Abstract
“Sense of number” refers to the classical idea that we perceive the number of items (numerosity) intuitively. However, whether the brain signals numerosity spontaneously, in the absence of learning, remains unknown; therefore, we recorded from neurons in the ventral intraparietal sulcus and the dorsolateral prefrontal cortex of numerically naive monkeys. Neurons in both brain areas responded maximally to a given number of items, showing tuning to a preferred numerosity. Numerosity was encoded earlier in area ventral intraparietal area, suggesting that numerical information is conveyed from the parietal to the frontal lobe. Visual numerosity is thus spontaneously represented as a perceptual category in a dedicated parietofrontal network. This network may form the biological foundation of a spontaneous number sense, allowing primates to intuitively estimate the number of visual items.
Keywords: association cortices, quantity, single-unit recording
The classical idea of a “sense of number” (1,2) suggests that we and animals are endowed with the faculty to perceive the number of items (i.e., numerosity) intuitively. Numerosity might be a perceptual category provided by hard-wired sensory brain processes, without the need to learn what numerosity refers to. Supporting this hypothesis, numerosity is represented by an approximate nonverbal system that allows wild animals (3,4), prelinguistic infants(5,6), and innumerate humans (7,8) to readily estimate set size. Evidence that the brain is set up to assess visual numerosity spontaneously was recently provided by psychophysical and computational experiments: numerosity—just like color or perceptual categories like faces—in humans is susceptible to adaptation (9,10), and is extracted en passant by computational network models (11).
So far, the neuronal foundations of a perceptual number sense were never tested because all experiments done so far were performed in animals that learned to discriminate numerosity (12–14). Work with behaviorally trained nonhuman primates identified a cortical network with individual neurons in the prefrontal (PFC) and posterior parietal cortex (PPC) selectively responding to the number of items. Such “number neurons” abstractly represent the number of items across space, time, and modalities (15,16,17). Number neurons have also been traced indirectly in the human brain using functional MRI (fMRI) (18,19).
However, because neurons can be trained to represent behaviorally meaningful categories (20,21,22), it has been argued (23) that the presence of previously described number neurons in trained animals might be a product of intense learning, rather than a reflection of a spontaneous number sense. For the same reason, the coding scheme for numerosity has been debated (23): Is the spontaneous neuronal code for numerosity a summation code, as evidenced by monotonic discharges as a function of quantity (14,24), or a labeled-line code as witnessed by numerosity-selective neurons tuned to preferred numerosities analogous to those found in monkeys performing numerical tasks (25,26)? Here, we tested the core idea of the number sense and explored whether numerosity-selective neurons do exist in the brains of numerically naive monkeys (i.e., monkeys that had never been trained to discriminate numerosity).
Results
To ensure that the monkeys paid attention to the stimulus displays (but not to numerosity) during recording, the monkeys were trained to discriminate color in variable dot displays in a delayed match-to-sample task (Fig. 1A). Monkeys watched two displays (first sample, then test) separated by a 1-s delay. They were trained to release a bar if the displays contained the same color of dots. Five colors (red, blue, green, yellow, purple) were used. All five colors were presented in displays containing one, two, three, four, or five dots, or numerosities (Fig. 1B). Importantly, the number of items in the displays was completely irrelevant to solve the task. All five colors and numerosities were displayed as standard stimuli with variable dot sizes and positions, with control stimuli equating the total area and the average density of all dots across numerosities. All parameters (e.g., colors, numerosities, stimulus protocols, match versus nonmatch trials) were balanced and pseudorandomly presented to the monkeys.
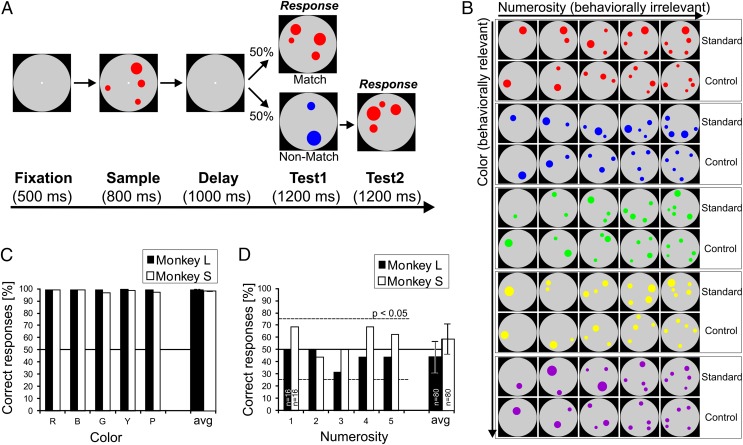
Fig. 1.
Task protocol and behavioral performance. (A) Delayed match-to-color task. A trial started when the monkey grasped a bar. The monkey had to release the bar if the items in the multidot displays of the sample period and test period were of the same color, and continue holding it if they were not (probability of match/nonmatch condition = 0.5). The sample display contained one to five dots of the same color. (B) Example stimulus displays showing the variations of stimulus parameters. Each of the five (behaviorally relevant) colors was shown in five different (behaviorally irrelevant) numerosities, and as standard vs. area and density control stimulus displays, resulting in a three-factorial stimulus design. (C) Behavioral color discrimination performance of both monkeys during all recording sessions (50% is chance level). Both monkeys performed equally well and almost perfectly to all five colors (red, blue, green, yellow, purple). Avg, average over all colors. (D) Behavioral performance in the generalization tests to numerosity the monkeys were not trained to discriminate (all displays only black dots). Both monkeys showed chance performance to each numerosity and the average (dotted lines indicate binomial threshold at P = 0.05), confirming that they did not pay attention to numerosity. Error bars indicate SEM.
Behavioral Performance.
Both animals were proficient in color discrimination and performed well above chance (monkey L: 99.19 ± 0.24%; monkey S: 97.93 ± 0.34%, binomial test, P < 0.001) for all color combinations (Fig. 1C). To ensure that the monkeys ignored the number of items that covaried with the color of the dots in the sample displays, we tested putative numerosity discrimination by inserting generalization trials of pure numerosity stimuli (only black dots) within the ongoing color discrimination task in two sessions after the end of the recording sessions. With an average numerosity discrimination of 43.8 ± 12.7% (monkey L) and 58.8 ± 12.4% (monkey S), both monkeys performed at chance level (two-tailed binomial test, P > 0.05) (Fig. 1D). The monkeys were thus exclusively discriminating color; they were ignorant of the numerosity information in the stimuli.
Single-Cell Responses.
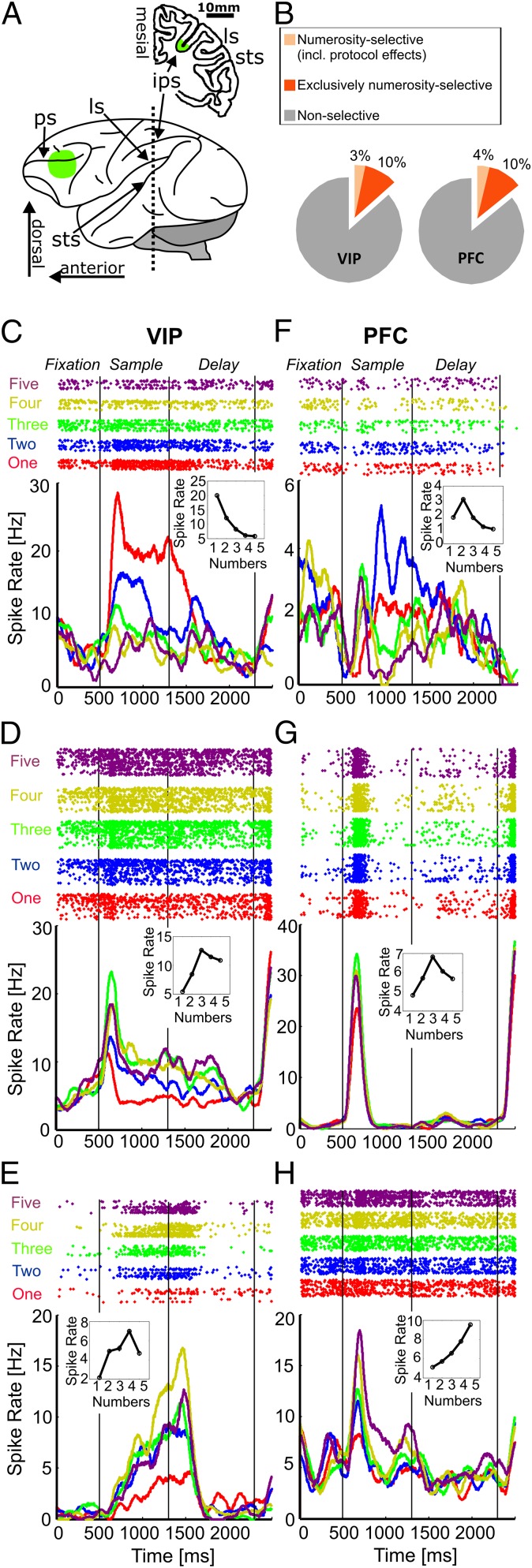
We recorded single-cell activity from randomly selected neurons in the ventral intraparietal area (VIP) in the fundus of the intraparietal sulcus (IPS) of the PPC and PFC while monkeys performed the color discrimination task (Fig. 1 A and B). A total of 238 neurons from VIP and 268 neurons from the dorsolateral prefrontal cortex around the principal sulcus were analyzed (Fig. 2A). To identify neurons selective to the varying stimulus parameters, we evaluated the average discharge rates of individual neurons during the sample presentation using a three-factor ANOVA, with factors (sample color) × (sample numerosity) × (stimulus protocol) (P < 0.01).
Fig. 2.
Recording sites and neural responses to numerosity. (A) Lateral view (Lower) of the left hemisphere of a monkey brain indicating the topographical relationships of cortical landmarks. Coronal section (Upper) at the level of the dotted line in the lateral view reconstructed from a structural MRI scan (Horsley-Clark coordinates 0 mm anterior/posterior). Green regions on the frontal lobe and in the fundus of the IPS mark the recording areas in the PFC and area VIP, respectively. LS, lateral sulcus; PS, principal sulcus; STS, superior temporal sulcus. (B) Pie chart depicting the absolute proportions of numerosity-selective neurons found in areas VIP and PFC. (C) Responses of an example VIP neuron selective to the numerosity one. (Upper) Dot-raster histograms (each dot represents an action potential); (Lower) averaged spike density functions (activity averaged and smoothed by a 150-ms Gauss-kernel). The first 500 ms represent the fixation period. Colors of dot histogram and spike density functions correspond to the number of items in the sample displays. (Inset) The neuron’s tuning function, the discharge rates as a function of the number of items. (C–E) Example VIP neurons tuned to numerosities one, three, and four (layout as in C). (F–H) Example PFC neurons tuned to numerosities two, three, and five (layout as in C).
Tuning to Visual Numerosity.
The behaviorally relevant parameter color significantly modulated neuronal activity in 12% (29/238) of VIP cells and 10% (26/268) of PFC neurons. However, a similar proportion of neurons spontaneously also encoded the number of items in the dot displays (numerosity) that was behaviorally irrelevant and not discriminated by the animals (Fig. 1D). In area VIP, 13% (32/238) of the neurons showed a significant main effect numerosity, and 14% (38/268) in PFC (Fig. 2B). Most of these numerosity-selective neurons, i.e., 10% (24/238) of all VIP neurons and 10% (28/268) of all PFC neurons, were not activated by covarying visual parameters but exclusively showed a main effect numerosity (no main effect stimulus protocol or interactions with factor stimulus protocol, P < 0.01). Responses of three example numerosity-selective neurons from area VIP and the PFC are depicted in Fig. 2 C–E for area VIP, and Fig. 2 F–H for PFC. The tuning functions of example VIP neurons showed peak activity for visual numerosity one (Fig. 2C), three (Fig. 2D), and four (Fig. 2E), whereas the PFC neurons had a preferred numerosity of two (Fig. 2F), three (Fig. 2G), and five (Fig. 2H).
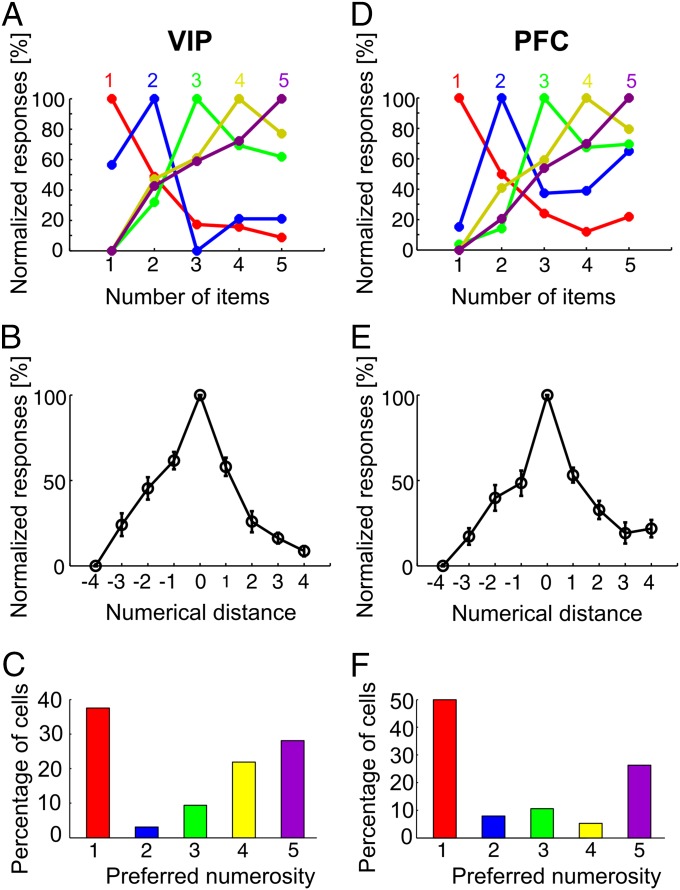
Population-tuning functions were calculated by averaging the normalized activity for all neurons that preferred a given numerosity. Similar response profiles were observed for all neurons tuned to numerosities one, two, three, four, and five in area VIP (Fig. 3A) and the PFC (Fig. 3D). All functions in VIP (Fig. 3B) and PFC (Fig. 3E) showed a systematic drop off of activity because the number of items in the dot displays varied from the preferred value. To evaluate this across the population of VIP and PFC neurons, we normalized the activity of each numerosity-selective neuron and plotted its activity as a function of distance from its preferred numerosity. A significant decrease of activity from numerical distance one and two was found, even when only considering numerosity-selective VIP neurons tuned to numerosity two, three, and four (paired samples t test, two-tailed, all tests P < 0.05), indicating peak-tuning functions with systematically falling flanks on both sides of the numerosity tuning curves. Numerosity one was by far the most frequent preferred numerosity in both VIP (Fig. 3C) and PFC (Fig. 3F). In summary, we find that both VIP and PFC neurons were tuned to preferred numerosities in the absence of numerosity training.
Fig. 3.
Normalized response rates of selective neurons as a function of numerical distance. (A and D) Normalized responses averaged for neurons preferring the same numerosity (color coded) in area VIP (A) and in PFC (D). (B and E) Normalized discharge rates of all numerosity-selective cells in (C) VIP and (E) PFC plotted against the numerical distance from the preferred number of items. Numbers closer to the preferred quantity elicited higher discharge rates. Error bars indicate SEM. (C and F) Frequency distributions of the preferred numerosities for VIP and PFC, respectively.
Comparing the Timing of Numerosity Signals in VIP and PFC.
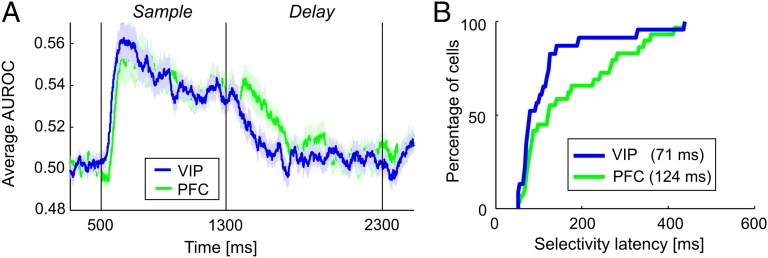
We examined the time course of numerosity selectivity in each brain area using a sliding version of the receiver operating characteristic (ROC) applied to the populations of numerosity-selective neurons in the sample epoch (Materials and Methods). This selectivity measure revealed that numerosity selectivity appeared with a shorter latency in VIP than PFC following the onset of the sample stimulus (Fig. 4A). We quantified the latency of category selectivity for each VIP and PFC neuron by evaluating the time at which the area under the ROC curve (AUROC values) crossed a predefined threshold (3.0 SD above the mean value during the fixation epoch for 20 consecutive 1-ms time bins) in the sample period. Across all neurons for which a latency could be defined with this method (VIP, n = 23; PFC, n = 29), numerosity selectivity emerged significantly earlier in VIP (median = 71 ms) than in PFC (median = 124 ms) (two-tailed Mann–Whitney U test, P < 0.05). The observed latency difference between VIP and PFC neurons could not be caused by differences in the strength of numerosity selectivity (as measured by AUROC values), which were comparable in both cortical areas (Fig. 4B).
Fig. 4.
Time course of VIP and PFC numerosity selectivity. (A) The time course of numerosity selectivity across numerosity-selective VIP and PFC populations was determined by a sliding ROC analysis. AUROC values of 0.5 indicate complete indiscriminability of the best and least preferred numerosities; AUROC values larger than 0.5 indicate stronger neuronal activity to the preferred numerosity. (B) Cumulative latency distributions across all neurons that showed significant numerosity selectivity after sample onset according to the sliding ROC analysis revealed the fraction of VIP and PFC neurons that had become numerosity selective by each time point. Shading indicates SEM.
Discussion
Our finding of numerosity-selective neurons in numerically naive monkeys supports the idea of a visual number sense, the faculty to perceive visual collections intuitively (1, 2). Because perceived numerosity is susceptible to adaptation just like color, contrast, or speed, Burr and Ross (9,10) recently suggested visual numerosity as a primary sensory attribute. This finding was recently supported by a computational network model in which sensitivity to numerosity spontaneously developed (11). However, because adaptation is not restricted to primary visual attributes, but also observed for high-level visual categories such as faces (27), the category visual numerosity may alternatively be appreciated as a special perceptual category represented spontaneously in a dedicated parietofrontal network. Other complex visual categories are also represented in the primate visual system up to the frontal lobe in a relatively specialized fashion: faces, places, and body parts appear to have dedicated neural substrates for their representation (28,29,30,31). Numerosity may thus be another visual category that is processed hierarchically, not within the ventral visual stream like faces, places, and body parts, but within the dorsal visual stream. Number neurons seem to develop spontaneously and naturally within visual neural structures of the primate brain, probably based on visual input that (unavoidably) contains (among a variety of other visual features) different numbers of objects and events. Such neurons likely provide the neurobiological substrate of the approximate nonverbal system, allowing wild animals (3,4), prelinguistic infants (5,6), and innumerate humans (7,8) to readily estimate set size.
In the absence of a numerosity task, 13% of all neurons in VIP and 14% of all neurons in PFC were numerosity selective. For the VIP, this value is close to the proportion of selective neurons (14% of the neurons) found on average in our previous studies with numerosity-discriminating monkeys (calculated over four different studies (15,32–34). For the PFC, the proportion of numerosity-selective neurons in numerically trained monkeys was about twice as large (around 30% of the neurons). Perhaps PFC representations get “amplified” with numerical training and experience in the sense that a greater number of neurons respond to numerosity if numerosity needs to be processed in a behaviorally relevant way. That such neurons are at all present in naive monkeys may seem at odds with the PFC supporting complex executive functions (35). However, the PFC may be able to exert its cognitive functions precisely because it also has access to sensory information required for goal-directed behavior.
Neurons in area VIP represented their preferred numerosity on average 53 ms earlier than PFC neurons, suggesting that numerosity selectivity evolves along the visual path (see also ref. 32). This suggests that visual numerosity is extracted first in the termination zone of the dorsal visual pathway of the parietal cortex based on bottom-up process and later conveyed to the functionally connected lateral PFC (36). Even in trained animals, parietal signals of visual numerical categories do not arise as a result of feedback from PFC (22).
The neuronal code for numerosity representations has remained debated since neurons selectively responding to the number of items were discovered (12,13). Neurons in monkeys performing an explicit numerosity task exclusively displayed tuning functions that peak at their respective preferred numerosity (labeled-line code), irrespective of (visual or auditory) numerosity modality (16), spatial or temporal numerosity layout (15), or sensory-motor task demands (12,26). The relevance of tuned neurons’ responses for the monkeys’ behavioral performance was demonstrated by both error trial analyses (13,16,25) and inactivation of the respective brain areas (26). Contrasting a labeled-line code, an electrophysiological study in which monkeys implicitly discriminated numerosity reported monotonic response functions (summation code) for numerosities in the lateral intraparietal area (LIP) of the parietal lobe (14). Monkeys perform an oculomotor task with numerosity predicting the amount of reward they would receive following a gaze shift. As the authors acknowledge, the monkeys were not numerically naive in this study either (14) because the authors observed significant and consistent effect of numerical values on saccade latency, and thus concluded that the monkeys attended to numerosity.
Roitman and colleagues (23) reasoned that tuning to specific numerosities might be a product of experience and/or task demands because an explicit numerosity task requires monkeys to categorize numerical values. Such a putative learning effect can now be ruled out. In the current study with monkeys that were never trained to discriminate numerosity, both VIP and PFC neurons were still tuned to preferred numerosities. In agreement with influential computational models of number processing (37,38), the labeled-line code is a genuine and spontaneous code for the inherently categorical property number of items of stimuli.
The current data also help to exclude putative nonnumerical factors that were suspected to modulate numerosity-selective neurons during explicit numerosity tasks. It has been argued that the observed overrepresentation of numerosity one in numerosity discriminating monkeys might have been caused by reward expectation (23): because behavioral accuracy was highest when the sample value was 1 (13), monkeys could be more certain of achieving rewards, and this might be reflected in the discharges of neurons preferring numerosity one. Because numerosity one was still overrepresented by the population of neurons in our numerically naive monkeys, this putative nonnumerical factor can be ruled out. Neurons tuned to numerosity one superficially resemble monotonically decreasing units; however, these neurons are too sharply tuned to convey information for numerosities three and higher (Figs. 2C and 3 A and D) and thus cannot be regarded as decreasing summation units. Perhaps numerosity 1 is indeed a special set and thus represented by an abundance of neurons; after all, numerosities are collections of single elements, i.e., multiples of numerosity 1. Interestingly, the special status of one element is omnipresent in the singular-plural dichotomy (or numerosity one vs. all-other-numerosities distinction) found in natural language. Moreover, the singular-plural distinction is suggested to have a nonlinguistic conceptual basis (39,40). Neurons tuned to numerosity five were also abundant, but most likely comprises neurons with larger numerosity preference than our experimentally restricted range from one to five.
Our single-cell data in the naive nonhuman primate are in good agreement with event-related potentials in human infants (41) and human functional imaging data. Using fMRI adaptation, peaked blood oxygenation level dependent activity-tuning profiles were found in bilateral IPS as an indirect measure of neuronal numerosity tuning (18) as well as IPS and PFC (19). Peak-tuned numerosity detectors are also postulated as final stage of numerosity processing by computational models on number processing (37,38). The recently reported summation units (11,14) operating at an intermediate stage of the model hierarchy give rise to numerosity detectors that may constitute a locally restricted precursor mechanism, but current discrepancies between the computational solutions and the biological realizations (monotonically increasing or decreasing functions, or both) need to be reconciled.
Materials and Methods
Behavioral Protocol.
Two male rhesus monkeys (Macaca mulatta) weighing between 5.5 and 6.3 kg served as subjects for this study. The animals were seated inside primate chairs in a chamber 57 cm away from a 15-inch flat screen monitor (with a resolution of 1,024 × 768 pixels and a refresh rate of 75 Hz). The National Institute of Mental Health Cortex program was used to present the stimuli and monitor behavior. The monkeys' eye gaze was tracked by an infrared eye tracking system (ISCAN).
To ensure that the monkeys paid attention to the stimulus displays (but not to numerosity), the animals were trained to discriminate color in dot displays in a delayed match-to-sample task (Fig. 1A). The trial was initiated by the monkeys holding a response bar and fixating within 3.5° of visual angle of a central fixation target throughout the trial. Upon successful fixation for 500 ms, the sample stimulus was shown for 800 ms, followed by a delay period of 1,000 ms without a stimulus. After the delay, a dot display (test 1) was presented, which in 50% of the cases had the same color as the sample (match) and required the monkey to release the response bar to receive a fluid reward. The match display displayed the same color (relevant parameter) and the same numerosity (irrelevant parameter) as the sample stimulus. In the other 50% of the trials, the first test display showed a different color (nonmatch; relevant parameter) as well as a different numerosity (irrelevant parameter); in this case, the monkey had to hold the response bar and wait for the second test that always matched the sample in color to release the bar. Based on this task design, chance performance was 50% correct in the trials.
Stimuli.
The visual stimuli were colored dot (diameter range 0.5°–0.9° of visual angle) displays (only one color per stimulus) on a gray background circle (diameter 6° of visual angle). Five colors (red, blue, green, yellow, purple) were chosen for maximum discriminability. All colors were presented in displays containing one, two, three, four, or five dots. This provided a balanced 5 × 5 stimulus design.
Dot patterns were generated using a custom-written MatLab script (MathWorks). These routines enabled the generation of new stimuli sets for each recording session. Moreover, this software provided for the control of parameters of the dot patterns. For the standard stimuli, each stimulus contained a defined set of equally colored dots that appeared at randomized locations within the gray background circle. The diameter of each dot was randomly varied within the given range. To prevent the monkeys from memorizing the visual patterns of the displays, each quantity was tested with many different images per session and the sample and test displays that appeared on each trial were never identical. In addition to the standard stimuli, control stimuli controlling for both the spatial low-level visual features total dot area (total area of all items in a display equated for all stimuli in a trial) and dot density (same mean density of dot patterns for all stimuli in a trial) were used in each session (Fig. 1B). All parameters (e.g., colors, numerosities, stimulus protocols, match versus nonmatch trials) were balanced and pseudorandomly presented to the monkeys.
Evaluation of Putative Discrimination Generalization to Numerosity.
We also tested whether the monkeys might have learned to discriminate the behaviorally irrelevant stimulus dimension numerosity. For monkey L, blocks (40 trials, eight trials per numerosity, pseudorandomized) of pure numerosity stimuli (only black dots, all other parameters as in the color task) were inserted during the ongoing color discrimination task in two sessions; rewards were delivered after correct numerosity matches. The same procedure was applied for monkey S, except that individual pure numerosity trials were randomly interspersed among ongoing color trails; all trials were rewarded irrespective of response). These tests were done immediately after the end of recording sessions in both monkeys. Percentage of correct performance to each numerosity was tested by a binomial test (P < 0.05). The data (Fig. 1D) showed that neither monkey was able to discriminate numerosity.
Surgery and Neuronal Recordings.
The animals were implanted with a head bolt to allow immobilization of the head during training so that eye movements could be monitored. After the animals reliably discriminated the color of the items in the delayed match-to-sample color task, two recording chambers were implanted over the right lateral prefrontal cortex, centered on the principal sulcus and the right intraparietal sulcus guided by anatomical MRI and stereotaxic measurements. All surgeries were performed under sterile conditions while the monkey was under general anesthesia and received antibiotics and analgesics postprocedure.
Neuronal recordings were made from the two monkeys while they performed the task using arrays of up to eight tungsten microelectrodes attached to screw microdrives in a grid with 1-mm spacing. Neurons were not preselected for any sensory or task-related parameter. To access area VIP, recordings were made exclusively at depths ranging from 9 to 13 mm below the cortical surface and the presence of visual and somatosensory responses was tested qualitatively. Electrophysiological signals were amplified and filtered and waveforms of action potentials sampled at 40 kHz from each channel were stored to disk. Single unit separation was performed offline based on waveform characteristics (Offline Sorter, Plexon Systems). Timestamps of trial events and action potentials were extracted for analysis. Only single units that exhibited a sufficient discharge rate (>1 Hz) during the relevant trial phases and were recorded for at least 30 trials per condition were analyzed. All experimental procedures were in accordance with the guidelines for animal experimentation approved by the Regierungspräsidium Tübingen, Tübingen, Germany.
Behavioral Data Analysis.
For the color discrimination task, the percent correct performance for each color in each session was calculated, averaged across all sessions, and statistically verified using a binomial test. To test numerosity discrimination, percent correct performance was derived during the generalization tests and again analyzed with a binomial test for each numerosity.
Neuronal Data Analysis.
We analyzed discharge rates during sample presentation in an 800-ms window. By default, the 800-ms windows of the sample phase were shifted by 50 ms after sample onset for area VIP cells and by 100 ms for PFC cells to account for typical response latency of these cells. To determine the selectivity of a neuron, a three-factor ANOVA (criterion P < 0.01) using single-trial discharge rates was calculated for each cell in the sample period, with main factors stimulus protocol (standard or control), sample color (red, blue, green, yellow, purple), and sample numerosity (one, two, three, four, five). Each neuron’s discharge rates are tested only once; no multiple comparisons are applied. Only neurons with a minimum of 30 stimulus presentations per numerosity were taken into account. To estimate the probability of false positives in our approach (i.e., the probability that a neuron was classified as numerosity selective by chance), we additionally performed a the same three-factor ANOVA (criterion P < 0.01) with shuffled data, using recorded single-trial discharge rates that were randomly assigned to the numerical labels (238 VIP cells × 1,000, and 268 PFC cells × 1,000, resulting in 506,000 tests). We found that 1% (5,306/506,000) of the tests with shuffled data resulted in a statistically significant evaluation (at P < 0.01), confirming that the reported proportion of numerosity-selective neurons cannot be explained by chance occurrences.
To derive averaged numerosity-tuning functions, the tuning functions of individual neurons were normalized by setting the maximum activity to the most preferred numerosity as 100% and the activity to the least preferred quantity as 0%. Pooling the resulting normalized tuning curves resulted in averaged numerosity-filter functions.
The latency of numerosity selectivity (i.e., not the visual response latency) was calculated using a sliding ROC analysis (42). The true-positive (spike rate for the preferred numerosity) and false-positive rates (spike rate for the least preferred numerosity) were calculated for each neuron to generate the ROC curve. The AUROC was calculated with a sliding kernel of 50 ms and incremented by 1 ms at each time point. To arrive at baseline and threshold AUC values, the last 200 ms of the fixation period (before sample onset) were used. The threshold for each neuron was defined as the mean AUC during the presample period plus 3 SDs. The numerosity selectivity latency per single neuron was the time point after sample onset where this threshold criterion was exceeded for at least 20 consecutive 1-ms bins.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant NI 618/2-1 (to A.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Danzig T. Number The Language of Science. New York: The Free Press; 1930. [Google Scholar]
- 2.Dehaene S. The Number Sense. Oxford, UK: Oxford Univ Press; 1997. [Google Scholar]
- 3.Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim Behav. 2012;83:277–291. [Google Scholar]
- 4.Cantlon JF, Brannon EM. How much does number matter to a monkey (Macaca mulatta)? J Exp Psychol Anim Behav Process. 2007;33(1):32–41. doi: 10.1037/0097-7403.33.1.32. [DOI] [PubMed] [Google Scholar]
- 5.Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends Cogn Sci. 2004;8(7):307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Piazza M. Neurocognitive start-up tools for symbolic number representations. Trends Cogn Sci. 2010;14(12):542–551. doi: 10.1016/j.tics.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Gordon P. Numerical cognition without words: Evidence from Amazonia. Science. 2004;306(5695):496–499. doi: 10.1126/science.1094492. [DOI] [PubMed] [Google Scholar]
- 8.Pica P, Lemer C, Izard V, Dehaene S. Exact and approximate arithmetic in an Amazonian indigene group. Science. 2004;306(5695):499–503. doi: 10.1126/science.1102085. [DOI] [PubMed] [Google Scholar]
- 9.Burr D, Ross J. A visual sense of number. Curr Biol. 2008;18(6):425–428. doi: 10.1016/j.cub.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Ross J, Burr DC. Vision senses number directly. J Vis. 2010;10(2):1–8. doi: 10.1167/10.2.10. [DOI] [PubMed] [Google Scholar]
- 11.Stoianov I, Zorzi M. Emergence of a ‘visual number sense’ in hierarchical generative models. Nat Neurosci. 2012;15(2):194–196. doi: 10.1038/nn.2996. [DOI] [PubMed] [Google Scholar]
- 12.Sawamura H, Shima K, Tanji J. Numerical representation for action in the parietal cortex of the monkey. Nature. 2002;415(6874):918–922. doi: 10.1038/415918a. [DOI] [PubMed] [Google Scholar]
- 13.Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297(5587):1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- 14.Roitman JD, Brannon EM, Platt ML. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 2007;5(8):e208. doi: 10.1371/journal.pbio.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313(5792):1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- 16.Nieder A. Supramodal numerosity selectivity of neurons in primate prefrontal and posterior parietal cortices. Proc Natl Acad Sci USA. 2012;109(29):11860–11865. doi: 10.1073/pnas.1204580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieder A. Coding of abstract quantity by ‘number neurons’ of the primate brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199(1):1–16. doi: 10.1007/s00359-012-0763-9. [DOI] [PubMed] [Google Scholar]
- 18.Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44(3):547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Jacob SN, Nieder A. Tuning to non-symbolic proportions in the human frontoparietal cortex. Eur J Neurosci. 2009;30(7):1432–1442. doi: 10.1111/j.1460-9568.2009.06932.x. [DOI] [PubMed] [Google Scholar]
- 20.Roy JE, Riesenhuber M, Poggio T, Miller EK. Prefrontal cortex activity during flexible categorization. J Neurosci. 2010;30(25):8519–8528. doi: 10.1523/JNEUROSCI.4837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443(7107):85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- 22.Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat Neurosci. 2012;15(2):315–320. doi: 10.1038/nn.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roitman JD, Brannon EM, Platt ML. Representation of numerosity in posterior parietal cortex. Front Integr Neurosci. 2012;6:25. doi: 10.3389/fnint.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399(6735):470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 25.Nieder A, Merten K. A labeled-line code for small and large numerosities in the monkey prefrontal cortex. J Neurosci. 2007;27(22):5986–5993. doi: 10.1523/JNEUROSCI.1056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawamura H, Shima K, Tanji J. Deficits in action selection based on numerical information after inactivation of the posterior parietal cortex in monkeys. J Neurophysiol. 2010;104(2):902–910. doi: 10.1152/jn.01014.2009. [DOI] [PubMed] [Google Scholar]
- 27.Webster MA, MacLeod DI. Visual adaptation and face perception. Philos Trans R Soc Lond B Biol Sci. 2011;366(1571):1702–1725. doi: 10.1098/rstb.2010.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105(49):19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11(8):877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinsk MA, et al. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101(5):2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell AH, et al. Relationship between functional magnetic resonance imaging-identified regions and neuronal category selectivity. J Neurosci. 2011;31(34):12229–12240. doi: 10.1523/JNEUROSCI.5865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieder A, Miller EK. A parieto-frontal network for visual numerical information in the monkey. Proc Natl Acad Sci USA. 2004;101(19):7457–7462. doi: 10.1073/pnas.0402239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diester I, Nieder A. Semantic associations between signs and numerical categories in the prefrontal cortex. PLoS Biol. 2007;5(11):e294. doi: 10.1371/journal.pbio.0050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tudusciuc O, Nieder A. Contributions of primate prefrontal and posterior parietal cortices to length and numerosity representation. J Neurophysiol. 2009;101(6):2984–2994. doi: 10.1152/jn.90713.2008. [DOI] [PubMed] [Google Scholar]
- 35.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 36.Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- 37.Dehaene S, Changeux JP. Development of elementary numerical abilities: A neural model. J Cogn Neurosci. 1993;5:390–407. doi: 10.1162/jocn.1993.5.4.390. [DOI] [PubMed] [Google Scholar]
- 38.Verguts T, Fias W. Representation of number in animals and humans: A neural model. J Cogn Neurosci. 2004;16(9):1493–1504. doi: 10.1162/0898929042568497. [DOI] [PubMed] [Google Scholar]
- 39.Barner D, Wood J, Hauser M, Carey S. Evidence for a non-linguistic distinction between singular and plural sets in rhesus monkeys. Cognition. 2008;107(2):603–622. doi: 10.1016/j.cognition.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Ogura T, Barner D, Yang SJ, Carey S. Does the conceptual distinction between singular and plural sets depend on language? Dev Psychol. 2009;45(6):1644–1653. doi: 10.1037/a0015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izard V, Dehaene-Lambertz G, Dehaene S. Distinct cerebral pathways for object identity and number in human infants. PLoS Biol. 2008;6(2):e11. doi: 10.1371/journal.pbio.0060011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: John Wiley and Sons; 1966. [Google Scholar]