In PNAS, an article by Tseng et al. continues to examine an important and unique therapeutic manipulation that controls the growth of tumor cells in mice (1). This manipulation consists of using a monoclonal antibody specific to the membrane protein CD47 to block its biological activity: blocking CD47 reduces tumor growth by enabling macrophages to phagocytose the cancer cells (2–4). In this study, a previously undescribed effect of the anti-CD47 antibody treatment is reported, which is that it results in the activation of the CD8 set of T lymphocytes, a set important for the killing of tumor cells.
What is CD47? It is a protein of about 50 kDa consisting of an extracellular Ig domain and five membrane-spanning segments with a small cytoplasmic tail (5). CD47 is expressed on the surface of all cells, but is particularly prominent in a variety of cancer cells (2, 5). Indeed, one of the first biochemical characterizations was made on ovarian carcinomas that express CD47 at high levels (6). Early studies called attention to CD47 as a protein promoting the interaction among integrins, adhesion molecules involved in cellular communications (7). Subsequent studies confirmed a number of interactions of CD47 with various adhesion molecules among leukocytes in diverse biological reactions (reviewed in ref. 5). An important breakthrough in identifying a key role of this molecule was a report by Oldenberg et al. when they examined cells from mutant mice that lacked the CD47 protein (8). Red blood cells lacking the CD47 proteins injected into normal mice were rapidly engulfed by the macrophages of the spleen, but those red cells expressing CD47 were not taken up. In other words, the presence of CD47 in a cell was a deterrent to phagocytosis.
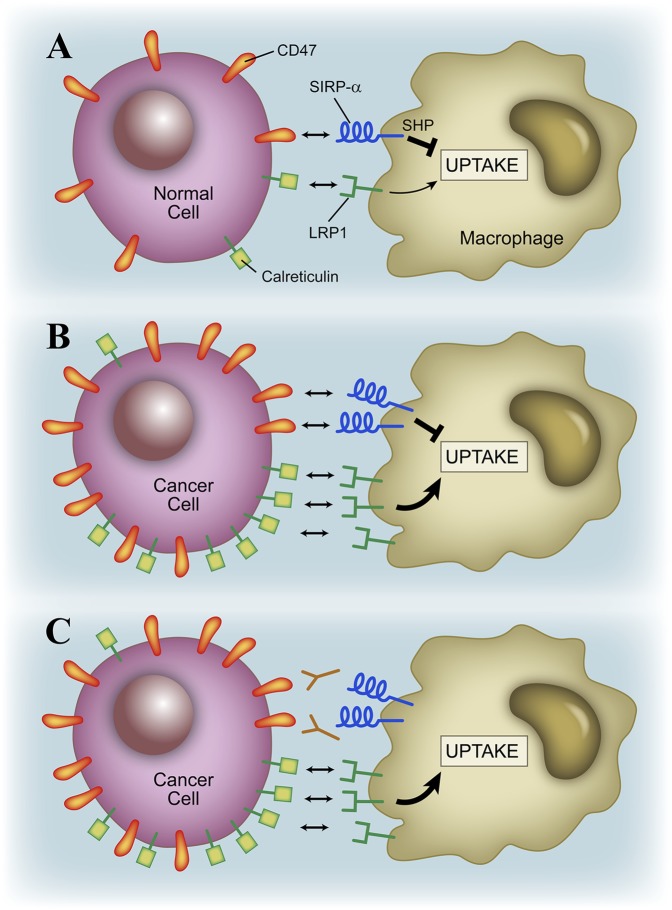
The study of Oldenberg et al. (8) was a breakthrough finding because it indicated that CD47 was acting as a molecule that regulated the recognition of “self.” Simply stated, the study indicates that expression of a level of CD47 by a cell will not favor its uptake by macrophages. Macrophages do not phagocytose their own cells: self-proteins avoid phagocytosis because they have CD47 as a “don’t eat me” signal, aside from lacking surface proteins that have complementary receptors on macrophages required for the uptake. CD47 is known to engage a receptor protein of the macrophage called signal regulatory protein-α (SIRP-α) (reviewed in ref. 5). Engagement of SIRP-α leads to inhibition of phagocytosis through an activation of a cellular phosphatase that inhibits cytoskeletal proteins. Phagocytosis of particle cells or pathogens is a complex process that involves membrane interactions and the rearrangement of intracellular organelles and cytoskeletal proteins to engulf the material (Fig. 1).
Fig. 1.
(A) Macrophages ignore normal cells as a result of negative interactions in which the CD47–SIRP-α pair promote the “don’t eat me” signal. Cancer cells show high levels of CD47 and avoid phagocytosis despite having a higher level of calreticulin (12) (B), but blocking CD47 with antibody favors their uptake (C).
CD47 is one of two molecular systems in which cells distinguish self from altered-self by the absence of a normally expressed protein. This capacity to distinguish self by the absence of a surface protein is the “missing self hypothesis” (9), first shown for natural killer cells, a cell lineage that patrols tissues and is important in early antiviral and antitumor resistance. In the case of natural killer cells, the cell-surface molecules are class I histocompatibility molecules interacting with receptors in the natural killer cells (10).
Studies in the Weissman laboratory recognized the features of many cancer cells in displaying large amounts of CD47, and by doing this evading recognition by the macrophages (2). Indeed, in experimental systems in which tumor cells were transplanted into mice, the growth of the tumor was controlled by injection of antibodies that neutralized CD47 (2–4, 11). These experiments only examined the interactions of the innate cellular system with the cancer cells: the cancer cells had been injected into immunodeficient mice. Thus, blocking the antiphagocytic properties of CD47, the molecules that confer the “don’t eat me” signal enabled the innate cell system represented mainly by macrophages to attack the tumor. (Macrophages, natural killer cells together with dendritic cells, granulocytes, and mast cells constitute the innate system, which participates in diverse reactions early in the immune response to pathogens as well as during the inflammatory reactions that follow. This innate system interacts in a truly symbiotic fashion with the adaptive cellular system of T and B cells.)
There is a second component of cancer cells that makes it possible for blockade of CD47 to be effective (12) (Fig. 1). As a result of stress or their intrinsic reactivity, many tumors abnormally express proteins that have complementary receptors on phagocytes, which favor their phagocytosis. One of these proteins is calreticulin, a protein normally expressed in the endoplasmic reticulum. Calreticulin acts as a chaperone of unfolded proteins in the endoplasmic reticulum, but it can be translocated to plasma membrane, where it is found in high levels in conditions of endoplasmic reticulum stress, cell death, and in cancer cells (13). Calreticulin interacts with the receptor protein LDL receptor-like protein on the macrophage: this is the “eat me” molecular pair (14). The combination of calreticulin together with the inhibition of the CD47-Sirp-α, the “don’t eat me” pair, promotes the effective phagocytosis of cells (14), and most prominently cancer cells (12) (Fig. 1).
The potential use of anti-CD47 antibody treatment as a therapeutic modality for human cancer would be greatly increased if lymphocytes reactive to the tumor would be recruited into the tumor-rejection reaction. This is the case for the anti-CD47 therapy, as the Tseng et al. (1) article now shows. Specifically, the new experiments indicated that in the presence of antibodies to CD47, macrophages engulfed tumor cells that expressed the protein ovalbumin (i.e., tumor cells were made to express this protein), which then served as a surrogate tumor antigen. CD4 and CD8 T cells were available that reacted with specific peptide segments in the ovalbumin protein so they could be tested and used as probes of a specific antitumor reaction. The macrophage phagocytosed the tumor cells and degraded the protein-selecting peptides, which were bound to histocompatibility molecules. Macrophages that phagocytosed the cancer cells then were able to stimulate the specific antiovalbumin CD8 T cells. This finding indicated that the macrophage had presented to the T-cell peptides from the phagocytosed cancer cells, in this case peptides bound to class I histocompatibility molecules. Such stimulation took place both in culture experiments but also in vivo. Indeed, the CD8 T cells stimulated by the macrophages that engulfed the tumors after the antibody treatment controlled the growth of the tumor by killing the cancer cell, reducing the tumor load. Interestingly, in the study (1) there was no stimulation of CD4 T cells, indicating that there was differential processing of the epitopes that gave rise to peptides, which stimulated one or the other T-cell.
However, the findings of Tseng et al. (1) bring up other important issues. The macrophage handled the protein ovalbumin and presented its immunogenic peptides into the class I MHC system, making the point that the cross presentation, the phenomenon in which engulfed proteins are able to be presented by class I molecules, pertains to more than one set of presenting cells and is not necessarily the exclusive property of one specialized set, such as the CD8 α-dendritic cell subset (15). The findings also make a point recently ignored: that macrophages, the first cells shown to present antigen (16), are in fact excellent presenting cells, depending much on the circumstances of the immunological reaction (17).
In sum, there is one major message that comes from the study by Tseng et al. (1): that the anti-CD47 interaction with cancer cells, together with macrophages, brings the adaptive T cells into the antitumor response, the symbiosis that is required between the innate and the adaptive system for the immune system to operate at its best.
Cancer cells create an environment that permits their growth and curbs the action of the innate and adaptive immune systems. Understanding the basis of these negative effects will permit a more rational attack on cancer. Immunotherapy against cancer is a burgeoning field in oncology involving, among several therapeutic maneuvers, the use of antibodies (18). These antibodies have diverse targets, such as blocking angiogenesis, or growth receptors on the tumors, or inhibitory interactions among lymphocytes. The end result is that the antibodies foster the activation of cancer-specific lymphocytes: cancer cells can display mutant proteins to which the immune system can react (19). The experimental studies establish that anti-CD47 therapy is effective in various experimental systems, setting the rationale for translating these findings into the cancer patient. Anti-CD47 therapy should be part of the immunotherapy armamentarium.
Footnotes
The author declares no conflict of interest.
See companion article on page 11103.
References
- 1.Tseng D, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, et al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26(12):2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 4.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldenborg PA. CD47: A cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. ISRN Hematol. 2013;2013:614619. doi: 10.1155/2013/614619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52(19):5416–5420. [PubMed] [Google Scholar]
- 7.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: A 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111(6 Pt 1):2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 9.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 11.Willingham SB, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao MP, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2(63):63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 14.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185(6):3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unanue ER. The regulatory role of macrophages in antigenic stimulation. Part Two: Symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- 17.Gray EE, Cyster JG. Lymph node macrophages. J Innate Immun. 2012;4(5-6):424–436. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]