Abstract
Polyphenism is the phenomenon in which alternative phenotypes are produced by a single genotype in response to environmental cues. An extreme case is found in social insects, in which reproductive queens and sterile workers that greatly differ in morphology and behavior can arise from a single genotype. Experimental evidence for maternal effects on caste determination, the differential larval development toward the queen or worker caste, was recently documented in Pogonomyrmex seed harvester ants, in which only colonies with a hibernated queen produce new queens. However, the proximate mechanisms behind these intergenerational effects have remained elusive. We used a combination of artificial hibernation, hormonal treatments, gene expression analyses, hormone measurements, and vitellogenin quantification to investigate how the combined effect of environmental cues and hormonal signaling affects the process of caste determination in Pogonomyrmex rugosus. The results show that the interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on the production of alternative phenotypes and set vitellogenin as a likely key player in the intergenerational transmission of information. This study reveals how hibernation triggers the production of new queens in Pogonomyrmex ant colonies. More generally, it provides important information on maternal effects by showing how environmental cues experienced by one generation can translate into phenotypic variation in the next generation.
Many plants and animals can express specific adaptive responses to their environment through phenotypic plasticity, whereby a given genotype can develop into different phenotypes depending on environmental conditions (1, 2). Maternal effects, through which the environmental conditions experienced by the mother are translated into phenotypic variation in the offspring (3, 4), contribute to many phenotypic traits in a wide variety of taxa (5, 6) and have important ecological and evolutionary consequences (7, 8). Investigating the mechanisms of cross-generational transmission of information underlying maternal effects is needed to better understand the optimization of phenotypes in changing environments (6) and, more generally, the evolution of life history strategies (9).
In many insect species, maternal effects are known to affect polyphenism (3, 10), an extreme form of phenotypic plasticity characterized by the production of alternative and discrete phenotypes from a single genotype (1, 11–13). Such maternal effects allow adequate responses to environmental cues such as temperature, photoperiod, nutrition, and population density in many species (10). Examples of maternal effects on insect polyphenism include the production of sexual versus parthenogenetic morphs in aphids (14, 15), winged versus wingless morphs in firebugs (16), and dispersal versus solitary morphs in locusts (17, 18). The endocrine system was found to play a role in the regulation of some maternal effects on insect polyphenisms (19–21), but the nature of the physiological and genetic pathways interacting with the hormonal system to translate environmental cues into offspring polyphenism remains mostly unknown (22).
The most striking example of polyphenism is found in insect societies (23), where a reproductive division of labor leads to the coexistence of fertile queens and sterile workers that greatly differ in morphology and behavior (24, 25). Even though recent studies revealed genetic influences on caste determination in social insects (reviewed in ref. 26), female caste fate is primarily influenced by environmental factors in most species studied (27–39). In ants, several studies suggested that maternal factors such as temperature or queen age may affect caste determination (40–44). However, it is only recently that the first example of maternal effects on female caste polyphenism was documented experimentally (45). Cross-fostering of eggs between hibernated and nonhibernated Pogonomyrmex colonies revealed strong maternal effects on caste production, as only eggs produced by a hibernated queen were able to develop into queens, irrespective of the hibernation status of the rest of the colony (45). Such maternal effects on the caste fate of the female offspring require that the hibernation triggers changes in the queen that affect polyphenism in the offspring. Hormones may be involved in this process in Pogonomyrmex ants, as Pogonomyrmex rugosus queen- and worker-destined eggs differed in their ecdysteroid content (45) and Pogonomyrmex barbatus mature queens treated with juvenile hormone (JH) were recently found to produce larger workers (46).
Studies on the mechanisms regulating insect polyphenisms (reviewed in ref. 10) suggest that the insulin/insulin-like growth factor signaling (IIS), JH, and vitellogenin (Vg) pathways, known to regulate reproduction in adult insects (47–51), play predominant roles in modulating larval development in response to environmental cues. A well-known example illustrating the role of these pathways is the caste fate of the female brood (queen or worker) in the honey bee Apis mellifera (52–58). In this species, worker-triggered differences in larval diet induce changes in IIS that affect JH (57), possibly through the release of neuropeptides (e.g., allatostatin and allatotropin) that influence JH production by the corpus allatum, as found in Drosophila (59). Changes in JH in turn affect the production of Vg (60–62), which may be involved in the process of caste determination (62, 63). Such effects of JH on Vg production, also reported in flies (64), locusts (65), and cockroaches (66), have been proposed to involve the action of ecdysteroids (62, 67–70). IIS, JH, and Vg may also play a role in the regulation of caste differentiation of larvae in ants, as caste-specific expressions of genes involved in the IIS pathway were documented in Solenopsis invicta (71) and Diacamma sp. (72). Interestingly, caste-specific differences in IIS, JH, and Vg were also documented in adult ants and bees (48, 73–78), suggesting further roles of these pathways in the regulation of social life (74, 79).
We propose that the interplay between IIS, JH, and Vg regulates maternal effects on caste polyphenism in ants by translating the environmental conditions experienced by the queen during hibernation into the production of alternative phenotypes in the offspring. Under this hypothesis, IIS would translate environmental cues into changes in JH, which would, in turn, affect the amount of Vg in queens and in eggs, thus possibly affecting the caste fate of the offspring (62, 63). This hypothesis makes four predictions. First, a pharmacological increase of JH in queens should mimic the effect of hibernation and stimulate the production of queens. Second, hibernation should affect IIS and the production of JH in queens. Third, both hibernation and a JH increase should stimulate the production of Vg in queens. Finally, Vg content should differ between queen- and worker-destined eggs. We tested these predictions by performing artificial hibernation, hormonal treatments, gene expression analyses, hormone measurements, and Vg quantification in Pogonomyrmex rugosus, an ant species in which temperature-triggered changes in the queen had previously been shown to affect the relative production of queens and workers. Each of the four predictions was confirmed by our experiments, thus revealing that the interplay between IIS, JH, and Vg regulates maternal effects on caste polyphenism in P. rugosus.
Results
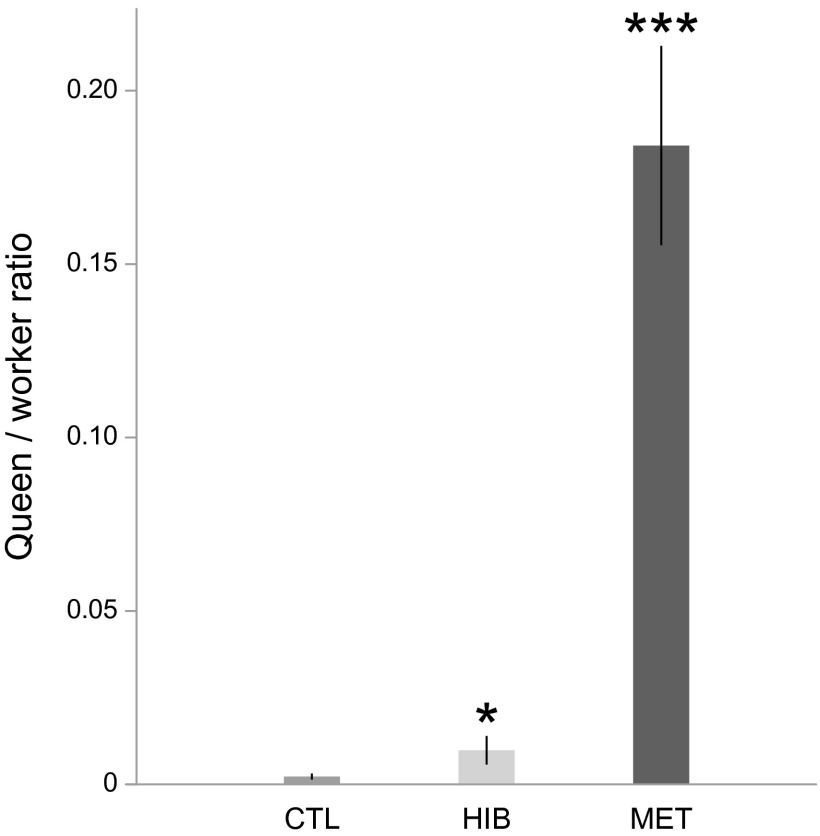
To investigate the mechanisms of caste allocation, we compared the production of queens between control, hibernated, and methoprene-treated P. rugosus colonies. There was a great variation among colonies in the proportion of queens among the offspring produced, ranging from 0 to 0.47 (0.05 ± 0.11, mean ± SD). There was a significant effect of the treatments on the proportion of queens produced [F(2,73) = 40.51, P < 0.001; Fig. 1]. Hibernation significantly increased the proportion of queens among the female offspring (t = 2.06, P = 0.04). The methoprene (JH analog) treatment had a similar—albeit stronger—effect, as the queen/worker ratio among the female offspring was significantly higher in colonies fed methoprene-treated food compared with control colonies (t = 5.39, P < 0.001). Interestingly, when only pupae that did not receive any treatment during larval development but were produced by treated queens (thus, those collected after week 11) were considered, there was also a significant difference between control and methoprene-treated colonies in the proportion of queens produced (t = 5.56, P < 0.001), showing that at least part of the observed effect of methoprene on caste determination was triggered by maternal effects.
Fig. 1.
The proportion of queens among the offspring produced (mean ± SE) was increased in hibernation (HIB) and methoprene (MET) treatments compared with control (CTL). *P < 0.05; ***P < 0.001.
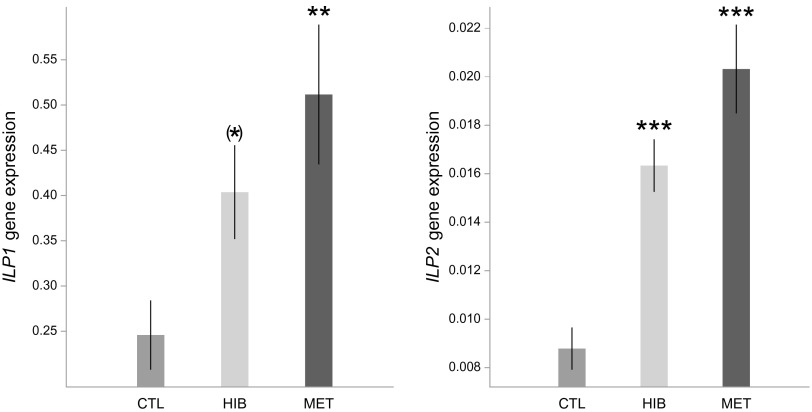
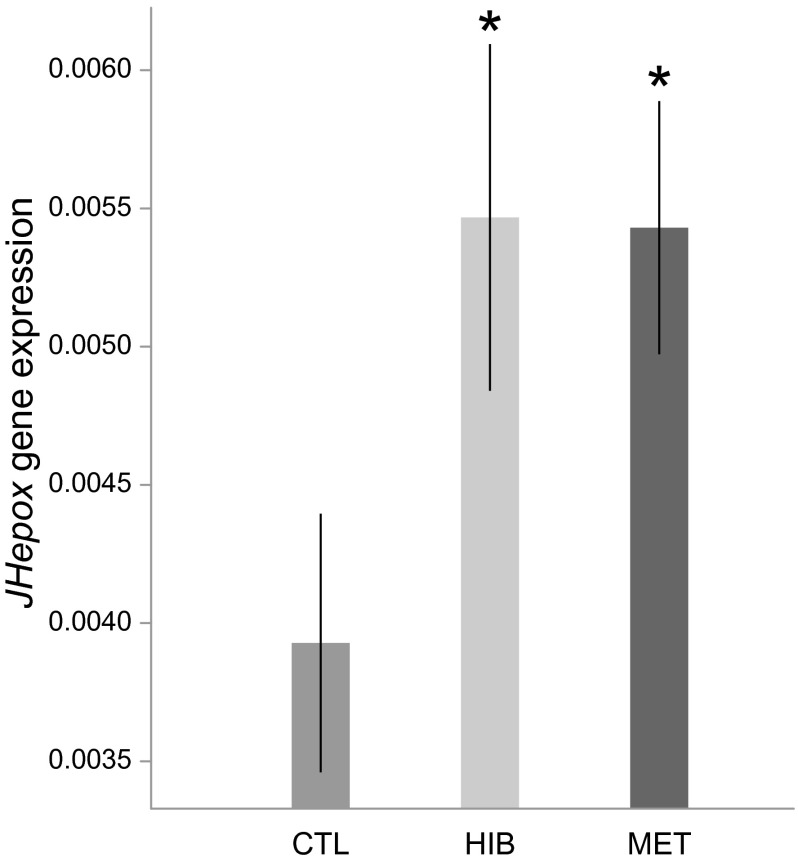
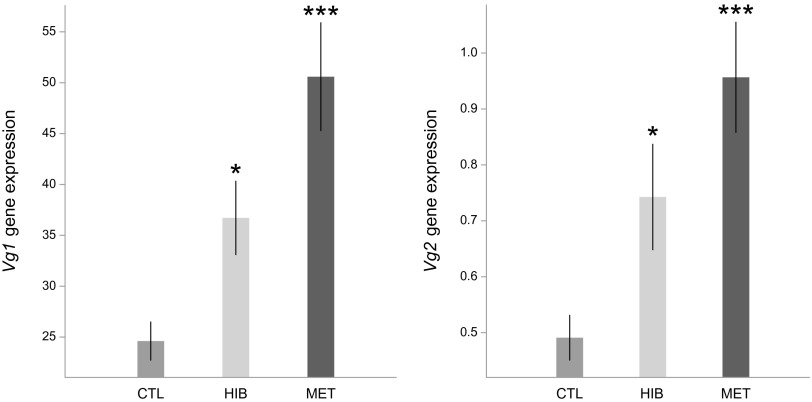
Whole-body queen samples were used to measure the expression of genes involved in the IIS pathway (two insulin-like peptide genes: ILP1 and ILP2), JH production (one gene coding for JH epoxidase: JHepox), and vitellogenesis (two Vg genes: Vg1 and Vg2). The treatments significantly affected the expression of all of the genes tested [ILP1: F(2,36) = 5.30, P = 0.01; ILP2: F(2,36) = 19.47, P < 0.001; JHepox: F(2,36) = 4.12, P = 0.02; Vg1: F(2,36) = 11.15, P < 0.001; Vg2: F(2,36) = 7.93, P = 0.001]. Compared with the control group, both hibernation and methoprene treatments up-regulated the expression of ILP1 (hibernation: t = 1.92, P = 0.06; methoprene: t = 3.24, P = 0.003; Fig. 2), ILP2 (hibernation: t = 4.02, P < 0.001; methoprene: t = 6.14, P < 0.001; Fig. 2), JHepox (hibernation: t = 2.28, P = 0.03; methoprene: t = 2.65, P = 0.01; Fig. 3), Vg1 (hibernation: t = 2.20, P = 0.03; methoprene: t = 4.72, P < 0.001; Fig. 4), and Vg2 (hibernation: t = 2.15, P = 0.04; methoprene: t = 3.98, P < 0.001; Fig. 4).
Fig. 2.
ILP1 and ILP2 were up-regulated in hibernation and methoprene treatments. The y axis indicates the relative gene expression in queens, corresponding to the ILP1 and ILP2 mRNA levels relative to the RP49 (control) mRNA level (mean ± SE). (*)P = 0.06; **P < 0.01; ***P < 0.001.
Fig. 3.
JHepox was up-regulated in hibernation and methoprene treatments. The y axis indicates the relative gene expression in queens, corresponding to the JHepox mRNA level relative to the RP49 (control) mRNA level (mean ± SE). *P < 0.05.
Fig. 4.
Vg1 and Vg2 were up-regulated in hibernation and methoprene treatments. The y axis indicates the relative gene expression in queens, corresponding to the Vg1 and Vg2 mRNA levels relative to the RP49 (control) mRNA level (mean ± SE). *P < 0.05; ***P < 0.001.
To determine whether ecdysteroids mediated the effect of JH on Vg genes expression, we compared the 20-hydroxyecdysone (20E) titer between queens from the control, hibernation, and methoprene groups. Although the 20E titer was lower in the methoprene group (3.38 ± 4.44 pg/mg) compared with the control (8.16 ± 8.47 pg/mg) and hibernation (8.18 ± 9.28 pg/mg) groups, the effect of the treatments was not significant (Kruskal–Wallis χ2 = 2.76, P = 0.25). However, there was a significant negative correlation between the 20E titer in queens and the proportion of queens in their brood (Spearman correlation test, ρ = −0.40, P = 0.01).
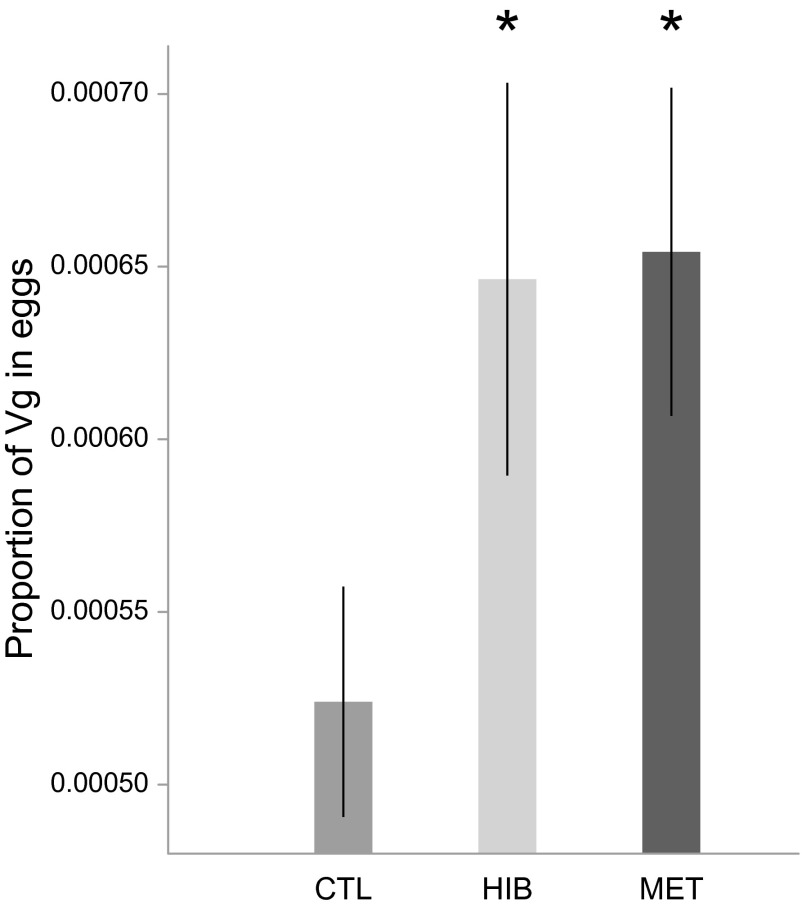
There was no significant difference between treatments in the number [F(2,72) = 1.35, P = 0.27] and weight [F(2,72) = 1.09, P = 0.34] of eggs produced. However, the treatments significantly affected the proportion of Vg among total proteins (Kruskal–Wallis χ2 = 6.63, P = 0.04; Fig. 5). The proportion of Vg in the protein content of eggs produced by both hibernated (U = 42, P = 0.038) and methoprene-treated (U = 53.5, P = 0.026) queens was significantly higher than in eggs produced by control queens. By contrast, this proportion did not differ significantly between eggs produced by hibernated and methoprene-treated queens (U = 79, P = 0.93).
Fig. 5.
The proportion of Vg among total proteins (mean ± SE) was increased in eggs produced in hibernation and methoprene treatments. *P < 0.05.
Discussion
Each of the four predictions developed under the hypothesis that the interplay between IIS, JH, and Vg regulates maternal effects on caste polyphenism in P. rugosus was confirmed by this study. In line with the first prediction that an artificial increase of JH in queens should stimulate the production of queens, the feeding of P. rugosus colonies with a JH analog (methoprene) mimicked the effect of hibernation, with both hibernated and methoprene-treated colonies showing an increased production of queens. These results reveal a role of JH in the regulation of caste polyphenism in P. rugosus. In this species, maternal effects were previously found to stimulate the production of queens in response to hibernation, as only colonies headed by a hibernated queen produced queens, whether or not the workers had been exposed to cold (45). The exposure to cold therefore triggers changes in queens that make them more likely to lay queen-destined eggs. In this study, the methoprene treatment also targeted the queen, as evidenced by an increase in the proportion of queens among the offspring developing in a non–methoprene-treated environment from eggs laid by methoprene-treated queens. Similar results were found in Pheidole pallidula, in which direct topical application of JH on the queen stimulated the production of queens (80), and in P. barbatus, in which it affected the size of the workers produced (46). Overall, the observed effects of hibernation and methoprene treatments show that hibernation-triggered JH changes in queens are involved in the production of queens in P. rugosus.
The second prediction was that hibernation should affect IIS and JH in queens. In line with this prediction, our results revealed that genes involved in IIS (ILP1 and ILP2) were up-regulated in P. rugosus hibernated queens. This suggests that hibernation can translate into changes in the IIS pathway. Low temperature or the associated photoperiod changes could directly affect IIS, as reported in the regulation of insect diapause (81). Alternatively, the effect of exposure to cold could have been mediated by a change in the queen nutritional status due to decreased activity and metabolism (82) or lower food intake during hibernation. Such effects of nutrition on IIS have been reported in Drosophila (83–85). Changes in IIS usually result in the release of neuropeptides (e.g., allatostatin, allatotropin) that influence the production of JH by the corpus allatum (59, 64). Accordingly, the exposure to cold also up-regulated the expression of the JHepox gene, which encodes JH epoxidase, the enzyme that catalyzes the oxidation of methyl farnesoate into JH III (86, 87), the last step in the JH biosynthesis in most insects (88–91).
The finding that the expression of IIS genes was also affected by the methoprene treatment could be explained by JH translating environmental cues into IIS changes rather than the opposite. This is consistent with the report that RNAi-mediated manipulation of JH production affects IIS in Tribolium beetles (49). However, the effect of methoprene on IIS is not incompatible with IIS regulating JH production, as it may have been mediated by the associated changes in Vg (48, 92), of which levels are known to affect IIS through the target-of-rapamycin pathway in bees (55, 57, 78). Furthermore, IIS is known to regulate the production of JH in flies (59, 64). Although our data and the available literature do not provide a definite answer on the directionality of the relationship between IIS and JH in ants, our results clearly show interactions between these pathways in response to environmental changes such as those experienced during hibernation.
The third prediction was that both hibernation and an artificial increase in JH should stimulate the production of Vg. In our experiments, both hibernation and methoprene treatments stimulated the production of queens and up-regulated the expression of Vg genes (Vg1 and Vg2) in queens. The effect of hibernation on vitellogenesis is likely to have been triggered by the increase in JH production. This is supported by the finding that the methoprene treatment also up-regulated Vg expression. These results show that JH-regulated vitellogenesis in adult P. rugosus queens is involved in the regulation of caste polyphenism.
In insects, effects of JH on Vg production have been proposed to be mediated by the ecdysteroid pathway (62, 67–70). Our results do not provide evidence for such a role of ecdysteroids, as the 20E titer in queens did not differ significantly among treatments. Interestingly, the results revealed a trend toward a reduction of 20E titer in methoprene-treated queens and a significant negative relationship between the 20E titer in queens and the proportion of queens in their offspring. This suggests that ecdysteroids may be involved in the process of caste determination (45, 93).
Finally, the fourth prediction was that the Vg content in eggs should correlate positively with their likelihood of developing into queens. This prediction was also supported by our data. Although neither the number nor the weight of eggs produced differed between control, hibernated, and methoprene-treated queens, the proportion of Vg in the protein content was significantly higher in eggs produced by both hibernated and methoprene-treated queens than by control queens. It is likely that the increased production of Vg in hibernated and methoprene-treated queens translated into a higher Vg content in the eggs, increasing their likelihood of developing into queens. How the Vg content in eggs alters the caste fate remains to be investigated, but, as Vg is thought to act as a nutritive source for the embryo (94), more Vg in the egg could result in more energy during early development, facilitating the path toward queen development. The finding of a higher proportion of Vg in eggs produced by queen-producing hibernated and methoprene-treated queens is consistent with our fourth prediction, and shows that the quantity of Vg injected in the eggs is involved in the early regulation of caste allocation and plays a role in the intergenerational transmission of information required for maternal effects on polyphenism to happen.
Overall, this study describes the mechanisms that allow the environmental cues experienced by one generation to be translated into phenotypic variation in the next generation. The interaction between IIS and JH in queens translates environmental cues into changes in Vg production. This affects the quantity of Vg injected into the eggs produced, influencing their development toward the queen or worker caste. In addition to the insights provided on the regulation of caste determination in social insects, this study raises the possibility that the interplay between IIS, JH, and Vg is also involved in the maternal regulation of other insect polyphenisms. More generally, this study provides routes to study the proximate mechanisms regulating maternal effects on any phenotypic traits.
Methods
Pogonomyrmex rugosus founding queens were collected during nuptial flights on July 15, 2008, in Bowie, AZ (N32°18′54″//W109°29′03″). Although it is affected by genetic compatibility effects, caste determination in this species remains mostly environmental with very strong maternal effects (26, 32, 45). After worker eclosion, the colonies were kept in laboratory conditions (30 °C, 60% humidity, and 12-h/12-h light:dark cycle) in plastic boxes containing a nest, a foraging area, and water tubes, and were fed once a week with grass seeds and a mixture of eggs, honey, and crushed mealworms. The experiments were performed on 92 2.5-y-old colonies that had never been exposed to cold and never produced queens. The colonies were divided in three groups: control (n = 26), hibernation (n = 25), and methoprene (n = 26).
The experimental manipulations were divided in two phases (Fig. S1). The first phase was set up to test the effect of an exposure to cold. Colonies from the hibernation group were kept for 2.5 mo in a dark climate chamber at 13 °C ± 1 °C and 60% humidity. The transition to and out of hibernation was done over a period of 2 wk by progressively decreasing or increasing temperature in a 8-h/16-h light:dark cycle. All of the other colonies (control and methoprene groups) were kept in the usual laboratory conditions (30 °C, 60% humidity, and 12-h/12-h light:dark cycle). The first phase terminated at week 0, when the second phase started. The second phase was set up to test the effect of JH treatment. To do so, we used methoprene (Sigma-Aldrich), a synthetic analog of JH. The colonies from the methoprene group were fed four mealworms crushed with 0.1 mg of methoprene in 0.1 mL of acetone each week for 8 wk (from week 0 to week 7), whereas colonies from the hibernation and control groups received four mealworms crushed in 0.1 mL of acetone. There is a drawback to this whole-colony approach because it affects all of the individuals in the colony. To circumvent this problem and determine whether the methoprene treatment acted on the queens to affect caste determination, we performed an additional analysis restricted to the offspring that pupated after week 11. These individuals were produced by methoprene-treated queens, but were not exposed to methoprene during larval development. The experimental design also required to control for a potential effect of acetone: feeding colonies mealworms crushed in acetone had no significant effect on the proportion of queens produced (food with acetone: n = 26; food without acetone: n = 15; Mann–Whitney U test: U = 204, P = 0.15).
Samples were collected in each colony to assess the proportion of queens among the female offspring produced, the number, weight, and Vg content of eggs produced, the expression of candidate genes, and the ecdysteroid titers in queens. All of the pupae produced were collected from week 3 until no brood remained (Fig. S1) and observations of size and morphology allowed the assignation of each pupa to the queen or worker caste. The proportion of queens among the offspring produced was then calculated for each colony (except one that did not produce enough offspring; control: n = 26; hibernation: n = 25; methoprene: n = 25). At week 4, the queen of each colony was isolated for 24 h in a 2-mL plastic tube closed with wire mesh and placed in the colony. Thus, the queen could still communicate with workers, reducing the stress of isolation. This method allowed us to collect and count the number of eggs produced by each queen in 24 h (control: n = 26; hibernation: n = 25; methoprene: n = 25). At week 5, a batch of eggs was collected in each colony (between 5 and 52 eggs per colony; 26.1 ± 8.9, mean ± SD) and weighed using a microbalance (Mettler Toledo MT5) to a precision of 1 μg (control: n = 26; hibernation: n = 25; methoprene: n = 25). The eggs were then stored at −80 °C for further measurement of Vg content, successfully performed on eggs produced by 40 colonies (control: n = 15; hibernation: n = 11; methoprene: n = 14). At week 7, the queen was collected in each colony. One-half of the queens were flash-frozen in liquid nitrogen and stored at −80 °C for later RNA extraction (control: n = 13; hibernation: n = 13; methoprene: n = 13), whereas the other half was used for ecdysteroid measurement (control: n = 12; hibernation: n = 12; methoprene: n = 13).
RNA extractions from whole-body queen samples were performed using a modified protocol including the use of TRIzol (Invitrogen) for the initial homogenization and the RNeasy Plus Micro extraction kit (Qiagen). For each individual queen, cDNAs were synthesized using 500 ng of total RNA, random hexamers, and Applied Biosystems reagents. Levels of mRNA were quantified by quantitative real-time PCR (qRT-PCR) using ABI Prism 7900 sequence detector and SYBR Green. All qRT-PCR assays were performed in triplicate and subjected to the heat-dissociation protocol following the final cycle of the qRT-PCR to check for amplification specificity. qRT-PCR values of each gene were normalized by using an internal control ribosomal protein 49 (RP49) gene. Paralog-specific primers (Table S1) were designed using sequence alignment (95) and primer analysis (96) programs. Primer sequences overlapped coding regions split by introns, allowing the specific amplification of cDNA levels over potential genomic DNA contaminations. Transcript quantification calculations were performed by using the ΔΔCT method (97).
The ecdysteroid titer in queens was determined using the liquid chromatography–mass spectrometry method developed by Westerlund and Hoffmann (98), with some minor modifications (see SI Text for details). The amount of Vg in eggs was measured by dot-blotting using Ectatomma tuberculatum (Formicidae: Ectatomminae) anti-Vg antibodies (99) (see SI Text for details).
To test for the effect of the treatments on the proportion of queens among the offspring, gene expression, and egg number and weight, we conducted ANOVAs on models optimized to fit our data. The proportion of queens was fit using a generalized linear model with quasi-binomial errors. The gene expression data were fit using a general linear model with normal errors. The ecdysteroid and Vg data could not be normalized and were analyzed using Kruskal–Wallis and Mann–Whitney nonparametric tests. The correlation between the ecdysteroid titer and the proportion of queens produced was tested using a Spearman rank correlation test. All statistical analyses were performed with R (www.R-project.org).
Supplementary Material
Acknowledgments
We thank Gro Amdam, Eric Lucas, and Gene Robinson for comments on this manuscript, and Tanja Schwander for the collection of founding queens. This research was supported by several grants from the Swiss National Science Foundation and a European Research Council Advanced Grant (to L.K.), and a Marie Curie Fellowship (to R.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221781110/-/DCSupplemental.
References
- 1.West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst. 1989;20:249–278. [Google Scholar]
- 2.West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 3.Mousseau TA, Dingle H. Maternal effects in insect life histories. Annu Rev Entomol. 1991;36(1):511–534. [Google Scholar]
- 4.Falconer D. Maternal effects and selection response. Genetics Today. 1965;3:763–774. [Google Scholar]
- 5.Bernardo J. Maternal effects in animal ecology. Am Zool. 1996;36(2):83–105. [Google Scholar]
- 6.Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13(10):403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43(3):485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. [DOI] [PubMed] [Google Scholar]
- 8.Mousseau TA, Uller T, Wapstra E, Badyaev AV. Evolution of maternal effects: Past and present. Philos Trans R Soc Lond B Biol Sci. 2009;364(1520):1035–1038. doi: 10.1098/rstb.2008.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parichy DM, Kaplan RH. Maternal effects on offspring growth and development depend on environmental quality in the frog Bombina orientalis. Oecologia. 1992;91(4):579–586. doi: 10.1007/BF00650334. [DOI] [PubMed] [Google Scholar]
- 10.Simpson SJ, Sword GA, Lo N. Polyphenism in insects. Curr Biol. 2011;21(18):R738–R749. doi: 10.1016/j.cub.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Michener CD. Social polymorphism in Hymenoptera. Symp R Entomol Soc Lond. 1961;1:43–56. [Google Scholar]
- 12.Mayr E. Animal Species and Evolution. Cambridge, MA: Belknap Press; 1963. [Google Scholar]
- 13.Stearns SC. The evolutionary significance of phenotypic plasticity. Bioscience. 1989;39(7):436–445. [Google Scholar]
- 14.Lees A. The control of polymorphism in aphids. Adv Insect Physiol. 1966;3:207–277. [Google Scholar]
- 15.Sutherland O. The role of crowding in the production of winged forms by two strains of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 1969;15(8):1385–1410. [Google Scholar]
- 16.Honek A. Maternal regulation of wing polymorphism in Pyrrhocoris apterus: Effect of cold activation. Cell Mol Life Sci. 1980;36(4):418–419. [Google Scholar]
- 17.Hunter-Jones P. 1958. Laboratory studies on the inheritance of phase characters in locusts. Anti-Locust Bull 29:1–32.
- 18.Saiful Islam M, Roessingh P, Simpson SJ, McCaffery AR. Parental effects on the behaviour and colouration of nymphs of the desert locust Schistocerca gregaria. J Insect Physiol. 1994;40(2):173–181. [Google Scholar]
- 19.Nijhout HF, Wheeler DE. Juvenile hormone and the physiological basis of insect polymorphisms. Q Rev Biol. 1982;57(2):109–133. [Google Scholar]
- 20.Lees A. The endocrine control of polymorphism in aphids. Endocrin Insects. 1983;1:369–377. [Google Scholar]
- 21.Hardie J, Lees A. Endocrine control of polymorphism and polyphenism. Comp Insect Physiol Biochem Pharmacol. 1985;8:441–490. [Google Scholar]
- 22.Miller GA, Islam MS, Claridge TD, Dodgson T, Simpson SJ. Swarm formation in the desert locust Schistocerca gregaria: Isolation and NMR analysis of the primary maternal gregarizing agent. J Exp Biol. 2008;211(Pt 3):370–376. doi: 10.1242/jeb.013458. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler D. Developmental and physiological determinants of caste in social Hymenoptera: Evolutionary implications. Am Nat. 1986;128(1):13–34. [Google Scholar]
- 24.Holldobler B, Wilson E. The Ants. Cambridge, MA: Belknap Press; 1990. [Google Scholar]
- 25.Wilson EO. The Insect Societies. Cambridge, MA: Harvard Univ Press; 1971. [Google Scholar]
- 26.Schwander T, Lo N, Beekman M, Oldroyd BP, Keller L. Nature versus nurture in social insect caste differentiation. Trends Ecol Evol. 2010;25(5):275–282. doi: 10.1016/j.tree.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Moritz RF, et al. Rare royal families in honeybees, Apis mellifera. Naturwissenschaften. 2005;92(10):488–491. doi: 10.1007/s00114-005-0025-6. [DOI] [PubMed] [Google Scholar]
- 28.Keller L, Sundstrom L, Chapuisat M. Male reproductive success: Paternity contribution to queens and workers in Formica ants. Behav Ecol Sociobiol. 1997;41(1):11–15. [Google Scholar]
- 29.Smith CR, Anderson KE, Tillberg CV, Gadau J, Suarez AV. Caste determination in a polymorphic social insect: Nutritional, social, and genetic factors. Am Nat. 2008;172(4):497–507. doi: 10.1086/590961. [DOI] [PubMed] [Google Scholar]
- 30.Libbrecht R, Schwander T, Keller L. Genetic components to caste allocation in a multiple-queen ant species. Evolution. 2011;65(10):2907–2915. doi: 10.1111/j.1558-5646.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- 31.Hughes WO, Boomsma JJ. Genetic royal cheats in leaf-cutting ant societies. Proc Natl Acad Sci USA. 2008;105(13):5150–5153. doi: 10.1073/pnas.0710262105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwander T, Keller L. Genetic compatibility affects queen and worker caste determination. Science. 2008;322(5901):552. doi: 10.1126/science.1162590. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, Lo N, Miyata H, Kitade O. Sex-linked genetic influence on caste determination in a termite. Science. 2007;318(5852):985–987. doi: 10.1126/science.1146711. [DOI] [PubMed] [Google Scholar]
- 34.Frohschammer S, Heinze J. A heritable component in sex ratio and caste determination in a Cardiocondyla ant. Front Zool. 2009;6(1):27–33. doi: 10.1186/1742-9994-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartfelder K, et al. Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie (Celle) 2006;37(2):144–163. [Google Scholar]
- 36.Koyama S, Takagi T, Martin S, Yoshida T, Takahashi J. Absence of reproductive conflict during queen rearing in Apis cerana. Insectes Soc. 2009;56(2):171–175. [Google Scholar]
- 37.Goodisman MAD, Kovacs JL, Hoffman EA. Lack of conflict during queen production in the social wasp Vespula maculifrons. Mol Ecol. 2007;16(12):2589–2595. doi: 10.1111/j.1365-294X.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 38.Rabeling C, et al. Thelytokous parthenogenesis in the fungus-gardening ant Mycocepurus smithii (Hymenoptera: Formicidae) PLoS One. 2009;4(8):e6781. doi: 10.1371/journal.pone.0006781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winter U, Buschinger A. Genetically mediated queen polymorphism and caste determination in the slave-making ant, Harpagoxenus sublaevis (Hymenoptera: Formicidae) Entomol Gen. 1986;11(3–4):125–137. [Google Scholar]
- 40.Passera L. The laying of biased eggs by the ant Pheidole pallidula (Nyl.) (Hymenoptera, Formicidae) Insectes Soc. 1980;27(1):79–95. [Google Scholar]
- 41.Gösswald K. Ueber den Lebenslauf von Kolonien der roten Waldameise. Zool Jb System Oekol u Geogr. 1951;80:27–63. [Google Scholar]
- 42.Bier K. Ueber den Saisondimorphismus des Oogenese von Formica rufa rufo-pratensis minor Gössw. und dessen Bedeutung für die Kastendetermination. Biol Zentralbl. 1954;73:170–190. [Google Scholar]
- 43.Vargo EL, Passera L. Gyne development in the Argentine ant Iridomyrmex humilis—role of overwintering and queen control. Physiol Entomol. 1992;17(2):193–201. [Google Scholar]
- 44.Petersen-Braun M. Untersuchungen zur sozialen Organisation der Pharaoameise Monomorium pharaonis L.(Hymenoptera, Formicidae) II. Die Kastendeterminierung. Insectes Soc. 1977;24(4):303–318. [Google Scholar]
- 45.Schwander T, et al. Maternal effect on female caste determination in a social insect. Curr Biol. 2008;18(4):265–269. doi: 10.1016/j.cub.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Cahan SH, Graves CJ, Brent CS. Intergenerational effect of juvenile hormone on offspring in Pogonomyrmex harvester ants. J Comp Physiol B. 2011;181(8):991–999. doi: 10.1007/s00360-011-0587-x. [DOI] [PubMed] [Google Scholar]
- 47.Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27(10):999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 48.Corona M, et al. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci USA. 2007;104(17):7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheng Z, Xu J, Bai H, Zhu F, Palli SR. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J Biol Chem. 2011;286(49):41924–41936. doi: 10.1074/jbc.M111.269845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parthasarathy R, Palli SR. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2011;41(5):294–305. doi: 10.1016/j.ibmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Süren-Castillo S, Abrisqueta M, Maestro JL. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol. 2012;42(7):491–498. doi: 10.1016/j.ibmb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Capella ICS, Hartfelder K. Juvenile hormone effect on DNA synthesis and apoptosis in caste-specific differentiation of the larval honey bee (Apis mellifera L.) ovary. J Insect Physiol. 1998;44(5–6):385–391. doi: 10.1016/s0022-1910(98)00027-4. [DOI] [PubMed] [Google Scholar]
- 53.Guidugli KR, Piulachs MD, Bellés X, Lourenço AP, Simões ZLP. Vitellogenin expression in queen ovaries and in larvae of both sexes of Apis mellifera. Arch Insect Biochem Physiol. 2005;59(4):211–218. doi: 10.1002/arch.20061. [DOI] [PubMed] [Google Scholar]
- 54.de Azevedo SV, Hartfelder K. The insulin signaling pathway in honey bee (Apis mellifera) caste development—differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J Insect Physiol. 2008;54(6):1064–1071. doi: 10.1016/j.jinsphys.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Patel A, et al. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS One. 2007;2(6):e509. doi: 10.1371/journal.pone.0000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler DE, Buck N, Evans JD. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol Biol. 2006;15(5):597–602. doi: 10.1111/j.1365-2583.2006.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mutti NS, et al. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J Exp Biol. 2011;214(Pt 23):3977–3984. doi: 10.1242/jeb.061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473(7348):478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 59.Tu MP, Yin CM, Tatar M. Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol. 2005;142(3):347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J Insect Physiol. 2000;46(2):153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- 61.Robinson GE, Vargo EL. Juvenile hormone in adult eusocial Hymenoptera: Gonadotropin and behavioral pacemaker. Arch Insect Biochem Physiol. 1997;35(4):559–583. doi: 10.1002/(SICI)1520-6327(1997)35:4<559::AID-ARCH13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Barchuk AR, Bitondi MMG, Simões ZLP. Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. J Insect Sci. 2002;2:1–8. doi: 10.1673/031.002.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engels W, et al. Honey bee reproduction: Vitellogenin and caste-specific regulation of fertility. Adv Invertebrate Repro. 1990;5:495–502. [Google Scholar]
- 64.Tatar M, et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 65.Dhadialla TS, Cook KE, Wyatt GR. Vitellogenin mRNA in locust fat body: Coordinate induction of two genes by a juvenile hormone analog. Dev Biol. 1987;123(1):108–114. doi: 10.1016/0012-1606(87)90432-5. [DOI] [PubMed] [Google Scholar]
- 66.Comas D, Piulachs MD, Bellés X. Fast induction of vitellogenin gene expression by juvenile hormone III in the cockroach Blattella germanica (L.) (Dictyoptera, Blattellidae) Insect Biochem Mol Biol. 1999;29(9):821–827. doi: 10.1016/s0965-1748(99)00058-2. [DOI] [PubMed] [Google Scholar]
- 67.Guidugli KR, et al. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005;579(22):4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- 68.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 69. Engelmann F (2002) Ecdysteroids, juvenile hormone and vitellogenesis in the cockroach Leucophaea maderae. J Insect Sci 2:20–27. [DOI] [PMC free article] [PubMed]
- 70.Parthasarathy R, Sun Z, Bai H, Palli SR. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2010;40(5):405–414. doi: 10.1016/j.ibmb.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu HL, Pietrantonio PV. Insect insulin receptors: Insights from sequence and caste expression analyses of two cloned hymenopteran insulin receptor cDNAs from the fire ant. Insect Mol Biol. 2011;20(5):637–649. doi: 10.1111/j.1365-2583.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 72.Okada Y, et al. Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp. J Insect Physiol. 2010;56(3):288–295. doi: 10.1016/j.jinsphys.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Amdam GV, Nilsen KA, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life history. Am Nat. 2007;170(1):37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amdam GV, Norberg K, Fondrk MK, Page RE., Jr Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Natl Acad Sci USA. 2004;101(31):11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5(3):e62. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nilsen KA, et al. Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J Exp Biol. 2011;214(Pt 9):1488–1497. doi: 10.1242/jeb.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wurm Y, et al. The genome of the fire ant Solenopsis invicta. Proc Natl Acad Sci USA. 2011;108(14):5679–5684. doi: 10.1073/pnas.1009690108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA. 2008;105(11):4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Natl Acad Sci USA. 2003;100(4):1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Passera L, Suzzoni JP. The role of the queen of Pheidole Pallidula (Nyl.) (Hymenoptera, Formicidae) in the brood sexualization after JH treatment. Insectes Soc. 1979;26(4):343–353. [Google Scholar]
- 81.Sim C, Denlinger DL. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA. 2008;105(18):6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mellanby K. Low temperature and insect activity. Proc R Soc Lond B Biol Sci. 1939;127(849):473–487. [Google Scholar]
- 83.Puig O, Tjian R. Nutrient availability and growth: Regulation of insulin signaling by dFOXO/FOXO1. Cell Cycle. 2006;5(5):503–505. doi: 10.4161/cc.5.5.2501. [DOI] [PubMed] [Google Scholar]
- 84.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2(2):239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 85.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12(15):1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 86.Claudianos C, et al. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol. 2006;15(5):615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Helvig C, Koener JF, Unnithan GC, Feyereisen R. CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA. 2004;101(12):4024–4029. doi: 10.1073/pnas.0306980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bellés X, Martín D, Piulachs M-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 2005;50:181–199. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- 89.Defelipe LA, et al. Juvenile hormone synthesis: “Esterify then epoxidize” or “epoxidize then esterify”? Insights from the structural characterization of juvenile hormone acid methyltransferase. Insect Biochem Mol Biol. 2011;41(4):228–235. doi: 10.1016/j.ibmb.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nouzova M, Edwards MJ, Mayoral JG, Noriega FG. A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem Mol Biol. 2011;41(9):660–669. doi: 10.1016/j.ibmb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tobe SS, Stay B. Structure and regulation of the corpus allatum. Adv Insect Physiol. 1985;18:305–432. [Google Scholar]
- 92.Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: The double repressor hypothesis. J Theor Biol. 2003;223(4):451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 93.Suzzoni J, Passera L, Strambi A. Ecdysteroid titre and caste determination in the ant, Pheidole pallidula (Nyl.) (Hymenoptera: Formicidae) Cell Mol Life Sci. 1980;36(10):1228–1229. [Google Scholar]
- 94.Hagedorn H, Kunkel J. Vitellogenin and vitellin in insects. Annu Rev Entomol. 1979;24(1):475–505. [Google Scholar]
- 95.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rychlik W. OLIGO 7 primer analysis software. Methods Mol Biol. 2007;402:35–60. doi: 10.1007/978-1-59745-528-2_2. [DOI] [PubMed] [Google Scholar]
- 97.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 98.Westerlund SA, Hoffmann KH. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography-mass spectrometry (LC-MS) Anal Bioanal Chem. 2004;379(3):540–543. doi: 10.1007/s00216-004-2598-x. [DOI] [PubMed] [Google Scholar]
- 99.Azevedo DO, Zanuncio JC, Delabie JHC, Serrão JE. Temporal variation of vitellogenin synthesis in Ectatomma tuberculatum (Formicidae: Ectatomminae) workers. J Insect Physiol. 2011;57(7):972–977. doi: 10.1016/j.jinsphys.2011.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.