Abstract
The activation of the satellite glial cells (SGCs) surrounding the dorsal root ganglion (DRG) neurons appears to play a role in pathological pain. We tested the hypothesis that fractalkine, which is constitutively expressed by primary nociceptive neurons, is the link between peripheral inflammation and the activation of SGCs and is thus responsible for the genesis of the inflammatory pain. The injection of carrageenin into the rat hind paw induced a decrease in the mechanical nociceptive threshold (hypernociception), which was associated with an increase in mRNA and GFAP protein expression in the DRG. Both events were inhibited by anti-fractalkine antibody administered directly into the DRG (L5) [intraganglionar (i.gl.)]. The administration of fractalkine into the DRG (L5) produced mechanical hypernociception in a dose-, time-, and CX3C receptor-1 (CX3CR1)–dependent manner. Fractalkine’s hypernociceptive effect appears to be indirect, as it was reduced by local treatment with anti–TNF-α antibody, IL-1–receptor antagonist, or indomethacin. Accordingly, the in vitro incubation of isolated and cultured SGC with fractalkine induced the production/release of TNF-α, IL-1β, and prostaglandin E2. Finally, treatment with i.gl. fluorocitrate blocked fractalkine (i.gl.)- and carrageenin (paw)-induced hypernociception. Overall, these results suggest that, during peripheral inflammation, fractalkine is released in the DRG and contributes to the genesis of inflammatory hypernociception. Fractalkine’s effect appears to be dependent on the activation of the SGCs, leading to the production of TNFα, IL-1β, and prostanoids, which are likely responsible for the maintenance of inflammatory pain. Thus, these results indicate that the inhibition of fractalkine/CX3CR1 signaling in SGCs may serve as a target to control inflammatory pain.
Keywords: cytokines, hyperalgesia, primary sensitization, nociception
Pain is one of the four classic signals of the inflammatory process. The sensitization of primary sensory neurons is accepted to be essential to inflammatory pain (1). Peripheral sensitization is mainly triggered by several inflammatory mediators produced during inflammation and tissue lesion (2). In this context, TNF-α appears to be pivotal to the stimulation of the prohypernociceptive cytokine cascade (3). The peripheral effect of pronociceptive cytokines depends on the local production/release of prostaglandins and sympathomimetic amines that, in turn, act directly on primary nociceptive neurons (4). There is growing evidence that, besides peripheral sensitization, inflammatory pain might be dependent on a process that occurs in the spinal cord, as well as in the dorsal root ganglion (DRG) and trigeminal ganglion (5, 6). In the spinal cord, neuronal plasticity is dynamically modulated by activated microglia- and astrocyte-derived mediators, which can increase pain responsiveness (7).
In the sensory ganglion, the most important type of glial cell is the satellite glial cell (SGC). The SGCs, which possess some characteristics of both astrocytes and microglia, normally form a single layer wrapped around each sensory neuron soma, a distinctive structure that is not found elsewhere in the central nervous system (CNS) (8). The SGCs respond to sensory neuron damage or inflammation by proliferating and expressing glial fibrillary acidic protein (GFAP), similar to glia responses in the spinal cord and brain. Upon activation, the SGCs can express neurotrophins, activated MAP kinases, cytokines, and other mediators that might contribute to abnormal pain sensitivity (8). However, perhaps due to the greater difficulty in administering drugs to the DRG in vivo, much less is known about the possible causal role of SGCs in pathological pain states or the mechanisms involved in their activation. In this situation, our research group developed a technique for the administration of drugs into the DRG (9). This method for DRG injection is simple, reliable, and nonsurgical, and permits repeated injections. The injected drug remains restricted to the ganglia, and the animals recover from anesthesia after only a few minutes, allowing for the rapid evaluation of drug-induced changes in their behavioral responses (9). Fractalkine [CX3C ligand-1 (CX3CL1)] is a unique chemokine (chemotactic cytokine) of the CX3C class, the structure of which is characterized by two cysteines separated by three amino acids (10, 11). Fractalkine is distinctive among the typically promiscuous chemokines in that it binds to only one known receptor, the CX3C receptor-1 (CX3CR1), and this receptor binds only to fractalkine. In addition, fractalkine is the only chemokine that is expressed extracellularly on neurons. In the hippocampus, neuronal excitation releases fractalkine, which then binds to and activates nearby glia (12). In the dorsal horn of the spinal cord, fractalkine is expressed in intrinsic neurons and sensory afferents whereas its receptor is expressed primarily in microglia (13). Therefore, in the CNS, fractalkine appears to function as a signal to inform glial cells about the state of neuronal excitation. In the DRG, fractalkine is constitutively expressed in the sensory neuron cell body whereas CX3CR1 expression is found mainly in SGCs (14).
Taking the above evidence into account, it is plausible to hypothesize that fractalkine may function as a neuron-to-SGC activation signal in the DRG, where it contributes to the genesis of inflammatory pain. Therefore, in the present study, we addressed whether the activation of SGCs by fractalkine in the DRG is involved in the genesis of inflammatory pain and investigated potential mechanisms involved in this process.
Results
Peripheral Inflammation-Induced Hypernociception Is Accompanied and Mediated by SGC Activation.
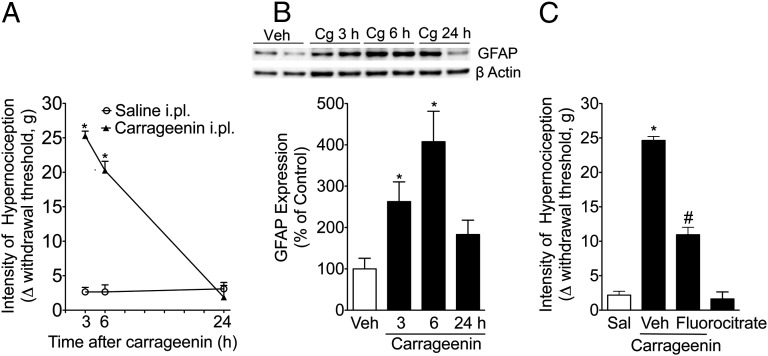
Previous studies have demonstrated that intermediate filaments in the cytoplasm, which are known as GFAP, are important markers for glial cells (astrocytes and SGCs) (15). Although the function of this protein is unknown, an increase in its expression may be correlated with the activation of satellite glial cells. Herein, it was observed that the peripheral inflammation induced by the intraplantar injection of carrageenin reduced the mechanical nociceptive threshold, which was accompanied by an increase in the expression of GFAP, suggesting the activation of the SGCs (Fig. 1 A and B). The GFAP expression levels peaked between 3 and 6 h after carragenin injection and returned to the basal level at 24 h, which coincided with the normalization of the mechanical nociceptive threshold (Fig. 1B). Recent evidence suggests that, in addition to inhibiting astrocyte function, fluorocitrate may also function as a tool to reduce SGCs’ activation and function (16). Therefore, to investigate the participation of SGCs in the development of inflammatory pain, fluorocitrate was administered directly to the DRG (L5). This treatment was able to reduce carrageenin-induced mechanical hypernociception (Fig. 1C), suggesting that SCG activation might play a role in the development of inflammatory pain. Fluorocitrate administration [intraganglionar (i.gl.)] alone did not produce any nociceptive effect (Fig. 1C). On the other hand, after carrageenin-induced paw inflammation, there is no significant increase in ionized calcium binding adaptor molecule-1 (IBA-1) expression (Fig. S1) in fifth lumbar vertebra (L5) DRGs.
Fig. 1.
Satellite glial cell activation during peripheral inflammation. The rats received an intraplantar (i.pl.) injection of carrageenan (Cg) (100 µg per paw) or saline. (A) At the indicated time points, mechanical hypernociception was evaluated. (B) Western blot analyses of GFAP expression were conducted on protein samples isolated from DRGs (L5) 3, 6, and 24 h after carrageenin or saline (Sal) injection in the rat paw. The Western blot analysis was performed using Image Lab Software, version 3.0 (n = 5). (C) The rats were pretreated with fluorocitrate (2 nmol per DRG) administered into the DRG (L5), followed by an intraplantar injection of carragennin. Mechanical hypernociception was evaluated 3 h after carrageenin administration. *P < 0.05 compared with the saline injection (paw) group. #P < 0.05 compared with the vehicle-treated group.
Fractalkine Released in the DRG Mediates Inflammatory Hyperalgesia.
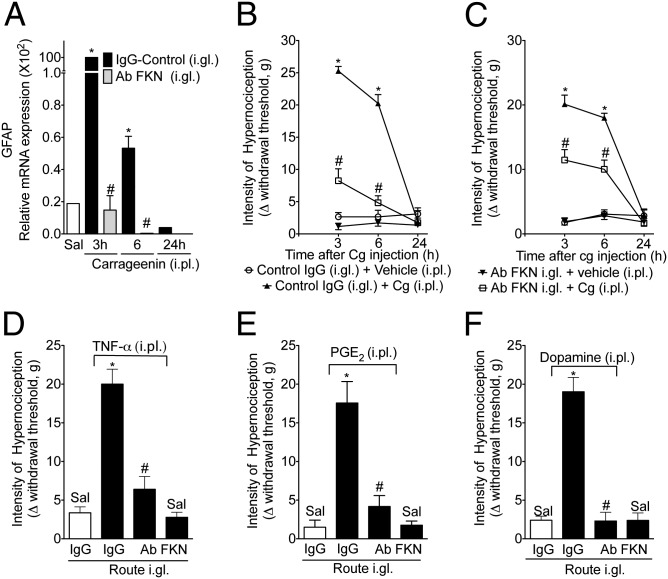
Next, the involvement of fractalkine in the activation of SGCs during carrageenin-induced paw inflammation and, consequently, in the genesis of inflammatory hypernociception was investigated. First, carrageenin-induced peripheral inflammation was observed to be associated with an increase in the mRNA expression of GFAP in the DRG (L5). The pretreatment (30 min before carrageenin injection) of rats with a neutralizing antibody against fractalkine (i.gl.) (17) abrogated this increase in GFAP mRNA expression (Fig. 2A). Second, the same pretreatment protocol was able to reduce the carrageenin-induced mechanical hypernociception (Fig. 2B). Third, in a therapeutic setting, the administration of anti-fractalkine, 1 h after carragenin injection, was also able to reduce mechanical hypernociception (Fig. 2C). It is noteworthy that the injection of neutralizing antibody against fractalkine into the contralateral DRG (L5) did not reduce carrageenin-induced mechanical hypernociception, suggesting a local effect (Fig. S2). Primary nociceptive neuron sensitization and, consequently, the genesis of inflammatory hypernociception depend on the local production of several pronociceptive cytokines, of which TNF-α is the most important (4). Moreover, the hypernociceptive effect of peripheral cytokines appears to be indirect, functioning through the stimulation of the production/release of hypernociceptive mediators such as prostaglandin (PG) E2 and sympathomimetic amines (dopamine) that act directly (2). The peripheral hypernociceptive effects of TNF-α, PGE2, and dopamine were reduced by the i.gl. injection of a neutralizing antibody against fractalkine (Fig. 2 D–F).
Fig. 2.
Effect of the neutralizing antibody against fractalkine (Ab FKN) in inflammatory hypernociception. The rats were pretreated (1 h) with Ab FKN (10 µg) or IgG control injected into the DRG (L5), followed by the injection of carrageenin (100 µg per paw) into the ipsilateral paw. At the indicated time points after carrageenin injection (A), mechanical hypernociception was evaluated, followed by the removal of the DRG (L5). (B) The expression of GFAP mRNA was determined. (C) Ab FKN (10 µg per DRG) or IgG control was also injected 1 h after carrageenin administration (100 µg per paw). Mechanical hypernociception was evaluated 3, 6, and 24 h after carrageenin injection. (D–F) The rats were pretreated (1 h) with Ab FKN (10 µg) or IgG control injected into the DRG (L5), followed by the injection of TNF-α (100 pg per paw), PGE2 (100 ng per paw), or dopamine (10 µg per paw) into the ipsilateral paw. Mechanical hypernociception was evaluated 3 h after the injection of the inflammatory mediators. In all experiments, the data are expressed as the mean ± SEM of 10 animals per group. “*” indicates statistical significance compared with the saline injection (paw) group; “#” indicates statistical significance compared with the IgG control-treated group.
Fractalkine Injection into the DRG Induces Mechanical Hypernociception: Role of SGCs.
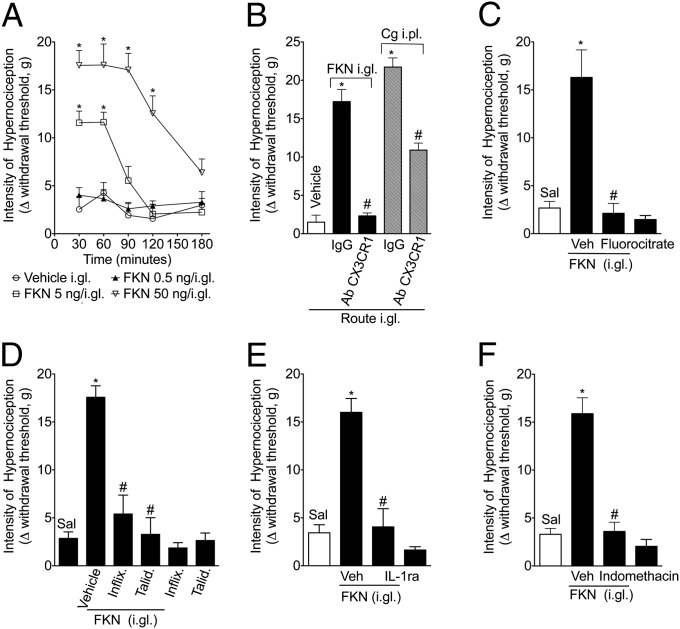
To confirm the pronociceptive effect of fractalkine release in the DRG, we examined the hypernociceptive response of rats that received an i.gl. injection of fractalkine. Exogenous fractalkine injected into the DRG (L5) produced paw mechanical hypernociception in a dose- and time-dependent manner (Fig. 3A). Mechanical hypernociception induced by i.gl. fractalkine was largely attenuated by treatment with a neutralizing antibody against CX3CR1 (Fig. 3B) (18), suggesting a specific- and receptor-dependent effect. The neutralizing antibody against CX3CR1 injected into the DRG also reduced carrageenin-induced mechanical hypernociception (Fig. 3B). As a control, neutralizing antibody against fractalkine was tested, and it inhibited fractalkine’s hypernociceptive effect, suggesting that this antibody could be an appropriate tool for the in vivo study of the fractalkine nociceptive role (Fig. S3). Interestingly, the role of DRG fractalkine/CX3CR1 on inflammatory pain appears to be dependent on SGC activation, as the hypernociceptive effect of i.gl. fractalkine was attenuated by pretreatment with fluorocitrate (i.gl.; Fig. 3C).
Fig. 3.
Pronociceptive effect of the i.gl. injection of fractalkine. (A) Fractalkine (FKN; 0.5–50 ng) was administered into the DRG (L5). Mechanical hypernociception was evaluated 30, 60, and 180 min after FKN injection. (B) A neutralizing antibody against the FKN receptor (AbCX3CR1; 10 µg per DRG) was administered into the DRG (L5). After 1 h, FKN (50 ng per DRG) or carrageenin (100 µg per i.pl.) was injected, and mechanical hypernociception was evaluated 1 and 3 h after injection. (C) The rats were pretreated with an i.gl. injection of fluorocitrate (2 nmol per DRG-L5). After 1 h, FKN (50 ng per DRG) was injected, and mechanical hypernociception was evaluated 1 h after FKN administration. The rats were pretreated with (D) infliximab (100 µg per DRG) and thalidomide (50 ng per DRG), (E) IL-1ra (300 ng per DRG), or (F) indomethacin (50 µg per DRG). After 1 h, FKN (50 ng per DRG) was injected, and mechanical hypernociception was evaluated 1 h after injection. The data are expressed as the mean ± SEM of 10 rats per group. “*” indicates statistical significance compared with the saline (paw or i.gl.) treatment group; “#” indicates statistical significance compared with the vehicle- or Ab control-treated group.
Fractalkine Mediates Inflammatory Pain Through the Stimulation of Proinflammatory Cytokines and PGE2 Production by SGCs.
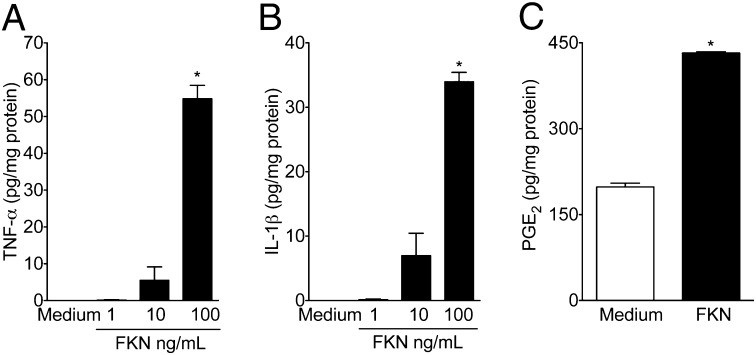
As previously mentioned, peripheral cytokines play a crucial role in the genesis of inflammatory pain (3, 4). Moreover, there is recent, indirect evidence suggesting that cytokines produced in the DRG may also be involved in the process of inflammatory pain (8). In this context, inflammatory hypernociception induced by the paw injection of carrageenin was found to be attenuated in a dose-dependent manner by the i.gl. injection of thalidomide and infliximab (anti–TNF-α therapies; Fig. S4 A and B) and IL-1 receptor antagonist (IL-1ra) (Fig. S4C), suggesting the participation of TNF-α and IL-1β. Furthermore, the i.gl. injection of indomethacin (cyclooxygenase inhibitor) also reduced carrageenin-induced mechanical hypernociception (Fig. S4D), indicating the participation of prostaglandins. Next, we evaluated whether the pronociceptive role of DRG fractalkine was dependent on these mediators. The prohypernociceptive effect of i.gl. fractalkine was reduced by i.gl injection of infliximab, thalidomide, IL-1ra, and indomethacin (Fig. 3 D–F). To reinforce this pharmacological data, we switched to an in vitro system of isolated and cultured SGCs. In corroboration with the in vivo results, the incubation of isolated SGCs with fractalkine induced the production/release of TNF-α (Fig. 4A), IL-1β (Fig. 4B), and PGE2 (Fig. 4C). These last results suggest that, during peripheral inflammation, fractalkine is released in the DRG, promoting the production of pronociceptive mediators such as cytokines and prostaglandins by SGCs.
Fig. 4.
Fractalkine stimulates cytokines and PGE2 release in a primary culture of satellite glial cells (SGCs). Isolated and cultured SGCs were incubated with fractalkine (FKN; 1–100 ng/mL). After 6 h, the supernatants were collected, and the concentrations of (A) TNF-α, (B) IL-1β, and (C) PGE2 were measured. The attached cells were lysed, and protein concentrations were determined for normalization. “*” indicates statistical significance compared with the medium-treated cells (n = 6).
Discussion
Several plastic changes in neuronal function across the nociceptive system have been found to be associated with the genesis of inflammatory pain, including the sensitization of primary and secondary sensory nociceptive neurons. Nevertheless, a large body of evidence has also suggested that this neuronal plasticity is not an intrinsic event of neurons but rather is dependent on other cells. The interaction between immune/glial cells and neurons during the induction of chronic pain is a novel concept that has gained much attention in the last years. For instance, during peripheral inflammation, the increase in neuronal inputs from the periphery to the CNS results in an intense activation of glial cells (microglia and astrocytes), mainly at the spinal cord and trigeminal nucleus (19–21). These cells then produce and release a great number of substances, including proinflammatory cytokines (TNF-α and IL1-β), which directly or indirectly act upon the neurons of the nociceptive system to amplify the pain process (7). Recently, another group of glial cells, SGCs, was found to be activated during peripheral inflammation and may be involved in the maintenance of inflammatory pain (8). The present study aimed to increase our understanding of the mechanisms involved in the activation of the SGCs during peripheral inflammation and its contribution to the genesis of inflammatory pain. The results provided strong evidence that, during peripheral inflammation, fractalkine is released in the DRG, probably by the body of primary sensory neurons, causing the activation of its receptor (CX3CR1), which is found on SGCs. Fractalkine promotes SGC activation, promoting the production/release of cytokines and prostaglandins that, in turn, are responsible for the genesis and/or maintenance of inflammatory pain.
Our group has worked for 30 y on the idea that the sensitization of primary nociceptive neurons at the site of inflammation would be sufficient to explain the induction of inflammatory hyperalgesia. The present data provide information indicating that the DRG might be an important structure in the cascade of events that sustains primary nociceptive neuron sensitization and subsequent inflammatory hypernociception. In this context, the local sensitization of primary sensory neurons likely spreads to the entire cell, reaching the cell body at the DRG, as well as the central terminal of the neuron in the spinal cord (22). Thus, it is conceivable that inflammatory pain is a phenomenon involving the whole neuron; this idea is supported by the fact that the i.gl. injection of morphine inhibits mechanical hypernociception induced by the injection of PGE2 into the hind paw (9).
Several studies correlate glial activation with an increase in the expression of different proteins, including GFAP (23, 24). Although the role of this molecule is still unknown, increased GFAP expression is a good marker of SGC activation. In this study, we found increased expression of GFAP in the DRG of rats after carrageenin-induced peripheral inflammation, suggesting that SCGs might be activated. In corroboration with our results, the inflammation of the temporomandibular joint, which causes trigeminal hypersensitivity, was found to increase the expression of GFAP in the trigeminal SGCs (21). However, SGC activation does not prove that they are involved in the genesis of inflammatory pain. To our knowledge, there has been only indirect evidence implicating SGCs in the genesis of inflammatory pain (25). Recently, the infusion of fluorocitrate into the DRG (L5) using a miniosmotic pump, which inhibited SGC activation, was shown to be able to reduce spinal nerve injury-induced pain behavior, suggesting the participation of SGCs in the genesis of neuropathic pain (16). Herein, we demonstrate that the direct injection of fluorocitrate into the DRG (L5) was also able to reduce inflammatory pain behavior. These findings were obtained through the use of a noninvasive technique developed in our laboratory (9) for direct DRG injections of agonists and antagonists in naive rats or those stimulated by peripheral inflammation. This technique does not involve surgery, is easy to learn, and allows behavioral testing within minutes after the injection.
Interestingly, SGCs appear to be rapidly activated after peripheral inflammation, as GFAP is already expressed in the DRG at least 3 h after carrageenin injection, which overlaps with paw hypernociception. Although little is known about the signaling mechanism between peripheral inflammation and SGC activation, there is speculation that SGC activation is dependent on signals released by primary neuron cell bodies in the DRG (8). In the case of glial cell activation in the spinal cord in chronic pain models, fractalkine is one of the main factors released by neuronal activation that contributes to this process (26). In fact, fractalkine is constitutively expressed in the central terminal of the primary sensory neurons whereas its receptor, CX3CR1, is found in intrinsic spinal microglia (13). During neuronal stimulation after a nerve lesion, fractalkine is suggested to be cleaved into its soluble form, which can in turn act on CX3CR1-expressing microglia (27). This interaction promotes the activation of microglia, which contributes to the genesis of chronic pain (28, 29). This evidence, together with the demonstration that fractalkine is also expressed in the cell bodies of the primary afferent neurons, whereas CX3CR1 expression was found in the SGCs, led us to suggest that the activation of SGCs during peripheral inflammation is dependent on fractalkine/CX3CR1 signaling. Giving support to our hypothesis, SGC activation during peripheral inflammation, as indirectly assessed by GFAP expression, was reduced by the blockage of fractalkine action in the DRG. This process appears to be important for the genesis and/or maintenance of inflammatory pain because the blockage of fractalkine also reduced inflammatory hypernociception and the pronociceptive effect of fractalkine was inhibited by fluorocitrate. Therefore, in addition to mediating spinal cord microglia activation (27), fractalkine/CX3CR1 signaling appears to also be involved in the activation of SGCs after peripheral inflammation and, consequently, in the genesis of inflammatory pain. Accordingly, CX3CR1-deficient mice present reduced inflammatory pain after the peripheral administration of zymosan (28).
One interesting question that emerges from our results is the mechanism underlying the ganglion release of fractalkine after peripheral inflammation. An intriguing result of the present study was the observation that hypernociception induced by PGE2, the effect of which depends on direct action on primary nociceptive neurons and is independent of the local production of other mediators, was also dependent on the fractalkine release in the DRG. This result, in fact, excluded the possibility that some substance produced during inflammation could reach the DRG and promote the release of fractalkine. Thus, it is likely that peripheral nociceptive sensitization (e.g., an increase in neuronal excitability) caused by PGE2 reaches the DRG and promotes the liberation of fractalkine. In line with this proposition, abnormal spontaneous activity in the sensory neurons has been implicated in the activation of SGCs after peripheral nerve injury. For instance, blocking spontaneous activity with tetrodotoxin, bupivacaine, or lidocaine prevented the activation of the glial cells in the DRG and the spinal cord in chronic pain models (30, 31). Furthermore, there is evidence in the literature suggesting that, during peripheral inflammation, IL1-β is released into the spinal cord by a mechanism dependent on neuronal activity, as this event was blocked when the sciatic nerve was anesthetized (32).
Another possibility could be related to possible calcium waves that appear to occur in peripheral nociceptive neurons during inflammatory sensitization (33). In this process, a modest rise in intracellular Ca2+ concentration is amplified by a secondary release of vesicular Ca2+, which propagates throughout the cell. In agreement, PGE2 elevated cytosolic calcium concentration in a subpopulation of nociceptive neurons (34). Therefore, Ca2+-induced Ca2+ release (CICR) could be a phenomenon involved in the integration of various segments of the primary sensory neurons (periphery terminal to DRG to central terminal), promoting the release of fractalkine and contributing to the maintenance of inflammatory pain.
Despite the hypothesis that CICR is involved in the communication between peripheral inflammation and the release of fractalkine in the DRG, the biochemical mechanism involved in this process has not been elucidated. Some possible candidates to promote the cleavage of this chemokine from DRG neuron membranes have been investigated. Matrix metalloproteinases (MMPs) might be one of the enzymes that promote fractalkine cleavage to its soluble form (12, 35). In corroboration of this hypothesis, the administration of doxycycline (an MMP inhibitor) into the DRG reduces inflammatory hypernociception induced by carrageenin injected into the rat paw (Fig. S5). Moreover, an increase in MMP expression and activity has been implicated in the cascade of events involved in the genesis of chronic pain (36). Another possible enzyme would be cathepsin S, which, at least in the spinal cord, is involved in the cleavage of fractalkine after the peripheral lesion of primary sensory neurons, contributing to the genesis of neuropathic pain (17).
Upon activation, glial cells are known to produce and release a great number of neuroactive substances, including proinflammatory cytokines, ATP, nitric oxide, and excitatory amino acids (26). There is also evidence in the literature suggesting that, during peripheral inflammation, cytokines are released into the DRG (37). In fact, the administration of Complete Freund's Adjuvant (CFA) into the rat paw induced an increase in the concentration of TNFα and IL-1β in the DRG tissue (38, 39). Herein, we demonstrated that these cytokines and prostaglandin produced in the DRG also participate in the genesis of inflammatory pain. Although which cell type is responsible for producing these cytokines and prostaglandins in the DRG was not addressed, our results suggest that SGCs are involved. Furthermore, the pronociceptive activity of fractalkine in the DRG was found to be dependent on cytokine and prostaglandin production, probably by SGCs. In agreement, the in vitro incubation of isolated SGCs with fractalkine promoted the production of TNF-α, IL-1β, and PGE2. There is also evidence in the literature suggesting that activated SGCs during peripheral inflammation can produce/release pronociceptive cytokines (39).
Although the present results indicate that pronociceptive activity of fractalkine on DRGs is manly dependent on CX3CR1-expressing SGC, that in turn, produced pronociceptive cytokines, we could not dismiss the participation of other cell types such as sensitive neurons themselves, Schwann cells, or infiltrating immune cells in this effect (40, 41). In agreement with this suggestion, there are resident CX3CR1-positive macrophages in the DRGs (42). Furthermore, this cell population increased after peripheral nerve lesion (42). However, in the present study, IBA-1 expression did not change significantly after carrageenin-induced paw inflammation, suggesting that, at least in the evaluated times, there is no activation and/or infiltration of macrophages in the DRGs. Moreover, because the fractalkine pronociceptive effect peaks at 30–60 min after injection, we could also speculate that it might be acting directly on primary sensitive neurons. In fact, there is a population of primary sensitive neurons that express CX3CR1 in their cell body (13).
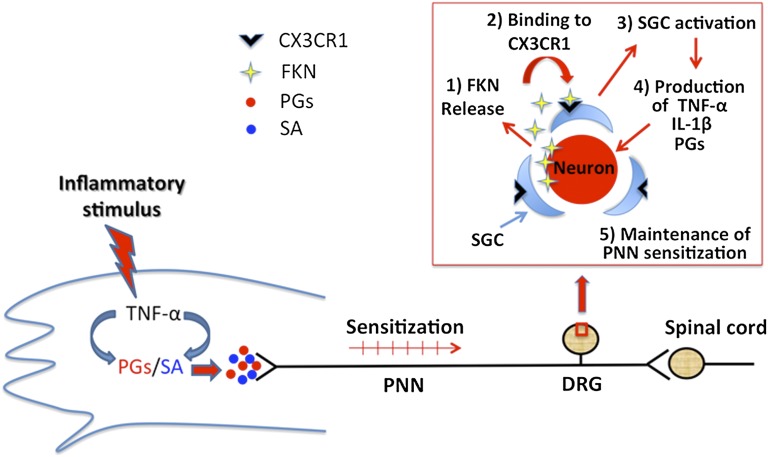
In conclusion, the results suggest that fractalkine/CX3CR1 signaling in the DRG is the key component linking peripheral inflammation and the activation of SGCs and that this process is crucial to the induction/maintenance of inflammatory pain. Fractalkine’s effect appears to be indirect and mediated by the production of TNFα, IL-1β, and prostanoids by the SGCs. These results are summarized in Fig. 5. We believe that the inhibition of fractalkine/CX3CR1 signaling in SGCs might constitute a target for the development of novel drugs to treat inflammatory pain.
Fig. 5.
Schematic representation of the mechanisms of fractalkine release/action in the DRGs during peripheral inflammation. Inflammatory insult stimulates the production/release of a cascade of cytokines in the site of inflammation, culminating in the production of directly acting hypernociceptive mediators [prostanoids and sympathetic amines (SA)]. Nociceptive neuron sensitization caused by these mediators reaches the DRG, promoting the release of fractalkine that, in turn, binds to CX3CR1 expressed on SGCs and stimulates the production of TNF-α, IL-β, and PGE2. These mediators might contribute to the maintenance of neuronal sensitization and, consequently, to inflammatory pain. PNN, primary nociceptive neuron.
Materials and Methods
For complete details, see SI Materials and Methods.
Animals.
The experiments were performed on Wistar male rats (180–220 g).
I.gl. Drug Administration.
The i.gl. drug administration was carried out in accordance with ref. 9. Briefly, the rats were anesthetized by the inhalation of 2% (vol/vol) isoflurane. The injecting needle was inserted through the punctured skin, toward the intervertebral space between the fifth and sixth lumbar vertebrae, until the tip touched the lateral region of the vertebrae. To reach the space between the transverse processes of the fifth and sixth vertebrae, smooth movements of the needle were made until the bone resistance was diminished and a paw flinch reflex was observed.
Nociceptive Test: Electronic Pressure-Meter Test.
Mechanical hypernociception was tested in rats as previously reported (43). In a quiet room, rats were placed in acrylic cages with wire grid floors. The test consisted of evoking a hind paw flexion reflex with a hand-held force transducer (electronic aesthesiometer; IITC Life Science) adapted with a 0.7-mm2 polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hind paw with a gradual increase in pressure.
SGC Isolation and Culture.
Rats were killed by decapitation under anesthesia. Dorsal root ganglia were collected and processed as previously described (44). Cells were dissociated and plated in six-well plastic plates coated with Matrigel (BD). The satellite cells were isolated according to an adapted version of a published protocol.
RNA Extraction and Real-Time PCR.
At 3, 6, and 24 h after the paw administration of carrageenin or saline, the rats were terminally anesthetized, and the ipsilateral DRGs (L5) were removed. The samples were homogenized in 1 mL of TRIzol (Invitrogen), and total RNA was extracted according to the manufacturer's instructions. Real-time PCR was performed using primers specific for the rat gene GFAP and for the rat housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Western Blot Analysis.
After the indicated stimulation, the DRG were homogenized in a lysis buffer containing a mixture of protease inhibitors and phosphatase inhibitors (Sigma-Aldrich). The protein concentrations of the lysate were determined using a BCA Protein Assay kit (Pierce). The protein samples were separated on an SDS/PAGE gel and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech) followed by the incubation with primary antibodies against GFAP (1:500; Millipore) and IBA-1 (1:300; Wako Chemicals) and then incubated for 1 h at room temperature with an HRP-conjugated secondary antibody (1:3,000; Jackson ImmunoResearch). The blots were visualized in an ECL solution (Amersham Pharmacia Biotech) and exposed in a ChemiDoc MP Imaging System (Bio-Rad Laboratories).
Cytokine Measurements.
The supernatants of isolated, cultured SGCs were collected 6 h after incubation with fractalkine (1–100 ng/mL) or medium (control) treatment. The supernatants were used to determine the levels of TNF-α and IL-1β by ELISA, as described previously (45).
Measurement of PGE2 in the Culture of Satellite Cells by RIA.
The concentration of PGE2 in supernatants of cultured SGCs was measured by RIA using a commercially available kit. The results are expressed as picograms of PGE2 per mg of protein from lysed cells (45).
Data Analyses and Statistics.
All results are presented as means ± SEM. The experiments were repeated at least twice. Two-way analysis of variance (ANOVA) was used to compare the groups and doses at all times (curves) when the hypernociceptive responses were measured at different times after the stimulus injection. The factors analyzed were treatments, time, and time-by-treatment interaction. When there was a significant time-by-treatment interaction, one-way ANOVA followed by Bonferroni’s t test was performed for each time. Alternatively, when the hypernociceptive responses were measured once after the stimulus injection, the differences between responses were evaluated by one-way ANOVA followed by Bonferroni’s t test. P < 0.05 was considered as significant.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of Fabiola Mestriner Giuliana Bertozzi, Ieda R. dos Santos, Sergio R. Rosa, and Tiago A. Bozzo. This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Pesquisa, Núcleo de Apoio a Pesquisa em Doenças Inflamatórias, and Centro de Pesquisas em Doenças Inflamatórias. G.R.S. was a recipient of a doctoral fellowship from FAPESP.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307445110/-/DCSupplemental.
References
- 1.Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972;240(102):200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- 2.Verri WA, Jr, et al. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha TM, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park CK, et al. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: Distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31(50):18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda M, Takahashi M, Nasu M, Matsumoto S. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain. 2011;152(9):2147–2156. doi: 10.1016/j.pain.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 8.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48(3):457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari LF, Cunha FQ, Parada CA, Ferreira SH. A novel technique to perform direct intraganglionar injections in rats. J Neurosci Methods. 2007;159(2):236–243. doi: 10.1016/j.jneumeth.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Bazan JF, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 12.Chapman GA, et al. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20(15):RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20(5):1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, et al. Selective activation of microglia in spinal cord but not higher cortical regions following nerve injury in adult mouse. Mol Pain. 2008;4:15. doi: 10.1186/1744-8069-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565(1):1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- 16.Liu FY, et al. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res. 2012;1427:65–77. doi: 10.1016/j.brainres.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Clark AK, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104(25):10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milligan ED, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20(9):2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27(22):6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa G, et al. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain. 2010;6:89. doi: 10.1186/1744-8069-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funez MI, et al. Teleantagonism: A pharmacodynamic property of the primary nociceptive neuron. Proc Natl Acad Sci USA. 2008;105(49):19038–19043. doi: 10.1073/pnas.0807922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemionow K, Klimczak A, Brzezicki G, Siemionow M, McLain RF. 2009. The effects of inflammation on glial fibrillary acidic protein expression in satellite cells of the dorsal root ganglion. Spine (Phila Pa 1976) 34(16):1631–1637. [DOI] [PubMed]
- 24.Zhang H, et al. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia. 2009;57(15):1588–1599. doi: 10.1002/glia.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda M, Takahashi M, Matsumoto S. Contribution of activated interleukin receptors in trigeminal ganglion neurons to hyperalgesia via satellite glial interleukin-1beta paracrine mechanism. Brain Behav Immun. 2008;22(7):1016–1023. doi: 10.1016/j.bbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: A role for fractalkine. J Neuroimmunol. 2008;198(1-2):113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci. 2009;29(21):6945–6954. doi: 10.1523/JNEUROSCI.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staniland AA, et al. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J Neurochem. 2010;114(4):1143–1157. doi: 10.1111/j.1471-4159.2010.06837.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang ZY, et al. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21(5):642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen YR, et al. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology. 2007;107(2):312–321. doi: 10.1097/01.anes.0000270759.11086.e7. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160(4):847–857. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samad TA, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 33.Usachev YM, Thayer SA. Controlling the urge for a Ca(2+) surge: All-or-none Ca(2+) release in neurons. Bioessays. 1999;21(9):743–750. doi: 10.1002/(SICI)1521-1878(199909)21:9<743::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Smith JA, Davis CL, Burgess GM. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. Eur J Neurosci. 2000;12(9):3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji RR, Xu ZZ, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30(7):336–340. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33(6):784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda M, et al. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129(1-2):155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112(1):23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 41.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: Expression in injured and non-injured nerves. Neuroscience. 1996;73(3):625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 42.Holmes FE, et al. Intra-neural administration of fractalkine attenuates neuropathic pain-related behaviour. J Neurochem. 2008;106(2):640–649. doi: 10.1111/j.1471-4159.2008.05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivancos GG, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37(3):391–399. doi: 10.1590/s0100-879x2004000300017. [DOI] [PubMed] [Google Scholar]
- 44.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118(1):69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 45.Cunha TM, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83(4):824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.