [PubMed] [Google Scholar]
- Johnson P. R., Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972 Jan;13(1):2–11. [PubMed] [Google Scholar]
- Klyde B. J., Hirsch J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J Lipid Res. 1979 Aug;20(6):705–715. [PubMed] [Google Scholar]
- Lafontan M., Dang-Tran L., Berlan M. Alpha-adrenergic antilipolytic effect of adrenaline in human fat cells of the thigh: comparison with adrenaline responsiveness of different fat deposits. Eur J Clin Invest. 1979 Aug;9(4):261–266. doi: 10.1111/j.1365-2362.1979.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Lemonnier D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest. 1972 Nov;51(11):2907–2915. doi: 10.1172/JCI107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairault J., Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M., Schneider E. L. Colony size distributions as a measure of in vivo and in vitro aging. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1353–1356. doi: 10.1073/pnas.75.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van R. L., Bayliss C. E., Roncari D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976 Sep;58(3):699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van R. L., Roncari D. A. Complete differentiation of adipocyte precursors. A culture system for studying the cellular nature of adipose tissue. Cell Tissue Res. 1978 Dec 28;195(2):317–329. doi: 10.1007/BF00236728. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Guzman R., Imagawa W., Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]