Abstract
Background
Typically, newborns with congenital hypothyroidism are asymptomatic at birth, having been exposed to euthyroid mothers. However, hypopituitarism may be associated with central hypothyroidism, preserved fertility, and autosomal dominant inheritance, requiring increased attention to thyroid management during pregnancy.
Patient Findings
A woman with a history of growth hormone deficiency and central hypothyroidism gave birth to a term male neonate appropriate for gestational age. Due to low thyrotropin (TSH) in the second trimester, the levothyroxine dose was decreased by the obstetrician, and free T4 was low throughout the latter half of pregnancy. The neonatal laboratory evaluation showed central hypothyroidism with a low T4 of 2.1 μg/dL (4.5–11.5) and an inappropriately normal TSH of 0.98 uIU/mL (0.5–4.5); undetectable growth hormone, IGF-I, and IGFBP3; a normal cortisol level; and a normal gonadotropin surge. After initiation of levothyroxine in the first week, both tone and feeding tolerance improved. However, the patient was found to have hearing loss, gross motor delay, and speech delay.
Summary
In this report, we review a case of vertical transmission of a dominant negative POU1F1 mutation in which fetal abnormalities due to the hypothyroxinemic state during gestation may have been exacerbated by a decrease in the mother's levothyroxine dose based on a low TSH in early gestation. Both mother and fetus were unable to synthesize sufficient thyroid hormone, which may be responsible for the patient's clinical presentation.
Conclusion
This case underscores several important points in the management of women with hypopituitarism. First, it is important that patients and clinicians are both aware of the differences in etiology, as well as appropriate screening and treatment, of primary versus central hypothyroidism. Second, it is necessary to monitor the thyroid hormone status closely during pregnancy to prevent fetal sequelae of maternal hypothyroidism. Third, genetic screening of patients with combined pituitary hormone deficiency is necessary, so that prenatal genetic counseling may be an option for expecting parents.
Introduction
Central hypothyroidism is characterized by low serum thyroid hormone levels due to insufficient stimulation by thyrotropin (TSH). Laboratory studies may reveal either low or inappropriately normal serum TSH levels in the presence of low thyroxine levels. Although relatively rare, this is most frequently seen in patients with combined pituitary hormone deficiencies (CPHD), who may also lack growth hormone (GH), prolactin, gonadotropins, and ACTH. Furthermore, central hypothyroidism should be suspected in any patient with a hypothalamic pituitary disorder. Gonadotropin deficiencies, however, may not occur with CPHD. Therefore these patients may remain fertile. Since TSH measurement may be normal in central hypothyroidism, using TSH to screen for thyroid function may lead to a false-negative result. In this report, we review a case of vertical transmission of a dominant negative POU1F1 mutation resulting in deficiencies of TSH, GH, and prolactin. Using the TSH testing strategy in pregnant women with central hypothyroidism to inform therapeutic decisions—that is, decreasing the dose of levothyroxine due to a low TSH—may lead to a hypothyroxinemic state during gestation and result in fetal abnormalities. This case underscores the importance of patients and clinicians being aware of the appropriate monitoring and treatment of central hypothyroidism during pregnancy to prevent fetal sequelae of maternal hypothyroidism.
Patient
Obstetric care of a G1P0 24-year-old woman with a history of hypothyroidism was transferred to a tertiary care facility because a routine ultrasound at 35 weeks gestation noted fetal macrocephaly, frontal bossing, small stomach, and polyhydramnios. A previous ultrasound at 20 weeks gestation indicated fetal growth appropriate for gestational age (AGA). Labor was induced at 40 weeks gestation; a C-section was performed for failure to progress, as well as fetal macrocephaly. APGAR scores were 5, 6, and 7 (at 1, 5, and 10 minutes), and the neonate was noted to have hypotonia and respiratory distress requiring continuous positive airway pressure for less than 24 hours. The infant also exhibited feeding intolerance in the first few days of life. A physical exam revealed a length of 51 cm (75%) and weight of 3.93 kg (85%), both AGA. Macrocephaly was noted (head circumference 39.5 cm; >97% +3.9 SDS) with a depressed nasal bridge, and widened, anteverted nostrils. Due to the maternal history, thyroid studies were obtained on day of life (DOL) 5. Studies showed a total T4 of 2.1 μg/dL (4.5–11.5) and a TSH of 0.98 uIU/mL (0.5–4.5) prompting initiation of levothyroxine replacement. The finding of central hypothyroidism prompted a complete pituitary evaluation, which revealed normal cortisol levels (17.4 μg/dL and 43.8 μg/dL; 6.0–26.0) and two undetectable GH levels of <0.1 ng/mL (0.1–3.1) on DOL 7. A magnetic resonance imaging (MRI) scan on DOL 13 showed a normal pituitary gland. A skeletal survey revealed absence of ossification centers at the proximal humerus, distal femur, and proximal tibia, consistent with delayed bone maturation due to hypothyroidism, with ossification centers absent that are typically present in newborn children (1). The infant required phototherapy for hyperbilirubinemia from DOL 5 to DOL 12, with a peak total bilirubin of 18.7 μg/dL (<12 μg/dL), and a direct component of 0.8 ng/dL (<0.6 mg/dL). On DOL 15, a total T4 was 10.9 μg/dL, and the patient was discharged home on a levothyroxine replacement dose of approximately 14 μg/kg/day. The patient had a normal gonadotropin surge at 12 weeks of age with FSH=1.5 IU/L (0–2.8) and LH=2.5 IU/L (0–2.1). The patient was noted to have failed his hearing screen in the neonatal intensive care unit (NICU), and bilateral mild to moderate sensorineural hearing loss was confirmed with Auditory Brainstem Response audiometry at five months of age.
Family history was notable for a maternal history of macrocephaly and hypoglycemia in the neonatal period. After exhibiting poor growth, she was referred to a pediatric endocrinologist. At six months of age, her length was −3.6 SDS, and she was found to have TSH deficiency, prompting initiation of levothyroxine. At 15 months of age, her length was −5.3 SDS, and GH deficiency was diagnosed with a peak GH of 0.9 ng/mL (reference range >10 ng/mL) following clonidine administration. A computed tomography (CT) scan was reported to be normal, without signs of a hypothalamic lesion. She received GH treatment until the age of 18, and reached an adult height of 64 inches, achieving her mid-parental target height. She had spontaneous menarche between 11 and 12 years of age, and had an unassisted pregnancy at the age of 24. Following childbirth, she was not able to lactate, presumably due to prolactin deficiency.
During pregnancy, her obstetrician obtained a history of hypothyroidism, and tests showed TSH was 0.058 uIU/mL (0.4–4.5) and free T4 was 0.8 ng/dL at 15 weeks (0.61–1.76). During a re-evaluation at 18 weeks, only a TSH level was evaluated and noted to be 0.067 uIU/mL; compliance was assured, and the levothyroxine dose was decreased from 88 to 75 μg. Evaluation by endocrinology was advised but did not occur. At 27 weeks, free T4 was 0.44 ng/dL, and levothyroxine was increased to 88 μg, on which she remained on until the end of pregnancy, with no further thyroid levels tested.
Following discharge from the NICU, the index patient was followed by pediatric endocrinology, with length and weight closely monitored. In the first months of life, the patient had very poor growth velocity, with a length +0.6 SDS at birth that decreased to −4.4 SDS at nine months of age; an IGF-1 level was <25 ng/mL (25–265), and IGFBP3 was <0.5 mg/L (0.7–3.6). GH replacement was discussed with the family at several visits, and started at nine months of age. Growth velocity improved from 13.5 cm/year (−6.4 SDS) over the first six months of life to 17 cm/year (+1.2 SDS) following six months of treatment. Length after 10 months of treatment was −3.6, SDS, and −1.6 SDS after 27 months of treatment. In addition, the patient was diagnosed with speech delay (no babbling at nine months, no words at 19 months) and gross motor delay (rolling at 9 months and pulling to stand/cruising at 15 months). His parents noted an improvement in gross motor skills and strength following initiation of GH therapy.
Methods
Informed consent was obtained to analyze the patient's and mother's DNA for candidate genes required for normal pituitary development and associated with the development of hypopituitarism as part of the Molecular Biology of Panhypopituitarism in Humans research study. Genes sequenced included HESX, SIX6, LHX3 LHX4, OTX2, PROP1, and POU1F1. This study has been approved by the Johns Hopkins University Institutional Review Board. DNA was isolated from maternal blood and the child's cheek swab using Qiagen DNeasy and QIAamp respectively (Qiagen, Valencia, CA). Polymerase chain reaction was performed using primers for exon 6 of the POU1F1 gene: sense 5′ CCG TGA CTC TCG TGT AAC TCT 3′ and antisense 5′ AAA ATA GAT AAT GTG GCT TCT GAG 3′. The product was cleaned with ExoSap-IT® (USB, Cleveland, OH), sequenced by Johns Hopkins Core Sequencing facility, and compared with the NCBI reference sequence NG_008225.1. SDS for length, weight, head circumference, and growth velocity are based on World Health Organization standards.
Results
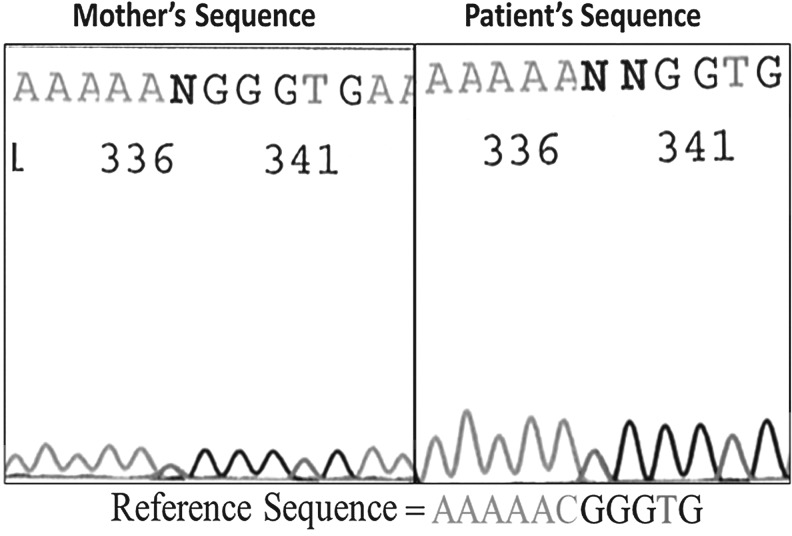
A heterozygous missense mutation c21629C>T, resulting in substitution of arginine 271 by tryptophane in codon 271 (R271W), was discovered (Fig. 1). Both mother and child were found to harbor this heterozygous R271W mutation of POU1F1, which has previously been described as a dominant negative mutation causing GH, TSH, and prolactin deficiency (2,3).
FIG. 1.
Comparison of POU1F1 DNA sequence of index patient and mother, compared to the reference sequence, shown below. The chromatogram shows a double peak (heterozygous) with C and T nucleotides at half the peak of surrounding nucleotides, whereas the reference sequence has only a C at this position.
Discussion
In this report, vertical transmission of a previously unknown dominant negative POU1F1 mutation caused maternal and fetal central hypothyroidism. The observed fetal and neonatal abnormalities may have been secondary to maternal and fetal hypothyroxinemia, illustrating the importance of proper monitoring and interpretation of thyroid tests during pregnancy. We propose that genetic diagnosis of the maternal hypopituitarism prior to pregnancy may have provided the family and the obstetrical healthcare team with the necessary information to manage the hypothyroidism during pregnancy properly.
Two cases of vertical transmission of the POU1F1 R271W mutation have been reported previously. In the index case of maternal–fetal POU1F1 (PIT1) deficiency reported by de Zegher et al. (4), the affected infant showed severe respiratory distress requiring high frequency oscillation for one week, cardiovascular instability requiring dopamine infusion, neurological and bone maturational delays, and severe developmental delay in the first year of follow-up. The first trimester T4 was 7 μg/dL. However, adherence to medication “appeared to be minimal in the latter half of gestation,” and maternal peripartum free T4 was 0.03 ng/dL (0.4 pmol/L). The child was followed for 17 years and continued to have hypotonia and severe developmental delay. This mother had a second child with an identical genotype, but received adequate maternal thyroxine replacement throughout pregnancy, and the second child had typical pre- and postnatal development (de Zegher, personal correspondence). In the case reported by Rainbow et al. (5), the mother received appropriate thyroxine replacement throughout pregnancy, and the newborn had no respiratory or feeding difficulties, as well as a normal developmental outcome with normal intelligence (Kirk, personal correspondence). The index patient in our case report had a phenotype intermediate between these two cases, which reflects the degree of hypothyroidism in the mother, who may have had insufficient levothyroxine replacement but sufficient thyroid hormone levels to pass the placenta and prevent severe sequelae in her child. The finding of an intermediate phenotype with a moderate decrease in thyroxine further underscores the important role of maternal thyroxine in neonatal development.
The patient and his mother described in this report share several clinical features, including features that have been described in other patients with the same POU1F1 mutation (R271W). These features include a prominent forehead, depressed nasal bridge, and a short nose with anteverted nostrils (6). However, there were several important distinctions between the clinical course of this mother and child. The patient had prenatal polyhydramnios with low tone and feeding intolerance, and sensorineural hearing loss. Previous descriptions of polyhydramnios in the literature have been associated with congenital/primary hypothyroidism with a goiter causing anatomical blockage and polyhydramnios, which has been relieved with intrauterine thyroxine therapy (7). We speculate that this patient's polyhydramnios was evidence of fetal/intrauterine hypothyroidism due to low intestinal tone, similar to postnatal constipation. This is further supported by the initial feeding difficulties with emesis and poor intestinal motility, which resolved after initiation of levothyroxine (an upper gastrointestinal exam was performed and was anatomically normal). The x-ray findings of the absence of ossification centers gives further evidence of fetal hypothyroidism, as the femoral epiphysis has usually developed by 36 weeks gestation but was absent in this patient. Sensorineural deafness associated with hypothyroidism has been reported (8). In a naturally occurring mouse model of secondary hypothyroidism, the Snell dwarf Pou1f1dw mouse, profound sensorineural deafness diagnosed by Auditory Brainstem Response testing was detected at three and six weeks. Interestingly, oral thyroid replacement of the dam in late gestation prevented hearing deficits, underscoring the importance of late gestational thyroid hormone for the developing fetus (9,10). The mother of the index patient in this report had normal hearing and intellectual development, and was presumably exposed to normal maternal thyroid levels during fetal development.
In the case reported here, the mother adhered to the prescribed therapy for hypothyroidism. However, a fundamental misunderstanding of the etiology of her hypothyroidism (central vs. primary), the lack of knowledge that her condition could be inherited, and the misinterpretation of a low TSH all eventually resulted in a lowering of thyroxine replacement during a period in pregnancy that required increased thyroxine administration. Due to a variety of physiological and hormonal changes in pregnancy, women with hypothyroidism have increased levothyroxine needs of 30–50% from as early as five weeks gestation (11–13). Additionally, laboratory reference ranges are not specific for pregnancy, and although there are published trimester-specific reference ranges for thyroid function tests, these are not universally agreed upon and not reported by laboratories (14–17). The mother's free T4 of 0.8 ng/dL at 15 weeks would be considered <2.5% of the second trimester population according to one study (17), whereas the FaSTER trial (16) would classify 0.77 ng/dL at the 5th percentile in the second trimester. It should be noted that the index patient's mother's laboratory result was considered within normal limits based on the reported reference range of 0.61–1.76 ng/dL. The free T4 of 0.44 ng/dL at 27 weeks was low according to both standard reference ranges and a trimester-specific range of 0.71–1.26 ng/dL (16).
The prevalence of hypothyroidism in pregnancy is high, with overt hypothyroidism occurring in 0.3–0.5% of pregnancies, and subclinical hypothyroidism in 2–3% of pregnancies (18). Conversely, central hypothyroidism is rare, estimated at 1:20,000–1:100,000 (19,20) in the general population. In addition, central hypothyroidism is frequently associated with multiple pituitary hormone deficiencies, including decreased fertility, making inherited central hypothyroidism that much more unusual. Assuring adequate thyroid hormone replacement in pregnancy is of utmost importance because studies have demonstrated a correlation between maternal hypothyroidism and poorer neuro-developmental outcome based on elevated TSH (21) or hypothyroxinemia (even in the presence of a normal TSH) (22,23).
The American Thyroid Association (ATA) and the Endocrine Society have published guidelines for the diagnosis and management of thyroid disease during pregnancy with the most recent guidelines released in 2012 (24,25). These guidelines emphasize the importance of providers recognizing the increased thyroxine needs in pregnancy, and although they do not recommend universal screening of healthy women for thyroid dysfunction before pregnancy, the guidelines advocate for caregivers to identify individuals who are at higher risk based on their medical history in order to screen either during pregnancy or even those women planning pregnancy. Interestingly, the guidelines do not clearly address the diagnostic criteria that distinguish pregnant women with central hypothyroidism; specifically that TSH levels are not reliable and free T4 or total T4 levels should be used in the decision making to titrate replacement doses. The recently published 2012 ATA guidelines for hypothyroidism in adults address pitfalls in interpreting TSH levels, and note that patients with central hypothyroidism may have only mildly elevated serum TSH levels secondary to secretion of bioinactive isoforms of TSH (26). Furthermore, these guidelines point out that patients with central hypothyroidism should have assessment of free T4 or free T4 index, not TSH, in order to guide treatment of hypothyroidism. These guidelines also advise consultation with an endocrinologist for women with hypothyroidism during pregnancy. Prior to these recent publications, there was no published consensus regarding screening for thyroid disorders during pregnancy, despite the recognized importance of maternal thyroid health for fetal development. The 2002 American Congress of Obstetricians and Gynecologists (ACOG) Practice Bulletin recommended targeted, rather than universal screening. The ACOG guidelines mention hypothalamic dysfunction as a rare cause of hypothyroidism, and recommend obtaining both TSH and free T4 in women with known hypothyroidism (27). The 2007 Endocrine Society clinical practice guidelines also refer to central hypothyroidism as a rare event, but all dose adjustment algorithms were based on TSH and not free T4—an approach that may miss cases such as the one described in our report (18). Fortunately, a heightened awareness for rare thyroid axis pathology such as central hypothyroidism has become more formally addressed in the most recent 2012 ATA guidelines. Our case clearly highlights the importance of not only awareness but also education regarding the timely approach required to manage central hypothyroidism; the clinical decision to decrease the dose of levothyroxine should never be made based on TSH without a confirmatory elevated T4 or FT4 to document overtreatment.
Our report highlights the need for guidelines that address thyroid issues in pregnant women, clearly address the need to understand different etiologies of hypothyroidism, and outline how to screen these women appropriately utilizing not just the TSH level for clinical decision but also obtaining confirmatory T4 or FT4 levels. A recent editorial underscores the point that hypothyroidism is commonly assumed to be primary hypothyroidism, with subsequent dose adjustments based only on TSH (28). Our report highlights an important example of why central hypothyroidism must be considered, and the consequences of this error.
The combination of hypothyroidism and GH deficiency should prompt physicians to consider congenital pituitary defects and underlying genetic mutations. Confusion over the etiology of the mother's hypothyroidism was not limited to her obstetrician, as many consult notes from different subspecialists referred to the mother's condition as “congenital hypothyroidism”, which is predominantly primary hypothyroidism and less commonly heritable (approximately 15% of congenital hypothyroidism is genetic and is more commonly autosomal recessive; an affected relative is identified in only 2% of cases of thyroid dysgenesis) (29,30). In particular, female patients with childhood pituitary hormone deficiencies should receive appropriate education to better understand the fetal risks during pregnancy and to allow them to advocate for appropriate monitoring.
Newborn state screening, which has been in place in Maryland since 1970, is designed to identify newborn children with hypothyroidism in a timely manner to avoid negative consequences on neurodevelopment and growth. As Maryland employs a screening strategy to find low T4 levels with a follow-up TSH, the child's low thyroxine level presumably would have prompted further investigation. Interestingly, the mother was found to have a low T4 level on newborn state screening at birth, but a repeat test was normal, and hypothyroidism was not diagnosed until the child presented with poor growth at six months of age. It is notable, however, that states relying on a TSH-only screening strategy would miss cases of children with central hypothyroidism (31). As central hypothyroidism has immediate effects on neurodevelopment and growth, and is a potentially heritable disorder that can affect subsequent generations, early identification is even more imperative.
The mother had been advised at diagnosis and early in her treatment course (prior to the first description of POU1F1 mutations) that her hormonal deficiencies were isolated and not heritable. Her GH deficiency and hypothyroidism were presumed by later physicians to be unrelated, as both are relatively more common than combined pituitary hormone deficiency. Prolactin deficiency was not presumed until failure of postpartum lactation.
Genetic diagnosis offers several benefits to this family. Knowing the genotype may help clinicians guide clinical care, as the POU1F1 mutations are not usually associated with gonadotropin or ACTH deficiency. The inheritance of the R271W mutation is dominant negative; therefore there is a 50% risk of each additional child inheriting this mutation. Genetic counseling and possible prenatal genetic diagnosis may be offered to the family. There is commercial testing available for both POU1F1 and PROP1, and research testing available at many academic institutions. Pediatric endocrinologists who treat patients with combined pituitary hormone deficiencies should strongly consider offering genetic testing, as the expanding reports of mutations may offer relevant clinical information.Mutations in the transcription factor LHX4 can be inherited in an autosomal fashion and result in variable anterior pituitary hormone deficiencies and preserved fertility (32,33). Endocrinologists who treat adult patients with idiopathic congenital hypopituitarism should also present the option of genetic testing to their patients, even in individuals in whom genetic mutations were not previously found, as novel mutations continue to be discovered. We propose that care of patients with hypopituitarism during the adolescent transition be formally transferred to a knowledgeable adult endocrinologist, as proper management of these patients and potential offspring require subspecialty expertise.
Conclusion
In summary, this case report identifies barriers that prevent appropriate care of the pregnant woman and her fetus with central hypothyroidism. Current guidelines detailing management of pregnant women with hypothyroidism do not address the special case of women with central hypothyroidism. In addition, pediatric and adult endocrinologists should be aware of genetic testing available to persons with congenital hypopituitarism, as it can have consequences for patients and future offspring. Finally, the importance of transition of care from pediatric to adult endocrinology expertise is necessary, and continued education of the patients about the nature and consequences of their rare condition is of utmost importance.
Acknowledgments
The authors would like to thank Dr. Sara DiVall for her help in preparation of this manuscript. The authors would also like to thank Dr. Francis de Zegher and Dr. Jeremy Kirk for providing clinical experience and advice.
Author Disclosure Statement
The authors have nothing to disclose. No competing financial interests exist.
References
- 1.Wilkins L. Epiphysial dysgenesis associated with hypothyroidism. Arch Pediatr Adolesc Med. 1941;61:13–34. [Google Scholar]
- 2.Radovick S. Nations M. Du Y. Berg LA. Weintraub BD. Wondisford FE. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992;257:1115–1118. doi: 10.1126/science.257.5073.1115. [DOI] [PubMed] [Google Scholar]
- 3.Ohta K. Nobukuni Y. Mitsubuchi H. Fujimoto S. Matsuo N. Inagaki H. Endo F. Matsuda I. Mutations in the Pit-1 gene in children with combined pituitary hormone deficiency. Biochem Biophys Res Commun. 1992;189:851–855. doi: 10.1016/0006-291x(92)92281-2. [DOI] [PubMed] [Google Scholar]
- 4.de Zegher F. Pernasetti F. Vanhole C. Devlieger H. Van den Berghe G. Martial JA. The prenatal role of thyroid hormone evidenced by fetomaternal Pit-1 deficiency. J Clin Endocrinol Metab. 1995;80:3127–3130. doi: 10.1210/jcem.80.11.7593413. [DOI] [PubMed] [Google Scholar]
- 5.Rainbow LA. Rees SA. Shaikh MG. Shaw NJ. Cole T. Barrett TG. Kirk JM. Mutation analysis of POUF-1, PROP-1 and HESX-1 show low frequency of mutations in children with sporadic forms of combined pituitary hormone deficiency and septo-optic dysplasia. Clin Endocrinol (Oxf) 2005;62:163–168. doi: 10.1111/j.1365-2265.2004.02189.x. [DOI] [PubMed] [Google Scholar]
- 6.Aarskog D. Eiken HG. Bjerknes R. Myking OL. Pituitary dwarfism in the R271W Pit-1 gene mutation. Eur J Pediatr. 1997;156:829–834. doi: 10.1007/s004310050722. [DOI] [PubMed] [Google Scholar]
- 7.Stoppa-Vaucher S. Francoeur D. Grignon A. Alos N. Pohlenz J. Hermanns P. Van Vliet G. Deladoey J. Non-immune goiter and hypothyroidism in a 19-week fetus: a plea for conservative treatment. J Pediatr. 2010;156:1026–1029. doi: 10.1016/j.jpeds.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Comer DM. McConnell EM. Hypothyroid-associated sensorineuronal deafness. Ir J Med Sci. 2010;179:621–622. doi: 10.1007/s11845-010-0579-y. [DOI] [PubMed] [Google Scholar]
- 9.Mustapha M. Fang Q. Gong TW. Dolan DF. Raphael Y. Camper SA. Duncan RK. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29:1212–1223. doi: 10.1523/JNEUROSCI.4957-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karolyi IJ. Dootz GA. Halsey K. Beyer L. Probst FJ. Johnson KR. Parlow AF. Raphael Y. Dolan DF. Camper SA. Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm Genome. 2007;18:596–608. doi: 10.1007/s00335-007-9038-0. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick DL. Russell MA. Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am. 2010;37:173–193. doi: 10.1016/j.ogc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Alexander EK. Marqusee E. Lawrence J. Jarolim P. Fischer GA. Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 13.Mandel SJ. Larsen PR. Seely EW. Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 14.Soldin OP. Tractenberg RE. Hollowell JG. Jonklaas J. Janicic N. Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–1090. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey BM. Dashe JS. Spong CY. McIntire DD. Leveno KJ. Cunningham GF. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol. 2007;109:1129–1135. doi: 10.1097/01.AOG.0000262054.03531.24. [DOI] [PubMed] [Google Scholar]
- 16.Lambert-Messerlian G. McClain M. Haddow JE. Palomaki GE. Canick JA. Cleary-Goldman J. Malone FD. Porter TF. Nyberg DA. Bernstein P. D'Alton ME FaSTER Research Consortium. First-, second-trimester thyroid hormone reference data in pregnant women: a FaSTER (First-, Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am J Obstet Gynecol. 2008;199:62.e1–62.e6. doi: 10.1016/j.ajog.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan YQ. Dong ZL. Dong L. Wang FR. Yang XM. Jin XY. Lin LX. Sun YN. Chen ZP. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf) 2011;74:262–269. doi: 10.1111/j.1365-2265.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 18.Abalovich M. Amino N. Barbour LA. Cobin RH. De Groot LJ. Glinoer D. Mandel SJ. Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2007;92:S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 19.Ascoli P. Cavagnini F. Hypopituitarism. Pituitary. 2006;9:335–342. doi: 10.1007/s11102-006-0416-5. [DOI] [PubMed] [Google Scholar]
- 20.Hanna CE. Krainz PL. Skeels MR. Miyahira RS. Sesser DE. LaFranchi SH. Detection of congenital hypopituitary hypothyroidism: ten-year experience in the Northwest Regional Screening Program. J Pediatr. 1986;109:959–964. doi: 10.1016/s0022-3476(86)80276-1. [DOI] [PubMed] [Google Scholar]
- 21.Haddow JE. Palomaki GE. Allan WC. Williams JR. Knight GJ. Gagnon J. O'Heir CE. Mitchell ML. Hermos RJ. Waisbren SE. Faix JD. Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 22.Pop VJ. Brouwers EP. Vader HL. Vulsma T. van Baar AL. de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 23.Pop VJ. Kuijpens JL. van Baar AL. Verkerk G. van Son MM. de Vijlder JJ. Vulsma T. Wiersinga WM. Drexhage HA. Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.Stagnaro-Green A. Abalovich M. Alexander E. Azizi F. Mestman J. Negro R. Nixon A. Pearce EN. Soldin OP. Sullivan S. Wiersinga W. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Groot L. Abalovich M. Alexander EK. Amino N. Barbour L. Cobin RH. Eastman CJ. Lazarus JH. Luton D. Mandel SJ. Mestman J. Rovet J. Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 26.Garber JR. Cobin RH. Gharib H. Hennessey JV. Klein I. Mechanick JI. Pessah-Pollack R. Singer PA. Woeber KA. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200–1235. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetrics and Gynecology. ACOG practice bulletin. Thyroid disease in pregnancy. Number 37, August 2002. American College of Obstetrics and Gynecology. Int J Gynaecol Obstet. 2002;79:171–180. doi: 10.1016/s0020-7292(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 28.Emerson CH. Diagnosis and treatment of hypothyroidism: rules, longstanding exceptions, and the emerging entity of thyroid receptor alpha resistance. Thyroid. 2012;22:1197–1199. doi: 10.1089/thy.2012.2212.ed. [DOI] [PubMed] [Google Scholar]
- 29.Castanet M. Polak M. Bonaiti-Pellie C. Lyonnet S. Czernichow P. Leger J. Nineteen years of national screening for congenital hypothyroidism: familial cases with thyroid dysgenesis suggest the involvement of genetic factors. J Clin Endocrinol Metab. 2001;86:2009–2014. doi: 10.1210/jcem.86.5.7501. [DOI] [PubMed] [Google Scholar]
- 30.LaFranchi SH. Congenital hypothyroidism: etiologies, diagnosis, and management. Thyroid. 1999;9:735–740. doi: 10.1089/thy.1999.9.735. [DOI] [PubMed] [Google Scholar]
- 31.LaFranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33:S225–S233. doi: 10.1007/s10545-010-9062-1. [DOI] [PubMed] [Google Scholar]
- 32.Castinetti F. Saveanu A. Reynaud R. Quentien MH. Buffin A. Brauner R. Kaffel N. Albarel F. Guedj AM. El Kholy M. Amin M. Enjalbert A. Barlier A. Brue T. A novel dysfunctional LHX4 mutation with high phenotypical variability in patients with hypopituitarism. J Clin Endocrinol Metab. 2008;93:2790–2799. doi: 10.1210/jc.2007-2389. [DOI] [PubMed] [Google Scholar]
- 33.Pfaeffle RW. Hunter CS. Savage JJ. Duran-Prado M. Mullen RD. Neeb ZP. Eiholzer U. Hesse V. Haddad NG. Stobbe HM. Blum WF. Weigel JF. Rhodes SJ. Three novel missense mutations within the LHX4 gene are associated with variable pituitary hormone deficiencies. J Clin Endocrinol Metab. 2008;93:1062–1071. doi: 10.1210/jc.2007-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]