Abstract
Abnormal assemblies formed by misfolded superoxide dismutase-1 (SOD1) proteins are the likely cause of SOD1-linked familial amyotrophic lateral sclerosis (fALS) and may be involved in some cases of sporadic ALS. To analyze the structure of the insoluble SOD1 amyloid fibrils, we first used limited proteolysis followed by mass spectrometric analysis. Digestion of amyloid fibrils formed from full-length N-acetylated WT SOD1 with trypsin, chymotrypsin, or Pronase revealed that the first 63 residues of the N terminus were protected from protease digestion by fibril formation. Furthermore, every tested ALS-mutant SOD1 protein (G37R, L38V, G41D, G93A, G93S, and D101N) showed a similar protected fragment after trypsin digestion. Our second approach to structural characterization used atomic force microscopy to image the SOD1 fibrils and revealed that WT and mutants showed similar twisted morphologies. WT fibrils had a consistent average helical pitch distance of 62.1 nm. The ALS-mutant SOD1 proteins L38V, G93A, and G93S formed fibrils with helical twist patterns very similar to those of WT, whereas small but significant structural deviations were observed for the mutant proteins G37R, G41D, and D101N. Overall, our studies suggest that human WT SOD1 and ALS-mutants tested have a common intrinsic propensity to fibrillate through the N terminus and that single amino acid substitutions can lead to changes in the helical twist pattern.
Keywords: aggregation, mass spectrometry, protein misfolding, neurodegeneration
Amyotrophic lateral sclerosis (ALS) or Lou Gehrig’s disease is a devastating motor neuron disease characterized by the formation of abnormal protein aggregates in neuronal cells. More than 100 different mutations in superoxide dismutase-1 (sod1) have been identified and linked to familial ALS (fALS). Although the precise mechanism(s) by which this diverse group of mutations causes fALS remains unclear, it generally is agreed that the ALS-mutant SOD1 proteins are prone to misfold and that they acquire toxic properties as a consequence (1–3). Abnormal protein deposits are seen frequently in protein misfolding diseases, and SOD1-containing aggregates have been found consistently in the spinal cords of ALS transgenic mice and fALS patients (4, 5). Moreover, in ALS transgenic mice, these proteinaceous deposits frequently have been shown to have amyloid-like properties such as filamentous structures and the ability to bind thioflavin-S (6–8). A number of other proteins that have been linked to neurodegenerative diseases form amyloid fibrils. Such fibrils are elongated, unbranched, and highly ordered protein aggregates composed mainly of cross–β-sheets, with parallel or anti-parallel β-strands stacking perpendicular to the axis of fibril growth (9–13).
Detergent-resistant aggregates isolated from the spinal cords of ALS transgenic mice contain full-length and metal-free human SOD1 (hSOD1) proteins, suggesting that it is full-length metal-free (apo) hSOD1 that acquires toxic properties in the disease mechanism (14). Moreover, as established earlier in our laboratory, full-length, soluble apo hSOD1 can be converted readily in vitro to a morphologically homogeneous preparation of amyloid fibrils by incubation with a small amount of reducing agent (15). SOD1 fibrils made under these conditions recently have been shown to induce cytokine expression in mononuclear cells, thus causing inflammation (16), and to activate microglial cells (17), suggesting that fibrils made under these mild physiologically relevant conditions have toxic properties that may be related to ALS. However, because of the insolubility and noncrystalline nature of these materials, the molecular structure(s) of such materials have not yet been determined by methods such as NMR and X-ray crystallography.
The goals of the present study were to determine which regions of the WT and ALS-mutant SOD1 proteins form the amyloid core of the SOD1 fibrils prepared using the conditions developed in our laboratory (15, 18) and to compare the structural morphologies of these WT and ALS-mutant SOD1 fibrils.
We used limited proteolysis coupled to LC-MS and MS/MS, which has been used widely to probe the structure of amyloid fibrils (19–24), and we also studied the fibrils using atomic force microscopy (AFM). We find that the core regions of both WT and mutant fibrils are consistently composed of the N terminus of the SOD1 protein, but the twist patterns of some of the mutant SOD1 fibrils may differ from those of the WT protein.
Results
The Most Protease-Resistant Region of WT SOD1 Is Formed by the N-Terminal Region of SOD1.
To determine the region of WT hSOD1 that is most resistant to protease digestion in the fibrils, soluble WT SOD1 was induced to form fibrils in the presence of 5 mM DTT, following the protocol previously published (15). Mature fibrils, as indicated by reaching the plateau of Thioflavin-T binding, were retrieved from the plate, collected by ultracentrifugation, and subjected to partial trypsin proteolysis. After digestion, the protease was quenched with 1 mM PMSF and was recentrifuged to separate the digested soluble peptides (S2) from the proteolytically resistant region of SOD1 that remained insoluble (P2). P2 was then solubilized by 7.2 M guanidine hydrochloride (GdnHCl) and 0.5 M DTT overnight at 37 °C. One major advantage of using trypsin is that peptides released in S2 are not digested to single amino acids and thus can be identified by MS. The compositions of P2 and S2 then were analyzed by HPLC-MS.
When soluble WT SOD1 was digested by trypsin under the same conditions, 12 peptides spanning the full-length sequence of SOD1 were recovered and identified by HPLC-MS (Table S1), demonstrating that soluble apo WT disulfide-oxidized SOD1 can be digested completely by this trypsin digestion condition. However, when WT SOD1 fibrils were digested, one polypeptide with a retention time of 30.49 min was found to be the primary product in P2, as shown by the total ion count (TIC) chromatograph (Fig. 1). This peptide has a measured average molecular mass of 7,285 Da, corresponding to the mass of the N-terminal acetylated tryptic peptide 1–69 in the sequence of WT SOD1 (numbered from the mature N terminus with the initiating Met residue removed; numbering starts from Ala at position 1). Note that five tryptic cleavage sites (K3, K9, K23, K30, and K36) in this region that were accessible in soluble WT protein became protected in the fibrillar configuration. In contrast, C-terminal peptides were cut and released to S2. The most abundant species from S2 were from the C-terminal end of the SOD1 sequence. Peptide bonds at residues R115, K122, and R143 were cleaved, resulting in the release of peptides 80–115, 116–122, and 144–153 to S2 (Fig. S1). These results suggest that the regions surrounding these corresponding cleavage sites were neither sterically nor conformationally hindered. Small amounts of tryptic peptides from the N-terminal region of SOD1 also were found in the soluble S2 fraction, possibly resulting from a small degree of heterogeneity of fibrils (subsets of fibrils with different conformations), overdigestion, or dissociation of SOD1 from the fibril because of the dynamic nature of amyloid fibrils (25).
Fig. 1.
LC-MS of WT hSOD1 fibrils partially digested by trypsin, chymotrypsin, and Pronase. (Left) Total ion chromatogram (TIC) from reverse-phase LCMS. (Center) Mass spectrum integrated across the entire chromatogram. A.u., arbitrary units. (Right) Measured average masses resulting from deconvolution. (A) Peptides from S2 are predominantly from the C terminus. (B–D) Peptides from the insoluble fibril core (P2). A major peptide composed of at least 63 continuous residues from the N terminus of SOD1 is recovered consistently from P2 with (B) trypsin, (C) chymotrypsin, and (D) Pronase. Theoretical average masses of the WT SOD1 peptides are as follows: 1–63 (6,571.36 Da), 1–64 (6,719.60 Da), 1–69 (7,287.05 Da), 80–115 (3,666.04 Da), 116–122 (825.99 Da), and 144–153 (945.18 Da). See Table S2 for a complete list of peptides found in S2 and P2.
To confirm our findings, Pronase and chymotrypsin, proteases with broader specificity, were used. In both cases, the protease-resistant region found in P2 was shortened to residues 1–63. The identity of the peptide was confirmed by high-resolution MS/MS (Figs. S2 and S3 and Table S3). Sixteen chymotryptic cleavage sites (L8, D11, N19, F20, E21, E24, N26, W32, L38, E40, L42, F45, E49, F50, D52, and N53) from the N terminus became protected in the fibrils. Because Pronase is a mixture of proteases that is capable of completely digesting the solvent-accessible region to single amino acid residues, the fact that residues 1–63 remained undigested strongly suggests that these residues comprise the minimal protease-resistant core region in WT SOD1 fibrils under these digestion conditions.
WT and Mutant Fibrils Share the Same N-Terminal Core.
Single point mutations in the SOD1 gene are responsible for causing SOD1-linked fALS. Most of these mutations result in SOD1 proteins with single amino acid substitutions and with biophysical properties very similar to the WT, making it difficult to postulate the disease-causing property conferred by these mutations. Moreover, it has been reported recently that not only mutant hSOD1 but also WT hSOD1 protein expressed at high levels in transgenic mice can lead to ALS (26). Large amounts of aggregated WT hSOD1 protein were found in the spinal cords and brains of these mice suggesting that WT hSOD1 protein also may become toxic in some forms. Thus, we aimed to determine whether ALS-mutant SOD1 protein fibrils prepared using our protocol have structures distinct from that of the WT SOD1 fibrils.
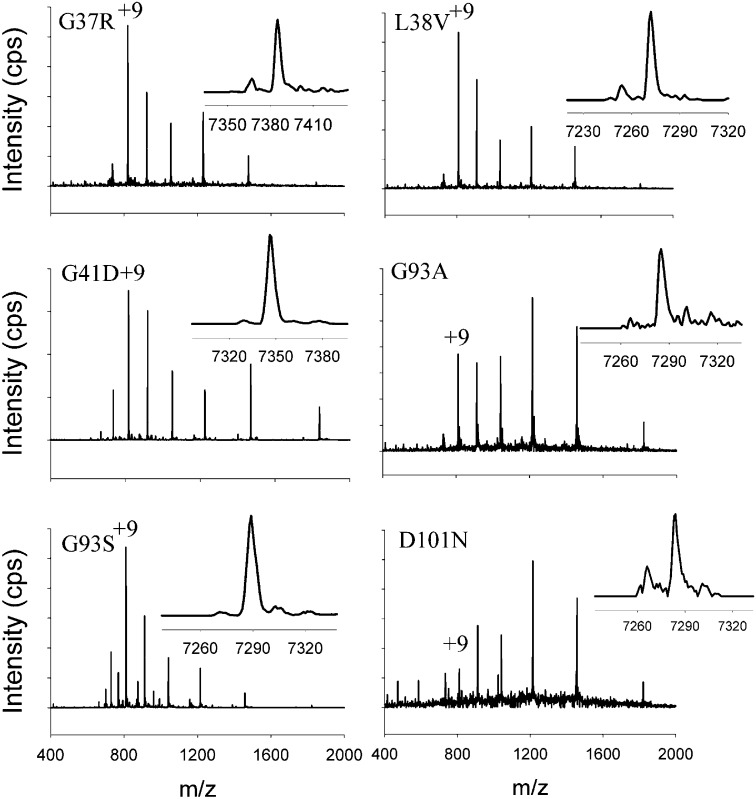
To minimize heterogeneity among different samples attributable to sample handling or ambient conditions, fibrils were made from the mutant proteins and WT SOD1 simultaneously and also were trypsin digested simultaneously. Three N-terminal mutants (G37R, L38V, and G41D) and three C-terminal mutants (G93A, G93S, and D101N) were tested. The P2 of each contained primarily one peak with an LC-MS retention time of around 30 min (Fig. S4). Peptide molecular mass deconvolution of each entire chromatogram revealed predominantly one species, the N-terminal residues 1–69 of each mutant, as in WT, with altered mass for the N-terminal mutations but unchanged mass for the C-terminal mutants (Fig. 2). Occasionally, a small amount of the C-terminal peptide 143–153 also was found in P2 (Table S4), although it always was more abundant in S2. These observations strongly suggest a unifying fibrillation mechanism in which the N-terminal end of SOD1 embedded in the core acquires protease resistance and forms the most inaccessible region of these fibrils similarly in WT and ALS-mutant sequences.
Fig. 2.
Mass spectrum of P2 peptide from mutant fibrils partially digested by trypsin. Mass spectrum integrated from the entire chromatogram. Theoretical average masses of the N-acetylated 1–69 SOD1 peptides are 7,287.5 Da (WT, G93A, G93S, and D101N), 7,386.39 Da (G37R), 7,273.23 Da (L38V), and 7,345.29 Da (G41D). Insets show measured average mass. See Table S4 for complete list of peptides detected.
AFM Analysis on WT and Mutant SOD1 Fibrils.
AFM in tapping mode was used to analyze the morphology of the SOD1 fibrils. It has been reported that fibril structures can evolve over time (27). Therefore, to achieve maximum consistency, fibril samples were taken from the plate after 45 h of incubation and were placed on the mica immediately. As has been observed in other amyloid fibrils, periodic twists that give the fibrils “bead-like” morphology were observed on the SOD1 fibrils (27–29). Because the width measurement of the fibril is subjected to variations of the AFM tip, height measurement was used as an indication of fibril diameter. The typical heights of the peaks of the fibrils were about 5–7 nm, and the heights of the trough were around 3.5–4.0 nm. The lengths of the fibrils vary from 200 nm to 3 µm. These measurements are within the range reported for other amyloid fibrils.
We were able to measure the pitch distances on the SOD1 fibrils to nanometer resolution (Fig. 3). By sectioning along the fibril using a scanning probe image processor, more than 200 helical twists on at least 30 fibrils for each WT and mutant were counted. Fibrils that were thinner than 4 nm or shorter than 500 nm were excluded from measurement because they might be protofibrils that had yet to adopt the final conformation. Histograms were constructed based on the measurement of each individual peak distance, and Gaussian curve fits on the histograms were constructed using OriginPro 8.1 (Fig. 4). The helical pitch distances along the WT fibrils are mostly consistent despite a small degree of variation. Nevertheless, general twist patterns are observed. The average twist distance for WT is ∼62.14 nm with an SD of ± 15.10 nm (Fig. S5). The average helical pitch distances for L38V, G93A, and G93S are not significantly different from WT. In contrast, G37R and G41D showed a substantial decrease in average helical pitch distance, to 35.80 nm (G37R) and 35.07 nm (G41D) compared with 62.14 nm (WT). The distribution of helical twists for D101N was more irregular, as is evident in the wider distribution in the histogram and the larger SD.
Fig. 3.
AFM of WT and mutant SOD1 fibrils. Insets show the magnified helical twist of the fibrils. WT, L38V, G93A, and G93S have and average pitch length around 55–65 nm. (Scale bar: 100 nm.) Periodicity profile along the fibril axis is shown on the right of each AFM image. (Vertical bar: 0.4 nm.) Distance between two peaks (pitch distance) is marked by the arrows.
Fig. 4.
Helical pitch distances for WT and mutant SOD1 fibrils. Histogram (bin size = 10 nm) of helical pitch distances for WT and mutants with Gaussian fit peak curve (black line). n > 200 counts. WT, L38V, G93A, and G93S have peak centers at 60.9 nm, 50.5 nm, 54.5 nm, and 49.3 nm, respectively. Peak centers for G37R and G41D are left shifted to shorter lengths, 33.9 nm and 33.6 nm. The distribution of helical pitch distances for D101N is more diverse, and the peak centers at 63.3 nm.
Discussion
The studies presented here provide two major observations. First, in our partial proteolysis experiments, we show that the most protease-resistant region of the WT and of each of the SOD1 mutant fibrils tested here are remarkably similar, being composed largely of the acetylated N terminus of the SOD1 polypeptide. For WT and across all of the mutants we tested, five trypsin cleavage sites within residues 1–69 consistently became inaccessible when the proteins were in the fibrillar form. When the fibrils were digested with Pronase, the minimal core region shortened further to residues 1–63. Because a flexible region of about 10 residues is required on the substrate for proteolysis to occur (30), the actual SOD1 sequence participated in the core might be shorter than 63 residues even though position 63–64 is the last peptide bond susceptible to digestion. Our results thus are consistent with the findings reported by Lang et al. (31) that loops IV and VII are not required for fibril formation.
The N-terminal 1–63 residues form β-strands 1–4 in the native SOD1 structure (Fig. 5), with β-strands 1–3 and 6 forming one β-sheet and β-strand 4 crossing over to the other side to form a second β-sheet with strands 5, 7, and 8, together forming the Greek-key β-barrel fold. Because strand 4, which is natively present in the second sheet, associates with strands 1–3 in the fibrillar state, our data demonstrate that the β-strand connectivity must undergo a major change in the process of fibril formation. In this respect, it is interesting to note that apo WT and ALS-mutant proteins (A4V, G93R, H48Q) are known to exist at physiological temperature as partially unfolded β-barrels, in which residues 21–53, which include strands 3 and 4, undergo rapid exchange of the backbone protons with deuterons (H/D exchange) (32). Increased H/D exchange in strands 3 and 4 implies a loosening of the hydrogen-bond networks with strands 2 and 6 and strands 5 and 7, respectively (Fig. 5). This increased flexibility might render hydrogen-bond donors and acceptors on strands 3 and 4 more available, possibly creating unprotected β-sheet edges that might drive the nonnative intermolecular interaction of the N terminus with other SOD1 subunits to form amyloid fibrils. (Residues 53–104 were not recovered from the reported H/D exchange experiment, and thus it is not known whether disruption of the hydrogen-bond network extends to this region.) It also should be noted that strands 3 and 4 contain the segment 33–38, which has been predicted to be amyloidogenic based on the steric zipper model (33). In addition, aromatic residues frequently are found within amyloid-forming proteins, and it is postulated that they play a role in fibril stability (34). Interestingly, one tryptophan and three phenylalanines are present in the N terminus, but there are no aromatic residues in the C terminus.
Fig. 5.
Structure of apo WT SOD1 (Protein Data Bank accession no. 1HL4) (65) and the hypothetical structural change leading to fibril formation. Residues that are protected from protease digestion in the fibrils are colored in magenta (strands 1–4). Significant rearrangement of the structure must accompany fibril formation so that strands 1–4 become tightly packed in the protease-resistant core.
The protected N-terminal core region we observe in our experiments is remarkably consistent in WT SOD1 and each of the six mutant SOD1 proteins we examined, in contrast to the wide assortment of protected regions reported by Furukawa et al. (35) for fibrils prepared by their method. This discrepancy likely is explained by their use of a nonnative N-terminal sequence and/or the different fibrillation conditions. The recombinant SOD1 used in the Furukawa study contained a four-amino acid residue extension at the N terminus and lacked the native N-terminal acetyl group. (The peptide Gly-Ser-His-Met was the remnant of a cleaved His6 tag.) Posttranslational modifications, such as N-terminal acetylation (36, 37) and C-terminal amidation (38, 39), have been reported in some instances to have profound effects on the structure and amyloidogenicity of peptides. In addition, because it is known that A4V is an ALS-causing mutation in SOD1, we thought it wise to ensure the complete fidelity of our SOD1 preparations by retaining the native N-acetyl Ala SOD1 N terminus. Our fibrillation conditions also were different from those reported by Furukawa et al. (35), potentially contributing to the differences in degrees of structural homogeneity of the fibril structures. Fibril structure can be strongly influenced by multiple factors such as buffer, pH, temperature, and mode and intensity of agitation (40–42). Our fibrils have consistent gross fibrillar morphology as seen in both AFM (Fig. 3) and EM (Fig. S6). In contrast, the EM images of the fibrils reported by Furakawa et al. show a wide assortment of widths and shapes, suggesting to us that these fibrils have diverse morphologies.
Although we found the C-terminal peptide 143–153 to be relatively abundant in the S2 fraction, we also occasionally found a small amount of this peptide in the P2 residual fibril. Its presence in substoichiometric amounts in P2 suggests that this region is not part of the amyloid core of the major fibrillar species but instead arises from a small fraction of the total fibrils, because a small degree of fibril polymorphism cannot be ruled out from our data. Another possibility is an attachment of this peptide to the N-terminal peptide present in the pellet through the native disulfide bond (C57—C146). As previously reported, disulfide-intact apo SOD1 can be recruited to the fibril by disulfide-reduced apo SOD1 (15). However, because this peptide (containing C146) is found mostly in S2, it suggests that the native disulfide bond in most proteins in the fibrils is not intact.
Our second set of findings concerns the morphology of the fibrils as visualized by our AFM experiments. Both mutants and WT protein form amyloid fibrils with a twisted morphology. For three of the mutant protein fibrils, L38V, G93A, and G93S, the morphology was strikingly similar to that of WT, but the other three showed significant differences in pitch length. Intriguingly, these latter mutations, G37R, G41D, and D101N, cause a net change in charge. Although the extended β-sheet of fibrils is largely stabilized by the hydrogen-bonding network of the polypeptide backbone, side-chain residues also can form hydrogen bonds and contribute to the fibril stability (29, 43). Additionally, the packing of the β-sheet is sequence dependent (44). In the steric zipper model developed from the crystal structures of microcrystals of amyloidogenic short polypeptides, the side-chains of β-sheets interdigitate to create a “dry zipper” in the core (44, 45). Inside the fibrils, β-sheets can be zipped together and stabilized by the noncovalent interactions (hydrophobic, π–π stacking, or salt-bridge) of the side chains. Therefore, mutations within the core β-sheets have a higher potential of altering the alignment and arrangement of β-sheet packing and the extent of the hydrogen-bond network, which dictate the twist and rigidity of a single strand of protofilament. Because the packing of protofilaments within the fibril is stabilized by many weak long-range electrostatic (or hydrophobic) interactions along the protofilaments, all of which must accommodate the twist and rigidity of the protofilaments, changes in the β-sheet packing pattern and the stability of the β-sheet induced by a single mutation are likely to propagate to the supermolecular packing and the final twist of the fibrils (46–48). This result is demonstrated for mutations such as G37R and G41D, both of which occur within the N-terminal region that forms the fibril core and constitute significant changes in size as well as in charge. Interestingly, D101N, a substitution with a change in charge that is outside the core region also changes the amyloid helical pitch. This result may be explained by the changes in electrostatic interactions on the surface of the protofilaments. On the other hand, L38V, another substitution within the core region that does not change the overall charge, does not cause a change in helical twist pattern, indicating that mutations within the core do not necessarily perturb the fibril superstructure.
Morphological differences such as those we have observed might be related to differences in the toxic properties of the fibrils. The origin of amyloid-associated toxicity is a highly debated topic, and multiple mechanisms, such as impaired mitochondrial function (49), proteasome inhibition (50), and permeation of the lipid bilayer (51, 52), have been proposed to explain how amyloid fibrils or associated structures can be toxic to a cell. These effects may be dependent on the structure of the fibrils or their fibrillar precursors. The toxicity of amyloid fibrils from huntingtin exon1 toward cultured cells was different for fibrils generated under various growth conditions that led to structural differences (53). Furthermore, seeding specificity in prions has been shown to be dependent on fibril structure (54). Although formation of in vivo aggregates seeded by in vitro SOD1 fibrils has not been reported, SOD1 fibrils or associated species might act as a template for the formation of the toxic species. It has been noted that SOD1 mutations resulting in a change of net charge tend to correlate with longer survival times (>5 y) for patients with SOD1-associated fALS (55). It is possible that these changes in survival times are related to the significantly changed fibril structures in mutants with a change in net charge.
The relevance of the N terminus of SOD1 in aggregation and toxicity in vivo also has been highlighted in several papers. A recent paper from Prudencio et al. (56) showed that an antibody raised against residues 24–36 could not recognize aggregated mutant SOD1 formed in human cell culture model, whereas antibody targeting the C-terminal residues 143–151 recognized both soluble and aggregated SOD1. Second, Cashman and colleagues reported that G127X and G85R induced misfolding of WT SOD1 in a Trp32-dependent manner (57); when Trp32 was altered to Ser, this ability was demolished. In addition, a Trp-to-Phe substitution (W32F) in G93A decreased motor neuron death caused by the G93A alteration (58). On the other hand, the C terminus appears to be nonessential in aggregation and toxicity related to ALS (59–61).
In summary, we provide evidence that WT and ALS-mutant SOD1 proteins, despite their different structural properties, have a strong tendency to form amyloid fibrils with the amino terminus as the core. Fibrils formed from ALS-mutant SOD1 proteins with an overall net charge different from that of WT appear to deviate more in overall fibril morphology than mutant proteins with the same overall net charge. It also is important to note here that, although the C terminus is not a major part of the core of the amyloid fibril structure, it potentially could play a secondary role in the amyloid-packing pattern and in the mechanism of amyloid propagation. Our work here also provides information that could be used for structure-based drug design, either to destabilize the fibril conformations or to stabilize them, depending on the relative toxicities of the SOD1 fibrils and their associated precursors. In any case, preventing the amino terminus from self-associating may be a goal for drug design, to prevent the formation of toxic fibrils or soluble intermediates formed during the amyloid formation cascade. We also show that substitutions at position 37 or 41 with charged residues significantly lowers the twist distances within the fibrils. Because different fibril morphologies might have different interacting partners and seeding ability in vivo, experiments designed to test the toxicity of different conformations of fibrils may be important in understanding of the role of SOD1 fibrils in ALS.
Materials and Methods
SOD1 Expression and Purification.
SOD1 were purified from Saccharomyces cerevisiae following the procedures from refs. 62–64. To convert to apo, purified SOD1 was dialyzed against EDTA as in ref. 15. Apo SOD1 then was filter-sterilized, flash-frozen in liquid nitrogen, and kept at −20 °C until use. Inductively coupled plasma (ICP)-MS was used to quantify metal status (14). All apo-proteins contain less than 0.10 equivalents of copper and zinc per dimer.
In Vitro Fibril Formation.
To make fibrils, 50 µM of apo SOD1 was prepared in 10 mM potassium phosphate (KPi), pH 7.0, with 5 mM DTT and 40 µM of thioflavin-T as described (15). Fibril assembly was monitored by fluorescence measurement at λem = 485 nm (λex = 444 nm) using a Fluoroskan plate-reader (Thermo Fisher). All solutions were made with chelexed metal-free distilled deionized water (ddH2O) and were filtered through a 0.22-µm filter.
Proteolysis of Fibrillar and Soluble SOD1.
Trypsin (porcine; Promega) proteolysis was in 50 mM NH4HCO3 at 37 °C for 30 min at 1:30 (wt:wt) enzyme:substrate. SOD1 fibrils were separated from soluble contaminants by ultracentrifugation in an Airfuge (Beckman Coulter) at 125,000 × g for 30 min; then the pellet was washed with 50 mM NH4HCO3. Soluble apo SOD1 was diluted to the same concentration as the fibril with 50 mM NH4HCO3 and was digested similarly. After proteolysis, the reaction was quenched by 1 mM PMSF, and the fibrils were spun down at 125,000 × g for 30 min to collect the S2 and P2. P2 was dissolved further in 7.2 M GdnHCl and 0.5 M DTT at 37 °C overnight. Supernatant was subjected to reduction by 250 mM DTT at 37 °C for 1 h. Proteolysis with chymotrypsin (bovine; Promega) was performed as above except in 100 mM Tris⋅HCl, 10 mM CaCl2, pH 8.0. Digestion with Pronase (Calbiochem) was at an 2:9 (wt/wt) E:S, 30 min at 37 °C in 50 mM Tris, 100 mM NaCl, 5 mM CaCl2, pH 8.0.
HPLC-Electrospray Ionization-MS and MS/MS.
Solubilized P2 was desalted using a C18 Zip-Tip (Millipore). After elution, samples were diluted to 7% acetonitrile (ACN) (vol/vol) with ddH2O, 0.1% formic acid (FA) before reversed-phase chromatography (Polymeric Reversed Phase S (PLRPS) column, 2.1 × 150 mm, 300 Å, 5 µm) (Agilent). A linear gradient was used to elute the column [5% B (ACN, 0.1% FA), 0–10 min; 21% B at 14 min; 46% B at 39 min; 95% B from 39.1–49 min]. Column eluent was directed to the electrospray ionization source (Ionmax; Thermo Scientific) of a linear ion trap mass spectrometer (LTQ; Thermo Scientific) operated in positive-ion mode. Peptide mass deconvolution used Promass (Novatia). Only peptides with intensity at least 10% of the most dominant peptide are reported. High-resolution MS/MS was performed on a static nanospray 7 T LTQ-FT system (Thermo Scientific) (15). MS/MS data analysis was done both manually and using Prosight PC (Thermo Scientific).
AFM.
SOD1 fibrils were diluted to 25 µM with 10 mM KPi, pH 7.0. Then 15 µL of fibril was added to the cleaved mica surface and allowed to adhere. After incubation at room temperature for 1 min, the mica was washed three times with 50 µL ddH2O and was allowed to air dry overnight. A scanning probe microscope (Dimension 5000; Bruker) was used to image SOD1 fibrils using an AppNano Aspire Conical Tapping (ACT) tip (Nanoscience Instruments) under tapping mode. Height images were collected at 1,024 × 1,024 pixels at a scan rate of 1.5 Hz. Image processing was performed using a scanning probe image processor.
Supplementary Material
Acknowledgments
We thank Dr. Herman Lelie for help with ICP-MS and Professor David Eisenberg and Dr. Magdalena Ivanova for helpful discussions. We acknowledge the use of the Scanning Probe Microscope facility at the Nano and Pico Characterization Laboratory at the California NanoSystems Institute at the University of California, Los Angeles. This work was supported by Korea Science and Engineering Foundation/Ministry of Education Science and Technology (KOSEF/MEST) through World Class University (WCU) project R31-2008- 000-10010-0 (to J.S.V.), National Institutes of Health Chemistry Biology Interface Training Grant 2 T32 GM008496 (to P.K.C.), and Program Project Grant P01 NS049134 (to J.S.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309613110/-/DCSupplemental.
References
- 1.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 2.Valentine JS, Hart PJ. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2003;100(7):3617–3622. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng Y, Chattopadhyay M, Whitelegge J, Valentine JS. SOD1 aggregation and ALS: Role of metallation states and disulfide status. Curr Top Med Chem. 2012;12(22):2560–2572. doi: 10.2174/1568026611212220010. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am J Pathol. 1997;151(2):611–620. [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata N, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994;179(1-2):149–152. doi: 10.1016/0304-3940(94)90956-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, et al. Copper-binding-site-null SOD1 causes ALS in transgenic mice: Aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12(21):2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 7.Basso M, et al. Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J Biol Chem. 2006;281(44):33325–33335. doi: 10.1074/jbc.M603489200. [DOI] [PubMed] [Google Scholar]
- 8.Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28(9):2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AS, Calkins E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature. 1959;183(4669):1202–1203. doi: 10.1038/1831202a0. [DOI] [PubMed] [Google Scholar]
- 10.Geddes AJ, Parker KD, Atkins ED, Beighton E. “Cross-beta” conformation in proteins. J Mol Biol. 1968;32(2):343–358. doi: 10.1016/0022-2836(68)90014-4. [DOI] [PubMed] [Google Scholar]
- 11.Sunde M, Blake C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem. 1997;50:123–159. doi: 10.1016/s0065-3233(08)60320-4. [DOI] [PubMed] [Google Scholar]
- 12.Sunde M, et al. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273(3):729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- 13.Eanes ED, Glenner GG. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- 14.Shaw BF, et al. Detergent-insoluble aggregates associated with amyotrophic lateral sclerosis in transgenic mice contain primarily full-length, unmodified superoxide dismutase-1. J Biol Chem. 2008;283(13):8340–8350. doi: 10.1074/jbc.M707751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattopadhyay M, et al. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci USA. 2008;105(48):18663–18668. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiala M, et al. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J Neuroinflammation. 2010;7:76. doi: 10.1186/1742-2094-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts K, et al. Extracellular aggregated Cu/Zn superoxide dismutase activates microglia to give a cytotoxic phenotype. Glia. 2013;61(3):409–419. doi: 10.1002/glia.22444. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay M, Valentine JS. Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS. Antioxid Redox Signal. 2009;11(7):1603–1614. doi: 10.1089/ars.2009.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277(21):19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- 20.Kheterpal I, Williams A, Murphy C, Bledsoe B, Wetzel R. Structural features of the Abeta amyloid fibril elucidated by limited proteolysis. Biochemistry. 2001;40(39):11757–11767. doi: 10.1021/bi010805z. [DOI] [PubMed] [Google Scholar]
- 21.Sajnani G, Pastrana MA, Dynin I, Onisko B, Requena JR. Scrapie prion protein structural constraints obtained by limited proteolysis and mass spectrometry. J Mol Biol. 2008;382(1):88–98. doi: 10.1016/j.jmb.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 22.Frare E, et al. Identification of the core structure of lysozyme amyloid fibrils by proteolysis. J Mol Biol. 2006;361(3):551–561. doi: 10.1016/j.jmb.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 23.Baxa U, et al. Architecture of Ure2p prion filaments: The N-terminal domains form a central core fiber. J Biol Chem. 2003;278(44):43717–43727. doi: 10.1074/jbc.M306004200. [DOI] [PubMed] [Google Scholar]
- 24.Polverino de Laureto P, et al. Protein aggregation and amyloid fibril formation by an SH3 domain probed by limited proteolysis. J Mol Biol. 2003;334(1):129–141. doi: 10.1016/j.jmb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Carulla N, et al. Molecular recycling within amyloid fibrils. Nature. 2005;436(7050):554–558. doi: 10.1038/nature03986. [DOI] [PubMed] [Google Scholar]
- 26.Graffmo KS, et al. Expression of wild-type human superoxide dismutase-1 in mice causes amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22(1):51–60. doi: 10.1093/hmg/dds399. [DOI] [PubMed] [Google Scholar]
- 27.Chiti F, et al. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci USA. 1999;96(7):3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tycko R. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem. 2011;62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles TP, et al. Role of intermolecular forces in defining material properties of protein nanofibrils. Science. 2007;318(5858):1900–1903. doi: 10.1126/science.1150057. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard SJ, Campbell SF, Thornton JM. Molecular recognition. Conformational analysis of limited proteolytic sites and serine proteinase protein inhibitors. J Mol Biol. 1991;220(2):507–530. doi: 10.1016/0022-2836(91)90027-4. [DOI] [PubMed] [Google Scholar]
- 31.Lang L, Kurnik M, Danielsson J, Oliveberg M. Fibrillation precursor of superoxide dismutase 1 revealed by gradual tuning of the protein-folding equilibrium. Proc Natl Acad Sci USA. 2012;109(44):17868–17873. doi: 10.1073/pnas.1201795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durazo A, et al. Metal-free superoxide dismutase-1 and three different amyotrophic lateral sclerosis variants share a similar partially unfolded beta-barrel at physiological temperature. J Biol Chem. 2009;284(49):34382–34389. doi: 10.1074/jbc.M109.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc Natl Acad Sci USA. 2010;107(8):3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16(1):77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa Y, Kaneko K, Yamanaka K, Nukina N. Mutation-dependent polymorphism of Cu,Zn-superoxide dismutase aggregates in the familial form of amyotrophic lateral sclerosis. J Biol Chem. 2010;285(29):22221–22231. doi: 10.1074/jbc.M110.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trexler AJ, Rhoades E. N-Terminal acetylation is critical for forming α-helical oligomer of α-synuclein. Protein Sci. 2012;21(5):601–605. doi: 10.1002/pro.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maltsev AS, Ying J, Bax A. Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties. Biochemistry. 2012;51(25):5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonemoto IT, Kroon GJ, Dyson HJ, Balch WE, Kelly JW. Amylin proprotein processing generates progressively more amyloidogenic peptides that initially sample the helical state. Biochemistry. 2008;47(37):9900–9910. doi: 10.1021/bi800828u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padrick SB, Miranker AD. Islet amyloid polypeptide: Identification of long-range contacts and local order on the fibrillogenesis pathway. J Mol Biol. 2001;308(4):783–794. doi: 10.1006/jmbi.2001.4608. [DOI] [PubMed] [Google Scholar]
- 40.Petkova AT, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307(5707):262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 41.Dzwolak W, Smirnovas V, Jansen R, Winter R. Insulin forms amyloid in a strain-dependent manner: An FT-IR spectroscopic study. Protein Sci. 2004;13(7):1927–1932. doi: 10.1110/ps.03607204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Relini A, et al. Detection of populations of amyloid-like protofibrils with different physical properties. Biophys J. 2010;98(7):1277–1284. doi: 10.1016/j.bpj.2009.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiltzius JJ, et al. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17(9):1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makin OS, Atkins E, Sikorski P, Johansson J, Serpell LC. Molecular basis for amyloid fibril formation and stability. Proc Natl Acad Sci USA. 2005;102(2):315–320. doi: 10.1073/pnas.0406847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson MJ, et al. The 3D profile method for identifying fibril-forming segments of proteins. Proc Natl Acad Sci USA. 2006;103(11):4074–4078. doi: 10.1073/pnas.0511295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiménez JL, et al. The protofilament structure of insulin amyloid fibrils. Proc Natl Acad Sci USA. 2002;99(14):9196–9201. doi: 10.1073/pnas.142459399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shammas SL, et al. Perturbation of the stability of amyloid fibrils through alteration of electrostatic interactions. Biophys J. 2011 doi: 10.1016/j.bpj.2011.04.039. 10.1039/C1SM05382E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamcik J, Mezzenga R. Adjustable twisting periodic pitch of amyloid fibrils. Soft Matter. 2011;7:5437–5443. [Google Scholar]
- 49.Eckert A, et al. Oligomeric and fibrillar species of beta-amyloid (A beta 42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med (Berl) 2008;86(11):1255–1267. doi: 10.1007/s00109-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 50.Urushitani M, Kurisu J, Tsukita K, Takahashi R. Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J Neurochem. 2002;83(5):1030–1042. doi: 10.1046/j.1471-4159.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 51.Williams TL, Day IJ, Serpell LC. The effect of Alzheimer’s Aβ aggregation state on the permeation of biomimetic lipid vesicles. Langmuir. 2010;26(22):17260–17268. doi: 10.1021/la101581g. [DOI] [PubMed] [Google Scholar]
- 52.Shaw BF, Valentine JS. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends Biochem Sci. 2007;32(2):78–85. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Nekooki-Machida Y, et al. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci USA. 2009;106(24):9679–9684. doi: 10.1073/pnas.0812083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121(1):63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Byström R, Andersen PM, Gröbner G, Oliveberg M. SOD1 mutations targeting surface hydrogen bonds promote amyotrophic lateral sclerosis without reducing apo-state stability. J Biol Chem. 2010;285(25):19544–19552. doi: 10.1074/jbc.M109.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prudencio M, Borchelt DR. Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states. Mol Neurodegener. 2011;6:77. doi: 10.1186/1750-1326-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grad LI, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci USA. 2011;108(39):16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor DM, et al. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2007;282(22):16329–16335. doi: 10.1074/jbc.M610119200. [DOI] [PubMed] [Google Scholar]
- 59.Ghadge GD, et al. Truncated wild-type SOD1 and FALS-linked mutant SOD1 cause neural cell death in the chick embryo spinal cord. Neurobiol Dis. 2006;21(1):194–205. doi: 10.1016/j.nbd.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, et al. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation. Hum Mol Genet. 2005;14(16):2335–2347. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- 61.Zu JS, et al. Exon 5 encoded domain is not required for the toxic function of mutant SOD1 but essential for the dismutase activity: Identification and characterization of two new SOD1 mutations associated with familial amyotrophic lateral sclerosis. Neurogenetics. 1997;1(1):65–71. doi: 10.1007/s100480050010. [DOI] [PubMed] [Google Scholar]
- 62.Hough MA, et al. Dimer destabilization in superoxide dismutase may result in disease-causing properties: Structures of motor neuron disease mutants. Proc Natl Acad Sci USA. 2004;101(16):5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiedau-Pazos M, et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996;271(5248):515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 64.Doucette PA, et al. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279(52):54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 65.Strange RW, et al. The structure of holo and metal-deficient wild-type human Cu, Zn superoxide dismutase and its relevance to familial amyotrophic lateral sclerosis. J Mol Biol. 2003;328(4):877–891. doi: 10.1016/s0022-2836(03)00355-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.