Abstract
The field of endothelial progenitor cell (EPC) biology is approaching a decade and a half since generating substantial promise as a potential reparative cell therapy for a spectrum of human clinical disorders. With considerable speed, scientists and clinicians moved from basic studies of isolating and characterizing the biologic properties of EPCs, to pre-clinical EPC treatment studies in rodent model systems of cardiovascular disease, and to the delivery of EPC or marrow-derived cells into selected human subjects (reviewed in [1, 2]). In some disease settings, patient benefits from the infused EPC or marrow-derived cells have been documented, though perhaps not to the extent hoped for or predicted by the results in the preclinical animal model systems [3]. In most human clinical trials, autologous bone marrow mononuclear cells have been infused into patients with cardiovascular disease in an attempt to provide certain presumed EPC subsets to ameliorate ischemic insult [4-7]. To provide some perspective on the advances to date, this review will begin by highlighting the major clarifications in EPC definitions that have occurred over the past 10 years and how this information has instructed changes to the selection of bone marrow subsets for patient use [8-11]. To bring perspective to the increased appreciation of the roles played by hematopoietic cells in vascular repair, we will provide an overview of the hematopoietic hierarchy in mouse and man and identify those subsets that display proangiogenic activities. This perspective may help the reader consider crucial milestones in the discovery and application of HSC and progenitor cells as a cell therapeutic that have not been well explored in the EPC field. The review will conclude with a list of issues that need to be addressed to permit a more quantitative and definable nomenclature for the cells that participate in vascular endothelial repair and replacement. This review will not address the role of those EPC comprised of resident or circulating endothelial cells or endothelial colony forming cells involved in vascular repair and regeneration under normal or pathological conditions (reviewed in [8-15]).
Introduction
The field of endothelial progenitor cell (EPC) biology is approaching a decade and a half since generating substantial promise as a potential reparative cell therapy for a spectrum of human clinical disorders. With considerable speed, scientists and clinicians moved from basic studies of isolating and characterizing the biologic properties of EPCs, to pre-clinical EPC treatment studies in rodent model systems of cardiovascular disease, and to the delivery of EPC or marrow-derived cells into selected human subjects (reviewed in [1, 2]). In some disease settings, patient benefits from the infused EPC or marrow-derived cells have been documented, though perhaps not to the extent hoped for or predicted by the results in the preclinical animal model systems [3]. In most human clinical trials, autologous bone marrow mononuclear cells have been infused into patients with cardiovascular disease in an attempt to provide certain presumed EPC subsets to ameliorate ischemic insult [4-7]. To provide some perspective on the advances to date, this review will begin by highlighting the major clarifications in EPC definitions that have occurred over the past 10 years and how this information has instructed changes to the selection of bone marrow subsets for patient use [8-11]. To bring perspective to the increased appreciation of the roles played by hematopoietic cells in vascular repair, we will provide an overview of the hematopoietic hierarchy in mouse and man and identify those subsets that display proangiogenic activities. This perspective may help the reader consider crucial milestones in the discovery and application of HSC and progenitor cells as a cell therapeutic that have not been well explored in the EPC field. The review will conclude with a list of issues that need to be addressed to permit a more quantitative and definable nomenclature for the cells that participate in vascular endothelial repair and replacement. This review will not address the role of those EPC comprised of resident or circulating endothelial cells or endothelial colony forming cells involved in vascular repair and regeneration under normal or pathological conditions (reviewed in [8-15]).
Clarifications in the definition of endothelial progenitor cells
As originally identified by Asahara and co-workers in 1997 [16], circulating blood cells derived from the bone marrow could migrate to the site of vascular injury and promote recovery of blood flow via formation of vessels in a process called postnatal vascularization. These blood cells which could also be demonstrated to upregulate numerous cell surface markers thought to be endothelial specific in vitro, were identified as endothelial progenitor cells (EPC). However, some of the earliest cell surface markers used to identify the putative EPC included markers that were co-expressed by endothelial and hematopoietic cells such as CD34, CD117, CD133, CD105, CD144, CD184, CD309, acetylated low density lipoprotein, and various plant lectins [16-20]. Work over the past decade has clarified that most of the cells identified as EPC have been shown to represent hematopoietic cells of various stages of differentiation [8, 21] that could be shown to play largely paracrine roles in providing vascular reparative functions in mice and man and in fact, EPC have recently been re-classified as hematopoietic or non-hematopoietic [9]. The reports of bone marrow derived cells that integrate as EPC into injured vasculature or tumor vessels [22-26] has been countered by other studies using more specific transgenic reporter systems and confocal microscopy to indicate that donor putative EPC if they are present in vessels, reside in a peri-endothelial location [27-31]. While numerous studies have attempted to utilize autologous bone marrow cells as a cell therapy to treat various forms of cardiovascular disease or peripheral arterial disease, only very modest improvements in patient outcomes have been reported in meta-analyses and none of the therapies have become standard of care [6, 7]. In fact, increasing evidence suggests that many cardiovascular disorders impair the function of the marrow-derived cells [32, 33] and thus, one would need to find a method to repair the function of the autologous cells before one could re-infuse them as a vascular reparative therapy [1]. Thus, additional work is required to learn more about the roles of hematopoietic cells in vascular repair and the mechanisms through which they promote vascular endothelial cell survival, proliferation, migration, and angiogenesis.
Introduction to hematopoietic cells as a clinical blood cell therapy
More than 50,000 HSC transplants are performed world-wide annually, making this one of the most common forms of tissue or organ transplantation. Successful translation of murine bone marrow transplantation techniques into human subjects has occurred over more than 5 decades of intense research and has been marked by significant changes in terminology and definitions over time as new methodologies and assays have evolved in the field. Much of the earliest work in the field rose out of basic research that attempted to understand the consequences of total body irradiation on host survival.
Murine HSC identification and the stem cell theory of hematopoiesis
Whole-body irradiation of animals was noted to depress hematopoiesis and cause life-threatening deficiencies in all circulating blood cells (pancytopenia) in exposed mice. However, shielding the spleen of the experimental animal from the radiation beam (or transplanting a normal spleen into an irradiated animal) resulted in recovery from an otherwise lethal radiation dose [34, 35]. In 1956, Ford et al. [36] provided definitive evidence that donor infused murine marrow cells gave rise to all the blood cells of the irradiated host. Soon after, Till and McCulloch [37] provided evidence that single multipotent hematopoietic progenitor cells could be identified in vivo by injecting donor marrow cells into a lethally irradiated recipient animal and examining the recipient spleen for hematopoietic colonies 8-12 days later; each cell clone forming a colony was called a colony-forming unit in spleen cell (CFU-S) [38]. This assay provided the first compelling evidence that hematopoietic cells were clonally derived, although CFU-S were later found not to represent a HSC assay [39].
Pluznik and Sachs [40] and Bradley and Metcalf [41] reported that murine hematopoietic cells could be quantified in vitro in clonal assays of hematopoietic progenitor cell differentiation. Furthermore, these investigators reported that each myeloid colony developing in vitro arose from a single precursor cell called the colony-forming unit in culture or CFU-C. Later, Axelrad and co-workers [42] succeeded in demonstrating that red blood cell colonies were also clonally derived. These assays subsequently proved useful in isolating CFU specific hematopoietic growth factors [43, 44] and proving that hematopoietic progenitor subsets segregate into specific lineage producing precursors [45]. The specific hematopoietic progenitor subsets are described in several recent reviews of the topic [46-48].
Murine HSC continue to be definable only as cells that proliferate and differentiate into all lineages of circulating peripheral blood cells for more than 4 months after transplantation into recipient animals and cells that possess the capacity for self-renew in vivo (testable by performing secondary or tertiary transplantation)[49]. The frequency of human and murine hematopoietic stem cells has been estimated to be 1/105-107 bone marrow cells. [50-55] The rarity of this population presented considerable impediments to HSC isolation until the later 1980’s and 1990’s with the availability of an array of monoclonal antibodies, fluorescence activated cell sorters (FACS), and a variety of congenic mouse strains.
Murine HSC and progenitor cells have been enriched (negative selection) using FACS by selecting bone marrow cells that fail to express cell surface antigens (lin−) typically displayed by mature B and T lymphocytes, neutrophils, macrophages, natural killer cells, and red blood cells [56]. Three commonly used phenotypic markers expressed by murine HSC (positive selection) include stem cell antigen-1 (Sca-1), c-kit, and Thy-1 [57]. Sca-1 is a cell surface molecule that is required for normal stem cell self-renewal and progenitor cell proliferation and lineage maturation in mice [58.] C-kit is a cell surface receptor tyrosine kinase that is necessary for murine HSC survival [59] and in utero murine embryo survival [60]. Thy-1 is a cell surface molecule expressed by stem and lymphoid cells [61]. CD34 is a cell surface sialomucin expressed by hematopoietic and endothelial cells [62]. CD34 is expressed on proliferating adult marrow stem cells but may be down-regulated and not detectable on the surface of quiescent marrow stem cells [63, 64]. Other antigens or enzymes that have been utilized to enrich (positive or negative) murine bone marrow stem cells include AC133, CD31, CD38, CD41, CD43, CD105, CD150, aldehyde dehydrogenase, and leukocyte function antigen-1 (LFA-1) (Figure 1) [46]. HSC may also be enriched from other marrow cells by the fact that quiescent stem cells retain the least amount of a DNA-binding dye (Hoechst 33342) and appear as a “side-population” distinguishable (by cell sorting) from other hematopoietic cells [47]. Use of several different combinations of these cell surface or metabolic markers has permitted enrichment of stem cells to homogeneity as evidenced by long-term repopulation of all blood lineages in recipient mice from a single transplanted cell [48]. It is important to stress, that the cell surface phenotype of most populations of cells fails to achieve HSC purity; most populations only enrich for those cells that functionally demonstrate multi-lineage repopulating ability upon transplantation. Therefore, one cannot quantitate the presence of a HSC by counting cells with a particular phenotype, but must transplant and enumerate the in vivo competitive repopulating ability of limiting dilutions of test cells in host animals to confirm HSC numbers that may be present in that population.
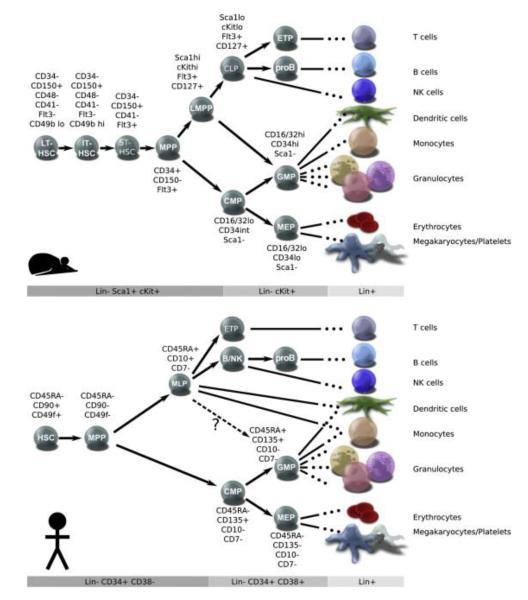
Figure 1.
Models of Lineage Determination in Mouse and Human Hematopoietic Hierarchies. The major classes of stem and progenitor cells are defined by cell surface phenotypes, which are listed next to each population and in the gray bars below each schematic. Terminally differentiated cells are shown on the right, and inferred lineage relationships are depicted with arrows. In mice (A), HSCs can be separated into long-term (LT), intermediate-term (IT), and short-term (ST) classes based on the duration of repopulation. In humans (B), HSCs are defined by the expression of CD49f and other markers, but their heterogeneity has not been investigated. In mice, differentiation of HSCs gives rise to transiently engrafting multipotent progenitors (MPPs), and a series of immature lymphoid-biased progenitors (such as LMPPs) that undergo gradual lymphoid specification. In humans, MPPs can be identified by the loss of CD49f expression; however, only one population of immature lymphoid progenitors (MLPs) has been described. Both mice and humans have well-defined populations of common myeloid progenitors (CMPs), granulocyte macrophage progenitors (GMPs), and megakaryocyte erythroid progenitors (MEPs). Lin: cocktail containing cell surface markers for all terminally differentiated populations (B cell; T cell; NK; dendritic cell, monocyte, granulocyte, megakaryocyte, and erythrocyte). Modified from Doulatov S, et al. Hematopoiesis: A human perspective. Cell Stem Cell 10:124, 2012.
The importance of functional HSC and progenitor cell (HSPC) niches in the murine bone marrow has been well described [65, 66]. While some HSPC are circulating throughout the systemic blood and lymphatic systems at homeostasis [67, 68], most HSC are maintained in quiescence within a niche in close approximation to the endosteal surface of bone and also near blood vessels that provide key survival factors [59, 65, 69]. Research over the last decade has led to the use of several growth factors and chemokine receptor antagonists to mobilize the HSPC into the systemic circulation and allow for collection of functional HSPC for stem cell transplantation [70-72]. Even if infused at high numbers, human and murine HSC will not substantially engraft in the host unless the host has received some form of bone marrow myeloablative conditioning to eradicate endogenous HSPC within their niches. Advancements in minimizing the bone marrow conditioning has permitting transplantation of HSC with minimal risks to the host and may provide new strategies for human stem cell transplantation for patients with genetic hematopoietic disorders (ie. sickle cell disease, thalassemia, Fanconi anemia) [73-75].
Human HSPC identification and assays for detection
Human HSPC have been isolated using monoclonal antibodies and flow cytometric cell sorting. (reviewed in [46, 76, 77]. Putative human HSC are enriched in and have been isolated from bone marrow, umbilical cord blood, fetal liver, and mobilized peripheral blood as cells expressing CD34, Thy-1, AC133, and c-kit, but not CD45RA, CD38, or mature blood cell lineage markers (lin−) (Figure 1). While CFC assays have proven useful in identifying the functional properties of flow cytometric sorted human progenitor cells, in vivo testing of selected HSPC populations in human subjects has not been possible for ethical reasons. Nonetheless, CD34+ selected hematopoietic cells have been proven to support patients long-term after bone marrow transplantation [77] and the dose of CD34+ cells collected for transplantation can be useful in predicting the time for donor cell repopulation of the neutrophil and platelet counts in the patients [78, 79].
In an attempt to develop an in vivo system for detection and quantitation of human HSC, several groups have developed xenotransplantation murine models. Non-obese diabetic (NOD) mice bred with severe combined immunodeficient (scid) mice result in NOD/scid mice that accept human hematopoietic grafts [80] and this model has permitted calculation of the frequency of human repopulating cells present in a donor sample [81]. Similarly, scid mice can be implanted with fragments of human fetal thymus and bone that will survive and function as a hematopoietic microenvironment [82]; sublethal irradiation of these scid-hu mice permits engraftment of intravenously administered human cells [83]. NOD-scid-IL2Rγc−/− female mice, lacking all endogenous B, T, and NK cells, display the highest levels of human donor cell repopulation with an 11-fold greater level of reconstitution of all blood cell lineages upon transplantation of limiting dilutions of HSC than in male littermates and many fold greater than NOD/scid hosts [84]. Human cord blood lin−CD34+CD38−CD45RA−Thy-1+Rhodaminelo CD49f+ cells infused at a single cell level were recently reported to give rise to multi-lineage human blood cell reconstitution in female NOD-scid-IL2Rγc−/− mice in primary and secondary transplants and at marrow and tissue sites other than the site of injection (suggesting systemic migration and engraftment) [85]. This remarkable finding suggests that progress may have been made in optimizing a murine xenogeneic transplant system that is capable of identifying the engraftment and multi-lineage differentiation of even a single injected human HSC (using limiting dilution infusions of donor cells), although this human HSC subset has not yet been validated in human subjects.
Summation of advances in HSC identification and understanding that have enhanced HSC use as a blood cell therapy
Progress over the past 5 decades has laid a foundation for quantitative functional analysis of HSPC in mouse and man. Once it was clear that the source of HSC was greatest in adult bone marrow and that infusion of donor marrow cells could rescue a lethally irradiated host, tools were available to progressively define the hematopoietic hierarchy. Wide availability of specific hematopoietic growth factors, clonal in vitro colony assays, monoclonal antibodies and flow cytometry sorting techniques permitted segregation of the hematopoietic hierarchy into discrete subsets of cells with characteristic cell surface markers and functional stem cell and progenitor stages with the capacity to give rise to specific lineages of cells. Translation of many of the concepts from the murine to human system has permitted establishment of a human hematopoietic hierarchy, though there are numerous differences in the specific markers used to define HSPC. In fact, there is not a single cell surface antigen that is expressed on the long term HSC in the mouse that is also present on the human long-term engrafting HSC. Given the availability of clonal in vitro hematopoietic progenitor assays and immunodeficient mouse models for human HSC engraftment, human HSPC can be functionally quantified following infusion and the differentiation potential of the cells followed over several months posttransplant. The availability of these tools has permitted practical study of the optimal reagents to mobilize human HSPC, cryopreserve the cells, and thaw adequate numbers of HSPC to engraft patients [84, 85].
Overview of the proangiogenic roles played by hematopoietic cells
HSC display proangiogenic activity
In the murine system, isolation of bone marrow populations enriched in HSC and transplantation of these cells into syngeneic irradiated recipient mice not only has been shown to regenerate all the blood cell lineages, but in some reports, donor HSC progeny upregulate endothelial cell antigens and take up residence as endothelial cells within numerous blood vessels in multiple tissues [86, 87]. HSC also readily engraft and are recruited to sites of endothelial injury following experimental retinal ischemia [87-90]. In some reports, the donor HSC appeared to form the endothelial lined vessels in the recovering retina [87, 89] while in other reports the HSC appeared to directly recruit the ingrowth of viable retinal endothelial cells or enhance retinal endothelial cell survival and regeneration via differentiation into microglia and secretion of growth promoting molecules for associated astrocytes [88, 90, 91]. Differences in the contributions of transplanted mouse HSC to endothelial lined vessels may be due to differences in the phenotypic subsets of cells used to enrich for the donor HSC, type of vascular injury, time of analysis (examination within the first two weeks after injury is more likely to show HSC contributions while examination months later is less likely to show HSC-derived resident endothelial cells), and type of transgenic reporter mouse used to identify the donor HSC [29, 92-94]. These and other variables are also relevant to the controversy as to why bone marrow cells may or may not integrate as endothelial cells in experimental tumor implants [95, 96]. Whether murine HSC actually change their fate to become endothelial cells may not yet be definitively resolved, however, it is clear that HSC can serve paracrine roles to enhance the process of neovascularization [97].
In the human system, transplantable HSC are typically collected as a mobilized peripheral blood fraction with or without further CD34 selection before administration to the transplant recipient. Nearly all of these CD34 expressing cells are also KDR+ and CD45+ but only some of the cells express AC133 [98-100]. As noted above, CD34+AC133+ cells engraft in immunodeficient mice and are enriched in hematopoietic CFU-C activity [99, 101, 102].Thus, the choice to use CD34 and AC133 and/or KDR as markers to identify circulating EPC [16, 103] was essentially an exercise in determining whether this population of cells known to contain human HSPC also displayed proangiogenic activity. Many reviews have highlighted the ability of human CD34 cells (with or without further enrichment into those subsets expressing AC133 and KDR) to play important roles as biomarkers for coronary disease, peripheral arterial disease, and tumor angiogenesis, as well as, potential therapy to treat ischemic cardiac and limb disorders (reviewed in [1, 8, 9, 13, 15]). Thus, there is ample evidence to define human HSPC as proangiogenic cells. Reports of transplanted human HSC contributing to cells of the endothelial lineage in vivo are limited [104, 105]. Several recent papers have highlighted novel methods of polychromatic flow cytometry that permit analysis of human CD14−CD235a− CD31+CD45+CD34+ cells into one subset expressing AC133 and another subset not expressing this antigen (Figure 2). Remarkably, the CD14−CD235a−CD31+CD45+CD34+AC133+ cells display proangiogenic activity while the CD14−CD235a−CD31+CD45+CD34+AC133− cells do not display angiogenic activity [99]. Of interest, patients with peripheral arterial disease who suffer from deficient angiogenic activity displayed a diminished ratio of the proangiogenic to the nonangiogenic circulating hematopoietic subsets [99] (Figure 3), while pediatric patients bearing solid tumors displayed a significantly higher ratio of proangiogenic to nonangiogenic hematopoietic cells compared to age-matched control subjects but, the patient proangiogenic to nonangiogenic ratio normalized to age-matched control levels after tumor treatment [106]. These data are of interest, as they suggest that patient CD34+ cells are heterogenous in proangiogenic activity and that the AC133+ subpopulation of the CD34+ cells may vary in frequency with human disease states. Would this also suggest that for optimal therapeutic benefits, the CD34+ cells should be further fractionated to enrich for proangiogenic cell effects [1]?
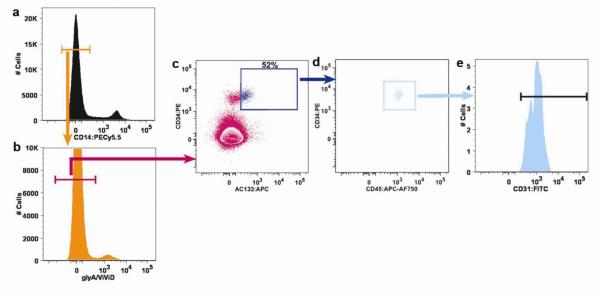
Figure 2.
Frequency analysis of CD31+CD34brightCD45dimAC133+ cells. Uncompensated raw data was collected on a digital flow cytometer, compensated after acquisition by using FlowJo software, and visualized in plots with bi-exponential scaling. Mononuclear cells were identified on a forward versus side scatter plot and then CD14− cells (orange gate in a) were identified. All CD14− cells were then assessed for viability and glycophorin A (CD235a) expression (b). CD14− glyA−ViViD− (viable cells do not retain this molecule) cells (pink gate in b) were subgated onto a bivariant antigen plot to identify CD14−glyA−ViViD−CD34brightAC133+ cells (dark blue gate in c). Viable CD14−glyA−CD34brightAC133+ cells are further subgated to identify the CD45dim subpopulation (light blue gate in d). CD31 expression on the resulting viable cells was confirmed on a CD31 histogram (e). Figure modified from Estes ML, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry Part A 77A:835, 2010.
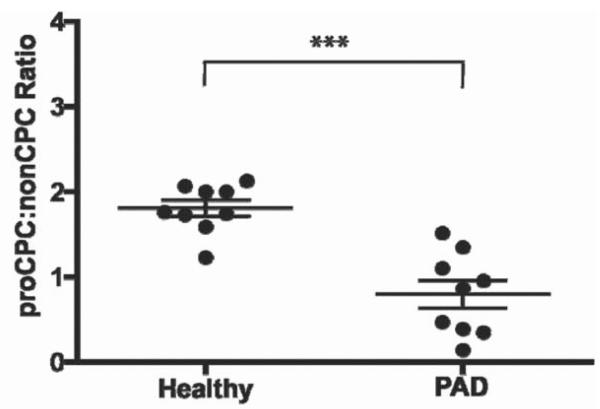
Figure 3.
Ratio of circulating progenitor subsets denotes disease state in peripheral arterial disease patients. The ratio of proangiogenic (CD31+CD34brightCD45dimAC133+) to nonangiogenic (CD31+CD34brightCD45dimAC133−) cells is depicted and patients with peripheral arterial disease (PAD) display a significant decrease when compared to age and gender matched control subjects. Figure modified from Estes ML, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry Part A 77A:837, 2010.
Myeloid progenitor cells display proangiogenic activity
As reviewed above, use of specific monoclonal antibodies and cell sorting permits isolation of subsets of murine bone marrow cells that are enriched for HSC or for various individual lineage progenitor stages (devoid of HSC activity) that can be validated using functional assays [46]. Wara and colleagues [107] recently reported significant differences in the ability of murine common myeloid progenitor (CMP) and granulocyte macrophage progenitor (GMP) cells to differentiate into proangiogenic cells (PAC) compared to murine HSC, megakaryocyte erythroid progenitor (MEP), or common lymphoid progenitor (CLP) cells. The murine CMP and GMP displayed significantly more upregulation of the vascular endothelial growth factor 2 receptor (VEGFR2) and a panel of other endothelial cell surface antigens upon in vitro culture, greater proliferation and migration on fibronectin, greater stimulation of umbilical vein endothelial cells to form capillary-like structures and enhanced adhesion to those endothelial tubes, and greater rescue of blood flow restitution in mice with experimentally induced hindlimb ischemic injury. Further analysis revealed a role for Kruppel-like factor 10 (KLF10) in promoting PAC generation from murine CMP and GMP. KLF10 expression in PAC was regulated by transforming growth factor-beta 1(TGF-β1) and upon TBF-β1 stimulation, KLF10 directly bound to a consensus nucleotide sequence within the VEGFR2 promoter to enhance VEGFR2 expression in PAC. Stimulated PAC displayed significantly greater adhesion, migration, and augmentation of recovery of blood flow in limbs of mice with experimentally induced ischemia compared to PAC generated from KLF10 deficient mice [108]. The significant recovery of the injured vessels and microvascular bed in the instrumented mice from the wild-type (WT) PAC was associated with 4% co-localization of PAC with CD31+ capillaries and no co-localization was seen in animals that received PAC derived from the KFL10 deficient mice. Thus, the benefit from infused PAC in the animals with hindlimb ischemia was largely related to paracrine effects of the KLF10 expressing CMP- and GMP-derived PAC. Of interest, PAC isolated from patients with peripheral arterial disease displayed lower levels of KLF10 expression similar to diminished KLF10 expression in mice undergoing hindlimb arterial ligation [108]. These largely paracrine effects displayed by the KLF10 expressing PAC are reminiscent of the vast number of publications reporting beneficial effects of murine bone marrow-derived EPC in various animal models of cardiovascular or skeletal muscle ischemic injury (reviewed in [3, 8, 9, 17, 20, 23]). It will be interesting to compare potency of the murine PAC derived from the sorted CMP and GMP compared to unsorted PAC derived in published “EPC” protocols to determine whether there are any differences in proangiogenic effects derived from these cells. This may be an important point since PAC tend to be non-proliferative cells while the CMP and GMP can be dramatically expanded in vitro and may serve as a method to generate far greater numbers of PAC than currently possible [9].
A detailed comparison of PAC generation and function from human HSC and progenitor subsets has not been reported. Wara and colleagues [107] have reported that human umbilical cord blood and adult bone marrow CMP and GMP gave rise to significantly more VEGFR2 expressing progeny than the HSC enriched subset, but no analyses of CMP or GMP-derived PAC progeny for in vitro or in vivo angiogenic activities were reported. Since human HSC and progenitor cell ex vivo expansion has now been reported by several groups [109-113], it would appear that focused analysis of the various hematopoietic subsets to determine the most optimal stem or progenitor cell precursors of the PAC would be appropriate in moving toward the cell numbers required for clinical applications.
Monocyte-macrophages display proangiogenic activity
Much has been published on the role of monocytes and macrophages in regulating the process of angiogenesis. A simple PubMed keyword search (12/09/12) for monocytes and angiogenesis resulted in 733 retrievals and a keyword search for macrophages and angiogenesis yielded 2134 results. A sampling of recent work reveals that different monocyte subsets appear more readily specified to proangiogenic macrophage fates [114-116], are key components of the tumor microenvironment [117, 118], can be targeted for specific anti-tumor angiogenic responses [119-121], employ both Wnt [122] and Notch [123-125] signaling pathways to interact with sprouting endothelial cells, and play key roles in some central nervous system pathophysiologic angiogenic processes [126]. It is now well accepted that many of the early outgrowth EPC isolation protocols enrich for monocytes and macrophages which under the conditions of the tissue culture medium and growth factor additives, upregulate endothelial cell surface proteins and downregulate typical activate macrophage antigens [127]. However, these monocytes and macrophages induced to display putative EPC characteristics never fully achieve the epigenetic changes in gene expression that permit the cells to display full endothelial cell gene expression patterns or functions [128] and therefore, putative EPC can be induced to display macrophage functions with standard forms of provocation [129, 130]. Thus, there is ample evidence that both mouse and human monocytes and macrophages display specific functions that promote angiogenesis during development, homeostasis, and disease. The capacity of monocytes and macrophages to upregulate endothelial antigens and appear to “look like EPC” may be a function of their microenvironment in vitro and in vivo [131-134].
Summation and recommendations
The hematopoietic hierarchy is well described in mice and man. Numerous in vitro and in vivo assays have been developed to specifically define the many discrete steps in differentiation from the HSC to the eight major derivative lineages. The detailed analysis of murine HSC homing, engraftment, differentiation, and self-renewal following transplantation have permitted the translation of HSC transplantation into human clinical practice despite obvious differences in some of the specifics for each of these hematopoietic hierarchies that differ between mouse and man. The field continues to move forward with advances in methods to mobilize cells, enhance HSC expansion, and minimize host conditioning to broaden the patient populations amenable to this therapy.
Unlike the hematopoietic system, the field of EPC biology continues to suffer from a lack of key research tools and concepts that include:
lack of recognition that most putative EPC are simply proangiogenic hematopoietic subsets that may be better studied through use of already established hematopoietic hierarchical subset phenotypes rather than attempting to define novel markers,
no discrete in vivo quantitative gold standard assay for any putative EPC population to be measured by (difficult to measure the quantitative end points of paracrine effects of an infused population since multiple molecules may be producing numerous effects concomitantly),
lack of detailed pathway analysis for the molecules through which the various hematopoietic subsets (putative EPC) interact with the host vascular endothelial cells (it is not clear whether all the cells work through similar pathways or if different putative EPC subsets use different pathways),
inadequate ability to measure the angiogenic status of the host vascular endothelium which represents the responding population to the infused putative EPC (cannot isolate the cells and infuse into a host to measure endothelial repopulation or regeneration and thus cannot quantitatively measure host responder status which may vary by age, sex, tissue, treatment, or disease),
inadequate ability to define the proangiogenic status of circulating putative EPC in patients with suspected angiogenic deficiency state or to sense the presence of excessive angiogenesis that may be occurring in a patient with a malignancy,
incomplete information to confirm a hematopoietic to endothelial lineage switch as is suspected to occur in instances when putative EPC are thought to integrate as qualified participants to regenerate vascular endothelial monolayer properties (need for unique lineage specific reporter systems to permit fate mapping studies to be performed in mice to permit identification of a hematopoietic to endothelial transition).
The field of EPC study is relatively new and numerous advancements may facilitate better understanding of how the hematopoietic and vascular endothelial systems interact to produce, maintain, and repair functional vessels during human development, throughout our lifespan, and under the duress of a host of pathophysiologic stressors that culminate in cardiovascular disease.
REFERENCES
- 1.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Yang YJ, Li CJ, Gao RL. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: a systematic review and meta-analysis for randomized controlled trials. Coron Artery Dis. 2008;19:327–335. doi: 10.1097/MCA.0b013e328300dbd3. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Sun T, Ye P. Age, gender and diabetic status are associated with effects of bone marrow cell therapy on recovery of left ventricular function after acute myocardial infarction: a systematic review and meta-analysis. Ageing Res Rev. 2010;9:418–423. doi: 10.1016/j.arr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 11.Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J Mol Cell Cardiol. 2011;50:266–272. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso P, Bertolini F. Circulating endothelial cells as biomarkers in clinical oncology. Microvasc Res. 2010;79:224–228. doi: 10.1016/j.mvr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, Simari RD. Resident vascular progenitor cells-diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4:161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells: an expanding universe. Hypertension. 2010;55:593–599. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 23.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 25.Davidoff AM, Leary MA, Ng CY, Spurbeck WW, Frare P, Vanhove M, Nienhuis AW, Vanin EF. Autocrine expression of both endostatin and green fluorescent protein provides a synergistic antitumor effect in a murine neuroblastoma model. Cancer Gene Ther. 2001;8:537–545. doi: 10.1038/sj.cgt.7700346. [DOI] [PubMed] [Google Scholar]
- 26.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 29.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry TE, Song M, Despres DJ, Kim SM, San H, Yu ZX, Raghavachari N, Schnermann J, Cannon RO, 3rd, Orlic D. Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res. 2009;84:317–325. doi: 10.1093/cvr/cvp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ieronimakis N, Hays A, Reyes M. Bone marrow-derived cells do not engraft into skeletal muscle microvasculature but promote angiogenesis after acute injury. Exp Hematol. 2012;40:238–249. e233. doi: 10.1016/j.exphem.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 33.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson L, Marks E, Gaston E, Robson M, Zirkle R. Role of the spleen in radiation injury. Proc Soc Exp Biol Med. 1949;70:7440–7442. doi: 10.3181/00379727-70-17053. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson L, Simmons E, Marks E, Eldredge J. Recovery from radiation injury. Science. 1951;113:510–511. doi: 10.1126/science.113.2940.510. [DOI] [PubMed] [Google Scholar]
- 36.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177:452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 37.Till J, McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 38.Becker A, McCulloch E, Till J. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–455. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 39.Uchida N, Jerabek L, Weissman I. Searching for hematopoietic stem cells. II. The heterogeneity of Thy-1.1 loLin-/loSca-1+ mouse hematopoietic stem cells separated by counterflow centrifugal elutriation. Exp Hematol. 1996;24:649–659. [PubMed] [Google Scholar]
- 40.Pluznik D, Sachs L. The cloning of normal “mast” cells in tissue culture. J Cell Comp Physiol. 1965;66:319–327. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- 41.Bradley T, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–300. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 42.Axelrad A, McLeod D, Shreeve M, Heath D. Properties of cells that produce erythrocytic colonies in vitro. In: Robinson W, editor. Hemopoiesis in Culture National Inst Health. Washington: 1974. pp. 226–237. [Google Scholar]
- 43.Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993;82:3515–3523. [PubMed] [Google Scholar]
- 44.Metcalf D. The granulocyte-macrophage regulators: reappraisal by gene inactivation. Exp Hematol. 1995;23:569–572. [PubMed] [Google Scholar]
- 45.Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991;254:529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- 46.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120–136. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Mayle A, Luo M, Jeong M, Goodell MA. Flow cytometry analysis of murine hematopoietic stem cells. Cytometry A. 2012 doi: 10.1002/cyto.a.22093. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Copley MR, Beer PA, Eaves CJ. Hematopoietic stem cell heterogeneity takes center stage. Cell Stem Cell. 2012;10:690–697. doi: 10.1016/j.stem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Orlic D, Bodine D. What defines a pluripotent hematopoietic stem cell (PHSC): Will the real PHSC please stand up! Blood. 1994;84:3991–3994. [PubMed] [Google Scholar]
- 50.Harrison DE, Astle CM, Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells. Effects of age. J Immunol. 1989;142:3833–3840. [PubMed] [Google Scholar]
- 51.Harrison DE. Competitive repopulation in unirradiated normal recipients. Blood. 1993;81:2473–2474. [PubMed] [Google Scholar]
- 52.Szilvassy SJ, Hoffman R. Enriched hematopoietic stem cells: basic biology and clinical utility. Biol Blood Marrow Transplant. 1995;1:3–17. [PubMed] [Google Scholar]
- 53.Sutherland HJ, Eaves CJ, Eaves AC, Dragowska W, Lansdorp PM. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- 54.Bhatia M, Bonnet D, Murdoch B, Gan O, Dick J. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nature Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 55.Abkowitz JL, Catlin SN, McCallie MT, Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- 56.Muller-Sieburg CE, Whitlock CA, Weissman IL. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986;44:653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- 57.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 58.Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood. 2003;101:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 59.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reith AD, Bernstein A. Molecular basis of mouse developmental mutants. Genes Dev. 1991;5:1115–1123. doi: 10.1101/gad.5.7.1115. [DOI] [PubMed] [Google Scholar]
- 61.Basch R, Berman J. Thy-1 determinants are present on many murine hematopoietic cells other than T cells. Eur J Immunol. 1982;12:359–364. doi: 10.1002/eji.1830120502. [DOI] [PubMed] [Google Scholar]
- 62.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- 63.Szilvassy S, Cory S. Phenotypic and functional characterization of competitive long-term repopulating hematopoietic stem cells enriched from 5-fluorouracil-treated murine marrow. Blood. 1993;81:2310–2320. [PubMed] [Google Scholar]
- 64.Sato T, Maekawa T, Watanabe S, Tsuji K, Nakahata T. Erythroid progenitors differentiate and mature in response to endogenous erythropoietin. J Clin Invest. 2000;106:263–270. doi: 10.1172/JCI9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 66.Hoggatt J, Scadden DT. The stem cell niche: tissue physiology at a single cell level. J Clin Invest. 2012;122:3029–3034. doi: 10.1172/JCI60238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 69.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, Nowlan B, Cisterne A, Bendall LJ, Sims NA, Levesque JP. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- 71.Mohty M, Ho AD. In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol. 2011;39:723–729. doi: 10.1016/j.exphem.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Damon LE. Mobilization of hematopoietic stem cells into the peripheral blood. Expert Rev Hematol. 2009;2:717–733. doi: 10.1586/ehm.09.54. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, Childs RW, Rodgers GP, Powell JD, Tisdale JF. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, Brodsky RA. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thakar MS, Kurre P, Storb R, Kletzel M, Frangoul H, Pulsipher MA, Leisenring W, Flowers ME, Sandmaier BM, Woolfrey A, Kiem HP. Treatment of Fanconi anemia patients using fludarabine and low-dose TBI, followed by unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:539–544. doi: 10.1038/bmt.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Payne KJ, Crooks GM. Human hematopoietic lineage commitment. Immunol Rev. 2002;187:48–64. doi: 10.1034/j.1600-065x.2002.18705.x. [DOI] [PubMed] [Google Scholar]
- 77.Verfaillie CM. Hematopoietic stem cells for transplantation. Nat Immunol. 2002;3:314–317. doi: 10.1038/ni0402-314. [DOI] [PubMed] [Google Scholar]
- 78.Alexander ET, Towery JA, Miller AN, Kramer C, Hogan KR, Squires JE, Stuart RK, Costa LJ. Beyond CD34+ cell dose: impact of method of peripheral blood hematopoietic stem cell mobilization (granulocyte-colony-stimulating factor [G-CSF], G-CSF plus plerixafor, or cyclophosphamide G-CSF/granulocyte-macrophage [GM]-CSF) on number of colony-forming unit-GM, engraftment, and Day +100 hematopoietic graft function. Transfusion. 2011;51:1995–2000. doi: 10.1111/j.1537-2995.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- 79.Arber C, Halter J, Stern M, Rovo A, Gratwohl A, Tichelli A. Graft source determines human hematopoietic progenitor distribution pattern within the CD34(+) compartment. Bone Marrow Transplant. 2011;46:650–658. doi: 10.1038/bmt.2010.193. [DOI] [PubMed] [Google Scholar]
- 80.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 81.Dick JE, Bhatia M, Gan O, Kapp U, Wang JC. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells. 1997;15(Suppl 1):199–203. doi: 10.1002/stem.5530150826. discussion 204-197. [DOI] [PubMed] [Google Scholar]
- 82.Heike Y, Ohira T, Takahashi M, Saijo N. Long-term human hematopoiesis in SCID-hu mice bearing transplanted fragments of adult bone and bone marrow cells. Blood. 1995;86:524–530. [PubMed] [Google Scholar]
- 83.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115:3704–3707. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- 85.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 86.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 87.Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB, Scott EW. The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood. 2005;105:1916–1922. doi: 10.1182/blood-2004-09-3415. [DOI] [PubMed] [Google Scholar]
- 88.Dorrell MI, Otani A, Aguilar E, Moreno SK, Friedlander M. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood. 2004;103:3420–3427. doi: 10.1182/blood-2003-09-3012. [DOI] [PubMed] [Google Scholar]
- 89.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 90.Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010;58:43–54. doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 93.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264–5272. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 95.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125:1771–1777. doi: 10.1002/ijc.24605. [DOI] [PubMed] [Google Scholar]
- 97.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 98.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 99.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, Pollok KE, Murphy MP, An CS, Srour EF, Ingram DA, Jr., Case J. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77:831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Muller R, Sgadari C, et al. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–3208. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 101.de Wynter EA, Buck D, Hart C, Heywood R, Coutinho LH, Clayton A, Rafferty JA, Burt D, Guenechea G, Bueren JA, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–396. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- 102.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 103.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 104.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramos AL, Darabi R, Akbarloo N, Borges L, Catanese J, Dineen SP, Brekken RA, Perlingeiro RC. Clonal analysis reveals a common progenitor for endothelial, myeloid, and lymphoid precursors in umbilical cord blood. Circ Res. 2010;107:1460–1469. doi: 10.1161/CIRCRESAHA.110.223669. [DOI] [PubMed] [Google Scholar]
- 106.Pradhan KR, Mund JA, Johnson C, Vik TA, Ingram DA, Case J. Polychromatic flow cytometry identifies novel subsets of circulating cells with angiogenic potential in pediatric solid tumors. Cytometry B Clin Cytom. 2011;80:335–338. doi: 10.1002/cyto.b.20602. [DOI] [PubMed] [Google Scholar]
- 107.Wara AK, Croce K, Foo S, Sun X, Icli B, Tesmenitsky Y, Esen F, Rosenzweig A, Feinberg MW. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood. 2011;118:6461–6464. doi: 10.1182/blood-2011-06-363457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wara AK, Foo S, Croce K, Sun X, Icli B, Tesmenitsky Y, Esen F, Lee JS, Subramaniam M, Spelsberg TC, et al. TGF-beta1 signaling and Kruppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization. Blood. 2011;118:6450–6460. doi: 10.1182/blood-2011-06-363713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blank U, Ehrnstrom B, Heinz N, Nilsson E, Brun A, Baum C, Schiedlmeier B, Karlsson S. Angptl4 maintains in vivo repopulation capacity of CD34+ human cord blood cells. Eur J Haematol. 2012;89:198–205. doi: 10.1111/j.1600-0609.2012.01812.x. [DOI] [PubMed] [Google Scholar]
- 110.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dahlberg A, Delaney C, Bernstein ID. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083–6090. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Palma M, Naldini L. Tie2-expressing monocytes (TEMs): Novel targets and vehicles of anticancer therapy? Biochim Biophys Acta. 2009;1796:5–10. doi: 10.1016/j.bbcan.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Laurent J, Touvrey C, Botta F, Kuonen F, Ruegg C. Emerging paradigms and questions on pro-angiogenic bone marrow-derived myelomonocytic cells. Int J Dev Biol. 2011;55:527–534. doi: 10.1387/ijdb.103228jl. [DOI] [PubMed] [Google Scholar]
- 116.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 117.Schmid MC, Varner JA. Myeloid cells in tumor inflammation. Vasc Cell. 2012;4:14. doi: 10.1186/2045-824X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin RZ, Klagsbrun M, Dudley AC. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15:481–495. doi: 10.1007/s10456-012-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Palma M, Naldini L. Angiopoietin-2 TIEs up macrophages in tumor angiogenesis. Clin Cancer Res. 2011;17:5226–5232. doi: 10.1158/1078-0432.CCR-10-0171. [DOI] [PubMed] [Google Scholar]
- 120.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 121.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep. 2012;1:141–154. doi: 10.1016/j.celrep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Newman AC, Hughes CC. Macrophages and angiogenesis: a role for Wnt signaling. Vasc Cell. 2012;4:13. doi: 10.1186/2045-824X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. 2011;2:1106–1116. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J Pathol. 2012 doi: 10.1002/path.4106. DOI 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 127.Favre J, Terborg N, Horrevoets AJ. The diverse identity of angiogenic monocytes. Eur J Clin Invest. 2012 doi: 10.1111/eci.12009. [DOI] [PubMed] [Google Scholar]
- 128.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel JN, Urbich C, Farcas R, Bonig H, Marquez VE, Zeiher AM, Dimmeler S. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res. 2011;109:1219–1229. doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 129.Rohde E, Schallmoser K, Reinisch A, Hofmann NA, Pfeifer T, Frohlich E, Rechberger G, Lanzer G, Kratky D, Strunk D. Pro-angiogenic induction of myeloid cells for therapeutic angiogenesis can induce mitogen-activated protein kinase p38-dependent foam cell formation. Cytotherapy. 2011;13:503–512. doi: 10.3109/14653249.2010.536214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoder M, Mead L, Prater D, Krier T, Mrough K, Li F, Krasich R, Temm C, Prchal J, Ingram D. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principles. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He H, Xu J, Warren CM, Duan D, Li X, Wu L, Iruela-Arispe ML. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120:3152–3162. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 133.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel(R) under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 134.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]