Abstract
Background
Biological sex differences may contribute to differential treatment outcomes for therapeutic products. This study tracks women's participation in late-phase clinical trials (LPCTs), where efficacy and safety of drugs and biologics are evaluated, of new molecular entity (NME) drugs and biologics approved by the U.S. Food and Drug Administration (FDA) in 2007–2009. Furthermore, presentations of sex-based analyses were assessed from the FDA reviews.
Methods
New drug applications (NDAs) and biologics license applications (BLAs) were accessed from the U.S. FDA database and evaluated for women's participation in LPCTs. Sex-based analyses for efficacy and safety contained in FDA reviews were surveyed. Ratios for women's LPCT participation (PROPORTION OF STUDY SUBJECTS) to their proportion in the disease population were calculated for each approved therapeutic product and grouped into therapeutic categories.
Results
Sex-specific (n=5) and pediatric (n=3) drug applications were excluded. Women's participation in LPCTs was 39%, 48%, and 42% in NDAs (n=50) and 49%, 62%, and 58% in BLAs (n=11) for 2007, 2008, and 2009, respectively. Sixty-four percent of NDAs and 91% of BLAs had participation to proportion ratios of ≥0.80. Seventy-four percent of NDA reviews and 64% of BLA reviews included safety and efficacy sex analysis. Ninety-six percent of NDA reviews and 100% of BLA reviews included efficacy sex analysis.
Conclusion
Women's participation in LPCTs averaged 43% for NDAs and 57% for BLAs in 2007–2009 and varied widely by indication. As a comparison, the 2001 U.S. Government Accountability Office (GAO) reported 52% of women's participation for drug clinical trials in1998–2000 and an FDA study reported 45% for BLAs approved from 1995 to 1999. This study showed that sex-analysis of both safety and efficacy in NDA has increased to 74% since the GAO report of 72%, while those for BLAs increased to 64% from 37% reported for therapeutic biologics approved in 1995–1999. Knowledge of disease prevalence and participation in clinical trials provides an understanding of recruitment and retention patterns of patients in these trials.
Introduction
The participation of both women and men in clinical trials is of paramount importance in determining potentially different treatment outcomes between sexes for medical products. Systemic exposure (pharmacokinetic, PK) differences between women and men may result from absorption, distribution, metabolism, and excretion differences and could cause dissimilar safety or efficacy responses.1 These sex differences in PK of a drug may be due to physiological and hormonal differences between men and women. Pharmacodynamic (PD) differences, independent of PK, have also been reported to result in response differences between men and women. For example, in men and women with similar plasma concentrations of quinidine, a higher QTc interval prolongation can be seen in women compared with men,2 and women tend to have a longer QTc interval compared with men at baseline.3,4 Higher fracture risk has been reported for women as compared with men during long-term use of hypoglycemic drugs of the thiazolidenedione class.5 A diabetes outcome progression trial showed that men's fracture risks were approximately 4% for rosiglitazone, 3.4% for metformin, and 3.4% for glyburide, and the risks in women were 9.3% for rosiglitazone, 5.1% for metformin, and 3.5% for glyburide. Further analysis in women showed that this trend for increased fracture risk with rosiglitazone occurred in both premenopausal and postmenopausal women, manifested after a year of therapy, and did not appear to be due to increased falls or accidental limb injury.5
Dosing strategies for antithrombotic agents have been defined to minimize bleeding risks while maintaining efficacy by individualizing dosing needs for this class of drugs. These are based on body weight and renal function, but due to the complexities associated with dosing of antithrombotic agents, a higher bleeding risk has been observed in women.6,7
In 2001, the U.S. Government Accountability Office (GAO) reported that 8 out of 10 drugs that were withdrawn from the U.S. market between January 1997 and December 2000 had a higher incidence of adverse effects in women.8 To gain a good understanding of sex differences in treatment response, information on basic biologic differences, participation of both women and men in clinical trials, and analysis of safety and efficacy data are important. Sex-based analyses of safety and efficacy in clinical trial data of drugs and biologics are essential to further increase this understanding so that a therapeutic product can be administered at doses that maximally benefit both sexes with minimal side effects. Adequate inclusion of women in early phase studies, where dosing regimens and dose finding are generally carried out, can provide this information.
Historically, women have been underrepresented or excluded from clinical trials. This was especially observed after the teratogenic effects of thalidomide were determined in the 1960s.9 This incident triggered the U.S. Food and Drug Administration (FDA) to issue the guidance document General Considerations for the Clinical Evaluation of Drugs in 1977, which recommended that females of childbearing potential (FCBP) should be excluded from participating in early dose-ranging studies.10 This recommendation was intended to protect the fetus from untoward outcomes of drug exposure but it inadvertently led to knowledge gaps in the understanding of sex differences as it was carried through all stages of drug development. The AIDS epidemic of the 1980s and the increased diagnoses of cancers in women escalated public concern that women's access to breakthrough treatments may be hampered if they were underrepresented or excluded from clinical trials.11 Thus, in 1993, the Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs, was issued by the FDA to effectively reverse the 1977 recommendation regarding exclusion of FCBP from early phase clinical studies.12 The 1993 guidance recommended the collection and analyses of data on sex differences in effectiveness, adverse events, and PK of a drug.12
Over the past 25 years, several studies have assessed women's participation in clinical trials and sex-based outcome differences in FDA approved drugs. In 1992, the GAO reported that 60% of the drugs approved between 1988 and 1991 had an underrepresentation of women in clinical trials when compared to the proportion of women in the disease population for which the drug is intended.13 When analyzed by therapeutic categories, certain drug types had notably lower participation of women than others (i.e., cardiovascular, HIV, and oncology drugs). In response, the Investigational New Drug (IND) and New Drug Application (NDA) regulations were amended by the FDA in 1998 requiring INDs and NDAs to tabulate the participation by gender (sex), age, and race and to evaluate safety and efficacy by gender, age, and race (Code of Federal Regulations Title 21[21CFR] sections 312.33 and 314.50). A subsequent study conducted by the GAO in 2001 concluded that women were well represented in clinical trials of drugs approved between 1998 and 2000, with more than 50% women participating in clinical trials overall, but there were still gaps in sex analyses on the clinical outcomes in the NDAs.14 An FDA internal review conducted by Evelyn, et al. showed that women's participation was 48% in clinical trials of new molecular entities (NMEs) approved between 1995 and 1999.15 More recently, an FDA study by Yang, et al. showed approximately equal participation of men and women in late-phase clinical trials of NME drugs approved during 2000 and 2002.16 In addition to participation, sex-based analyses were also surveyed by these studies. It was shown by these study investigators that sex-based analyses were present in 47% of the NMEs approved between 1988 and 1991, and approximately 70% in the NMEs approved during both 1998–2000 and 2000–2002.14,16 These studies demonstrated an increasing trend towards women's participation and sex-based analyses. Another internal review conducted by the FDA's Center for Biologic Evaluation and Research (CBER) showed that women's participation in biologics clinical trials for new product or biologic license applications approved from 1995 to 1999 was about 45%.17
This study is an update on the current status of the participation of women in late-phase clinical trials (LPCTs) submitted to support the approval of new drugs and biologics by the FDA. Phase 1 and phase 2 studies make up the early-phase clinical trials in which the dose tolerability, clinical pharmacology assessments, exploratory efficacy, and dose-related side effects are usually determined in healthy subjects and/or a small number of patients. LPCTs consist of phase 3 and some late phase 2 studies that generally confirm the efficacy outcome and safety profiles of a drug or biologic product in the targeted patient population. This study specifically evaluates the participation of women in LPCTs and also determines whether sex analysis is present in FDA reviews on safety and efficacy data for the approved products (2007–2009). This study uses the LPCT-reported information in FDA medical and statistical reviews of NME drugs and therapeutic biologics products approved by the FDA between January 2007 and December 2009. Unlike past participation studies,13–16 both drugs and therapeutic biologic products are included in this survey.
Materials and Methods
Participation data collection
A search of NME drugs and biologics approved by U.S. FDA from January 2007 to December 2009 on the Drugs@FDA website (www.accessdata.fda.gov/scripts/cder/drugsatfda) yielded a list of approved NME NDAs and NME biologics license applications (BLAs) for this study. LPCT data submitted to the FDA supporting the approval of these NDAs and BLAs were accessed from the FDA electronic document room. For this study, a LPCT was considered for inclusion if it was a phase 2, phase 2/3, or phase 3 study of safety and/or efficacy of the therapeutic products in a patient population. Therapeutic products that were approved for pediatric or sex-specific indications, as well as those without clinical data were excluded from the study. For each clinical trial, the total number of participants and participation by sex was determined from the study reports. All participants who had received at least one dose of the therapeutic product during the study were included. The number of women participating in the LPCTs within each NDA or BLA was summed to determine total women's participation. Women's participation for each approved NDA or BLA was calculated as a percentage of the total number of participants.
Average women's participation was determined for each year and NME type (NDA or BLA) by calculating the mean of all the women's participation percentages for each NDA or BLA approved each year. The number of participants whose sex was unspecified or not reported by the sponsor was determined by subtracting the sum of male and female participants from the total number of participants.
Participation data compared with proportion in disease population by sex
Sex differences in disease prevalence can contribute to the disproportionate participation by sex in clinical trials. To address the contribution of this factor, the participation of women in the LPCTs for that NME was compared with the proportion of women in the disease population for the NME indication. The background rate was determined using disease (e.g., HIV) or procedure (e.g., medical imaging) prevalence or incidence data in women obtained through a comprehensive literature search of the most recent peer-reviewed journals on PubMED and U.S. government databases from National Institutes of Health or Centers for Disease Control and Prevention (CDC). Prevalence data was used for chronic diseases, while incidence data was used for acute diseases or frequency of procedures. Data from the most recent studies conducted in North America and Europe, when available, were preferred. Search keywords that were used included women, sex, gender, prevalence, incidence, or epidemiology, and disease names or procedures. For therapeutic products indicated for specific disease symptoms, background rate was determined for the specific disease as a whole. For example, if an NME is indicated for treating chorea associated with Huntington's disease, the prevalence data was searched for Huntington's disease, not for chorea, which can be a symptom of various other conditions. Examples are shown in Table 1, where indications have been abbreviated from approved indications per package inserts accessed from Drugs@FDA.
Table 1.
Search Terms for Background Rate by Sex for Selected New Drug Applications
| Indication in the package inserts | Disease/diagnostic prevalence search term |
|---|---|
| Opioid used to accelerate gastrointestinal recovery after partial large or small bowel resection surgery with primary anastomosis | Bowel resection surgery |
| Topical corticosteroid indicated for the treatment of inflammation and pain associated with ocular surgery | Cataract surgery |
| Treats the involuntary movements (chorea) of Huntington's Disease | Huntington's disease |
| Magnetic Resonance Imaging contrast agent to detect and characterize lesions in adults with known or suspected focal liver disease | Non-alcoholic fatty liver disease |
| Indicated in combination with granulocyte colony-stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma | Non-Hodgkin's lymphoma |
Estimation of women's proportion in the disease population
The proportion of women in the disease population was estimated from prevalence or incidence data obtained from published literature as described above. The incidence or prevalence data by sex were reported in various forms, and the women's proportion in the disease population was then estimated as shown in Table 2 (with additional data in Table A1).
Table 2.
Examples of Estimation of Women's Proportion in the Disease Population
| Disease | Unit | Male | Female | Sum | %P | Source | Notes |
|---|---|---|---|---|---|---|---|
| Hypertension | Prevalence (%) | 23.4 | 23.5 | 46.9 | 50.1 | Wolz M, Cutler J, Roccella EJ, Rode F, Thom T, Burt V. Statement from the National High Blood Pressure Education Program: Prevalence of hypertension. Am J Hypertens 2000;13:102–104. | Using NHANES data for prevalence |
| Hypercholesteremia (>200mg/dL) | Prevalence (millions) | 47.7 | 54.5 | 102.2 | 53.3 | Lloyd-Jones D, Adams RJ, Brown TM, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215. | Using CDC and NIH data for prevalence |
| Parkinson's disease | Cases | 358 | 230 | 588 | 39.1 | Van Den Eeden S K, Tanner C M, Bernstein A L, et al. Incidence of Parkinson's disease: Variation by age, gender, and race/ ethnicity. Am J Epidemiol 2003;157:1015–1022. | Using Kaiser data |
| Depression | Prevalence (%) | 4.6 | 8.1 | 12.7 | 63.8 | National Institute for Mental Health. Major depressive disorder among adults. Retrieved from: www.nimh.nih.gov/statistics/1MDD_ADULT.shtml Accessed August 20, 2012. | Data from SAMHSA National Survey on Drug Use and Health |
| Non-Hodgkin's lymphoma | Cases | 226,855 | 211,470 | 438,325 | 48.3 | Surveillance Epidemiology and End Results (SEER) 2010 Registry http://seer.cancer.gov/statfacts/html/nhl.html | NCI SEER data |
| Overactive bladder | Cases | 2,469 | 2,735 | 5,204 | 52.6 | Stewart W F, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327–336. | Population sample |
| Schizophrenia | Incidence (per 100,000) | 21.8 | 21.3 | 43.1 | 49.4 | McGrath J, Saha S, Chant D, Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008;30:67–76. | Review of 383 studies |
| Acute coronary syndrome | Cases | 401,000 | 332,000 | 733,000 | 45.3 | Lloyd-Jones D, Adams RJ, Brown TM, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215. | Using CDC and NIH data for prevalence |
| Diabetes mellitus | Prevalence (%) | 7 | 6.1 | 13.1 | 46.6 | Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population. Diabetes Care 2006;29:1263–1268. | Data from NHANE's (standardized to 2000 U.S. census) |
| Pulmonary hypertension | Cases | 29 | 66 | 95 | 69.5 | Abenhaim L, Moride Y, Brenot F, et al.; International Primary Pulmonary Hypertension Study Group. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med 1996;335:609–616. | Health care data in several countries (rare disease) |
| Muckle-wells syndrome | Ratio | 1 | 1 | 2 | 50 | Shinawi M, Scaglia F. Hereditary periodic fever syndrome. Retrieved from Medscape Reference website: http://emedicine.medscape.com/article/952254-overview#a1 Accessed July 16, 2012. | Rare disease (males and females equally affected) |
%P, proportion (estimated) of women in the disease population; CDC, Centers for Disease Control; NCI, National Cancer Institute; NHANES, National Health and Nutrition Examination Survey; NIH, National Institutes of Health; SAMHSA, Substance Abuse and Mental Health Services Administration.
Women's participation relative to their proportion in the disease population was determined using the following equation:
Ratio=Women's Participation in LPCTs/Women's Proportion in the Disease Population
To evaluate the average of these ratios by therapeutic category, NME NDAs and BLAs were grouped by therapeutic category, and the average of the ratios in each therapeutic category was calculated. Therapeutic categories were based on the FDA review divisions that evaluated the submission package of the NDA or BLA as previously used by Yang's study on women's participation.16
Presentation of sex-based analysis on safety or efficacy
The FDA reviews were surveyed for sex analyses conducted by the agency. FDA medical and statistical reviews were accessed for each NDA and BLA from Drugs@FDA. A coding criterion similar to that used by the GAO 2001 report was employed in this study.14 Any analysis consisting of at least one sentence that summarized the safety or efficacy data by sex was coded as presentation of sex analysis. Furthermore, if sex analyses were done, but any analysis was indicated as exploratory or not statistically powered to draw conclusion, it was subcategorically coded as exploratory sex analysis. Conversely, if a conclusive statement was included without any mention that the analysis was exploratory, then it was subcategorically coded as conclusive sex analysis. Finally, if no efficacy or safety analyses by sex/gender were found in the NME's reviews, it was noted as no sex analysis.
Sponsors' clinical study reports were not examined for performance of analyses of safety and efficacy by sex.
Results
The FDA approved 57 NME NDAs and 11 NME BLAs between January 2007 and December 2009. Seven NME NDAs were excluded from further study because they were either approved for pediatric indications (n=2) or had sex-specific indications (n=4), or as a paper NDA supported by literature reports (n=1). No BLAs were excluded.
Women's participation in late-phase clinical trials
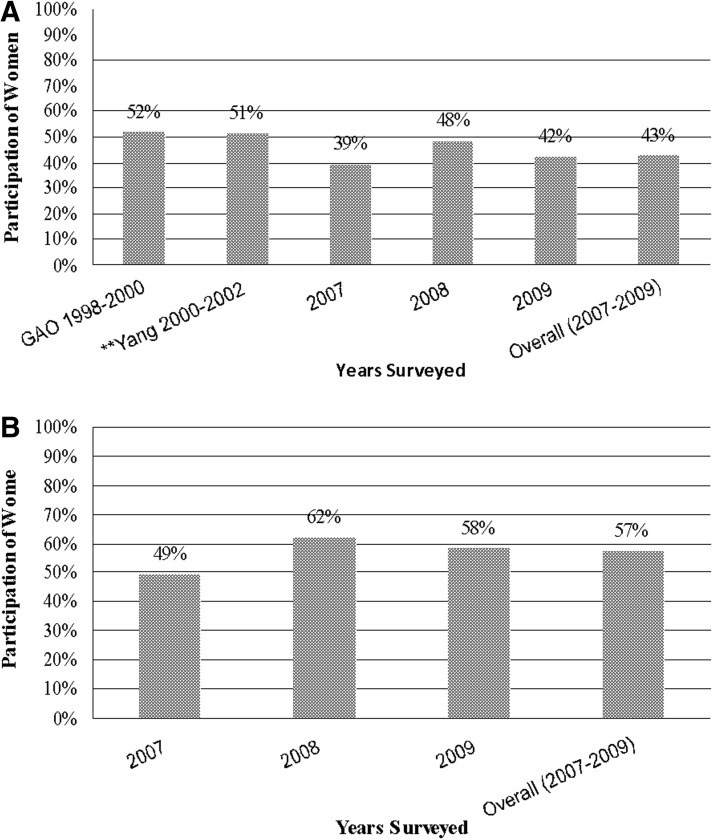
The remaining 50 NME NDAs and 11 NME BLAs contained 252 and 37 LPCTs respectively. Of the 252 NDA LPCTs evaluated for participation data, 240 (95.2%) LPCTs reported participants' sex information. In these LPCTs for NDAs, there were 113,420 enrolled participants, of which 60,420 (53.3%) were males, 49,057 (43.3%) were females, and 3.5% of the LPCTs had unspecified sex. All 37 (100%) of the BLA LPCTs reported participants' sex information. In the LPCTs of 11 NME BLAs surveyed, there were 12,886 enrolled participants, of which 5,441 (42.2%) were males, 7,407 (57.5%) were females, and 37 (0.3%) were unspecified. Women's participation was analyzed by year to determine trends. Average NDA LPCT participation was found to be 39.3% in 2007, 48.0% in 2008, and 42.0% in 2009 (Fig. 1A). Average participation in BLA LPCT was 48.5% in 2007, 61.6% in 2008, and 58.1% in 2009 (Fig. 1B).
FIG. 1.
Women's participation in new drug application (NDA) (A) and biologics license application (BLA) (B) late-phase clinical trials. **Yang et al. also included phase 4 (post-marketing) studies as LPCTs.16 GAO, U.S. Government Accountability Office.
Women's participation by indication
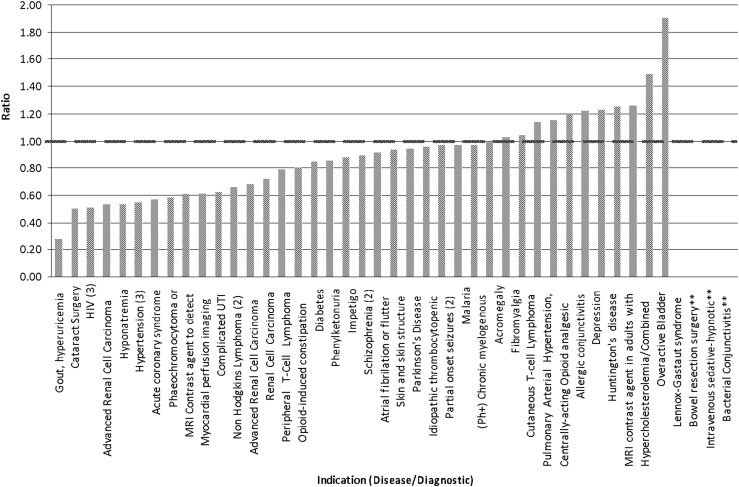
The prevalence/incidence data by sex obtained from the published literature was used to estimate the proportion of women in each disease population and the ratio described above for each NME (Table 2 and Table A1). A calculated ratio of 1 indicated that women's LPCT participation for a particular NME was equal to the proportion of women in the intended patient population of the NME. The ratios for NMEs that had the same indication were averaged prior to analysis. Four drugs (alvimopan, fospropofol disodium, besifloxacin HCl, and benzyl alcohol) were excluded from the ratio analysis because epidemiology data by sex for their indications could not be found (Table 3). Ratios of women's participation in NDA LPCTs compared with the estimated proportion of women in the disease population varied widely between 0.18 and 1.91, depending on the indication for the drug (Fig. 2). The gout drug febuxostat had the lowest participation to proportion ratio in women (ratio=0.28, proportion=18.9%, participation=5.3%). The ratio was also low for the three approved HIV drugs, with an average ratio of 0.48±0.03 (prevalence=25%, participation=12.0%). The highest ratio of women's participation to proportion in the disease population was for rufinimide, which is indicated for the treatment of seizures associated with Lennox-Gastaut syndrome (ratio=1.91, proportion=27.45%, participation=52.3%) and for fesoterodine fumarate, which is indicated for the treatment of overactive bladder (ratio=1.49, proportion=52.56%, participation=78.5%). Ten of the fifty (20%) NDAs had ratios of ≥1, indicating that the proportion of women enrolled in these NDA LPCTs was the same or higher than the proportion of women likely to use the products. Overall, 32 of the 50 NDAs (64%) had ratios of ≥0.80 indicating that the participation of women in these clinical trials were similar to or higher than the proportion of women in the disease populations in 64% of the NDAs.
Table 3:
New Molecular Entities for Which Sex Prevalence Data Were Unavailable
| Drug | Indication | Reason data was unavailable |
|---|---|---|
| Alvimopan | Peripherally acting μ-opioid receptor antagonist indicated to accelerate the time to upper and lower gastrointestinal recovery following partial large or small bowel resection surgery with primary anastomosis | Data could not be found for the sex prevalence of bowel resection surgery with primary anastomosis |
| Fospropofol disodium | Sedative-hypnotic agent indicated for monitored anesthesia care (MAC) sedation in adult patients undergoing diagnostic or therapeutic procedures | No specific disease or type of procedure indicated |
| Besifloxacin HCl | Indicated for the treatment of bacterial conjunctivitis | Data could not be found for the sex prevalence of bacterial conjunctivitis |
| Benzyl alcohol | Pediculocide indicated for the topical treatment of head lice infestation in patients 6 months of age and older | According to a CDC 2008 report, prevalence was higher in females in the Americas, but an exact figure was not reported. Ratio calculations could not be done.18 |
Indications have been abbreviated for this table; full, approved indications can be accessed from respective package inserts at drugs@FDA.gov.
FIG. 2.
Ratio of women's late-phase clinical trial (LPCT) participation to women's proportion in the disease population for approved NDAs by indication from 2007 to 2009. **Indicates that the background rate has not been studied or could not be determined for the disease. Ratio of 1 (dashed line) indicates that women's participation and proportion are equal. (2 or 3) indicates number of NMES, no number indicates 1 NME.
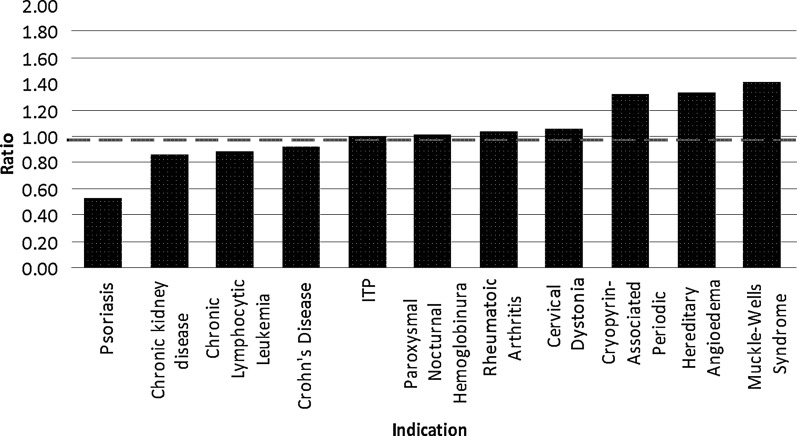
Similarly, the ratio of women's participation in BLA LPCTs compared with the women's proportion in the intended population varied between 0.53 and 1.42 (Fig. 3). Six biologics enrolled 65% or more women in their LPCTs. Seven of the eleven (64%) BLAs had ratios of ≥1, indicating that the proportion of women enrolled in BLA LPCTs is the same or higher than the proportion of women likely to use these products. The biologic indicated for the treatment of psoriasis, ustekinumab, had the lowest ratio of women's participation to proportion (0.53) while the biologic developed for the treatment of Muckle-Wells Syndrome, rilonacept, had the highest ratio (1.42). Ten of the eleven biologics had ratios ≥0.80 indicating that about 90% of the BLAs had women's participation similar to or higher than the proportion of women in the disease populations.
FIG. 3.
Ratio of women's LPCT participation to women's proportion in the disease population for approved BLAs by indication from 2007 to 2009. Ratio of 1 (dashed line) indicates that women's participation and proportion are equal. ITP, idiopathic thrombocytopenic purpura.
Women's participation by therapeutic category
The NDAs and BLAs approved during 2007 and 2009 were grouped in 16 therapeutic categories. There were between 1 and 8 drugs in each of the 16 therapeutic categories (Table 4). Women's participation ranged between 5.3% (rheumatology, gout) and 62.1% (special pathogens) of the participants in LPCTs when NDAs were categorized by therapeutic categories. NDAs approved for neurology, ophthalmology, psychiatry, and reproductive/urology indications had an average participation to proportion ratio greater than or equal to 1, indicating that the proportion of women represented in LPCTs was greater than or equal to the proportion of women expected to use the drugs approved in those categories. Drugs for antiviral and rheumatology indications (one NDA for gout) had women's participation (12% and 5.3% respectively) lower than the proportions of women in the disease populations (participation to proportion ratios of 0.48 and 0.28 respectively) (Table 4). The biologics were sorted into six therapeutic categories with one to four biologics in each category. Participation of women in BLA LPCTs ranged between 30.5% (oncology) and 83.2% (neurology). Of the therapeutic categories evaluated, five of the six categories had participation to proportion ratios of at least 0.88, with the exception of the dermatology category (ratio 0.53), demonstrating that BLA LPCTs enrolled study populations closely resembling the proportion of women in the intended patient populations in each drug category (Table 4).
Table 4.
Women's Participation in New Drug Application and Biologics License Application Late-Phase Clinical Trials by Therapeutic Category
| Therapeutic category (No. of NDAs) | Women participation% | Ratio* | Standard error | 95% confidence interval |
|---|---|---|---|---|
| Reproductive and urology (1) | 48.2 | 1.49 | N/A | N/A |
| Neurology (5) | 49.92 | 1.21 | 0.09 | 0.71–1.71 |
| Ophthalmology (1) | 59.4 | 1.19 | N/A | N/A |
| Psychiatry (3) | 50.73 | 1.0 | 0.12 | 0.50–1.50 |
| Special pathogens (1) | 62.1 | 0.96 | N/A | N/A |
| Hematology (1) | 61.4 | 0.96 | N/A | N/A |
| Metabolism and endocrinology (5) | 39.76 | 0.9 | 0.12 | 0.58–1.22 |
| Anesthesia/analgesia (3) | 60.73 | 0.89 | 0.20 | 0.05–1.73 |
| Drug oncology (8) | 35.65 | 0.83 | 0.09 | 0.61–1.03 |
| Anti-infective (3) | 40.4 | 0.81 | 0.10 | 0.39–1.23 |
| Gastroenterology (1) | 56.4 | 0.8 | N/A | N/A |
| Cardiovascular (6) | 42.65 | 0.79 | 0.09 | 0.55–1.03 |
| Medical imaging (4) | 32.95 | 0.76 | 0.17 | 0.23–1.29 |
| Antiviral (HIV) (3) | 12 | 0.48 | 0.03 | 0.35–0.61 |
| Rheumatology (gout) (1) | 5.3 | 0.28 | N/A | N/A |
| Therapeutic category (No. of BLAs) | ||||
|---|---|---|---|---|
| Rheumatology (4) | 67.3 | 1.28 | 0.08 | 1.03–1.53 |
| Neurology (1) | 83.2 | 1.05 | N/A | N/A |
| Hematology (3) | 53.9 | 0.96 | 0.05 | 0.76–1.16 |
| Gastroenterology (1) | 54.2 | 0.92 | N/A | N/A |
| Oncology (1) | 30.5 | 0.88 | N/A | N/A |
| Dermatology (1) | 31.3 | 0.53 | N/A | N/A |
Ratio comparing women's participation in studies to the prevalence/proportion of in the disease population.
NDAs, new drug applications; BLAs, biologics license applications.
Presentation of sex analyses
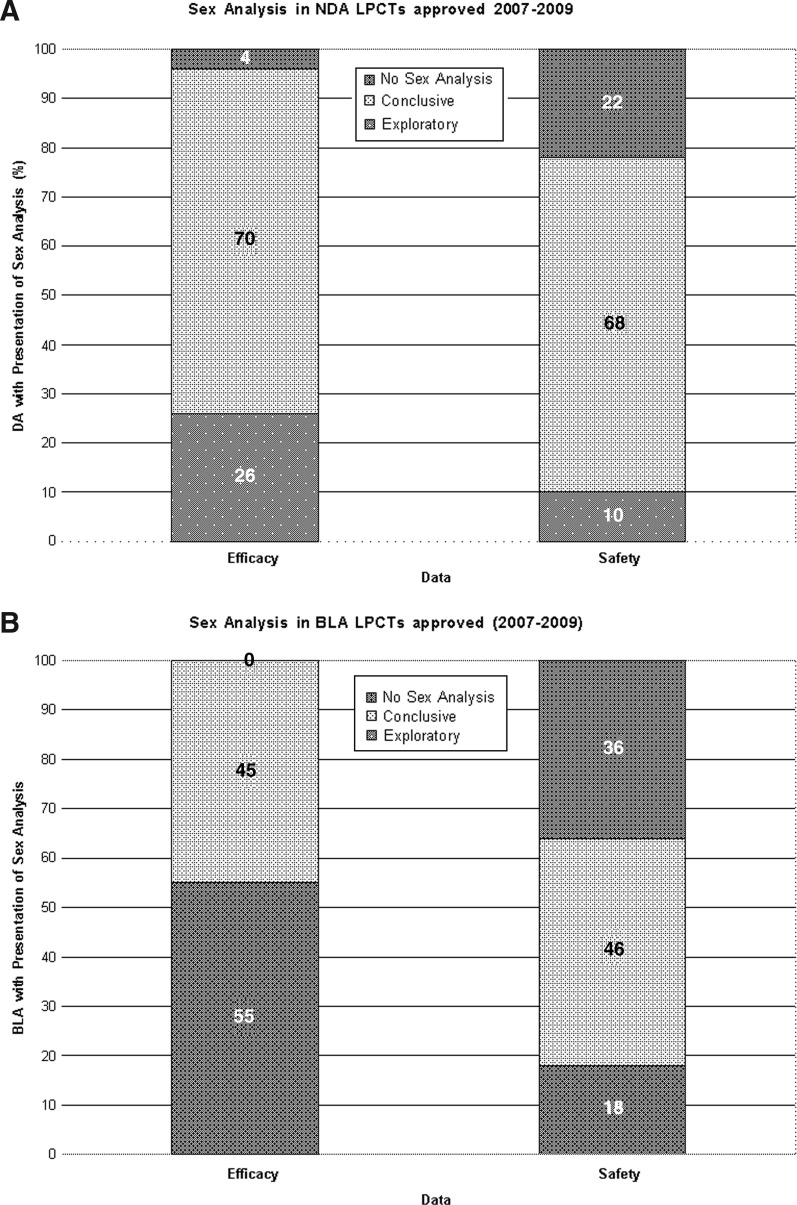
FDA medical and statistical reviews were evaluated for the presence of safety and efficacy analyses by sex (Table 5, Fig. 4). Out of 50 NDA reviews that were examined for sex analysis, 48 (96%) had sex-based efficacy analyses, 39 (78%) had sex-based safety analyses, and 37 (74%) had both safety and efficacy analyses by sex. All NDA reviews had at least one type (safety or efficacy) of analysis by sex. Of the 48 NDA reviews with efficacy analysis by sex, 13 (27%) had analyses coded as exploratory, and 35 (73%) had analyses coded as conclusive. Of the 39 NDA reviews with safety analysis by sex, 5 (13%) had analyses coded as exploratory, while 34 (87%) had analyses coded as conclusive. In 11 BLA reviews examined for sex analysis, all 11 (100%) had sex-based efficacy analyses, 7 (64%) had sex-based safety analyses, and 7 (64%) had both safety and efficacy analyses by sex. Of the 11 BLAs with efficacy analysis, 6 (54%) were coded as exploratory, and 5 (46%) had analyses coded as conclusive (Table 5). Of the 7 BLA reviews with safety analysis, 2 (29%) had analyses coded as exploratory while 5 (71%) had analyses coded as conclusive. As previously mentioned, these coding criteria were based on the FDA reviewers' comments on sex analyses found in medical and statistical reviews accessed from Drugs@FDA.
Table 5.
Presentation of Sex Analyses in Food and Drug Administration Reviews
| NDA (n=50) | Safety | Efficacy |
|---|---|---|
| Presentation of sex analysis | 39 (78%) | 48 (96%) |
| • Exploratory analysis | 5 | 13 |
| • Conclusive analysis | 34 | 35 |
| No sex analysis | 11 (22%) | 2 (4%) |
| Both safety and efficacy | 37 (74%) | |
| BLA (n=11) | Safety | Efficacy |
|---|---|---|
| Presentation of sex analysis | 7 (64%) | 11 (100%) |
| • Exploratory analysis | 2 | 6 |
| • Conclusive analysis | 5 | 5 |
| No sex analysis | 4 (36%) | 0 (0%) |
| Both safety and efficacy | 7 (64%) | |
FIG. 4.
Presentation of sex analyses in U.S. Food and Drug Administration reviews for NDAs (A) and BLAs (B) approved between 2007 and 2009.
Discussion
Participation of women in NDA late-phase clinical trials
Reports on the participation of women in clinical trials have often expressed the extent of participation as a percentage of the total number of participants included in the trial. In the current study we have—in addition to this—included a ratio that relates the women's proportion in the disease population to their participation in the LPCTs, which may give a better measure of the relative participation of women. Women's participation in LPCTs of approved NME drugs has remained between 40% and 50% based on reports in the last 10 years (Fig. 1). Differences in women's participation for NMEs approved each year may partly be due to sex differences in disease prevalence related to drug indications for drugs approved in each year. Compared with previous reports by GAO 2001 (52%)14 and Yang, et al. (51%),16 women's participation in LPCTs as determined by the current study (43.3%) showed a slight decrease. However, when participation was compared with the proportion of women in the disease population for the drugs indicated, 64% of the drugs had ratios ≥0.80, and 20% of the drugs had ratios ≥1.0 (Table 4). In 1992, the GAO determined that for more than 60% of the drugs, the representation of women in the study population was less than the representation of women in the population with the corresponding disease.13 These data indicate that although the overall average participation appears lower for the study period (2007–2009) compared with previous reports, a majority of the clinical trials enrolled numbers of women similar to the proportion of women expected to use the approved drug. Of note, between 2007 and 2009, three new HIV drugs were approved by the FDA, with an average of 12% women participating in the LPCTs of these drugs. The data indicated that participation of women in HIV drug trials is low (12%) and has decreased compared with the 20% that was reported for randomized controlled clinical trials of antiretroviral therapy products approved by the FDA from 2000 to 2008 by Soon et al.,19 and the participation rate is lower than the disease prevalence; in 2008, the CDC reported that women make up 25% of HIV patients living in the United States.20 Although gout is a disease that predominately affects men, disease prevalence in women has increased in the last few decades, and a 2004 study reported that about 30% of the patients are women.21 However, a gout drug approved in 2009 enrolled a study population of 5.3% female patients. At the time of review and approval of the drug, the FDA recommended that post-marketing studies be conducted and stated, “The trial should enroll adequate numbers of women because the safety data on febuxostat in women is limited; only 5% of the enrolled patients were women. While there are a variety of reasons why relatively few women were enrolled in phase 3 trials, a post-marketing study could enroll larger numbers of women.”22
Participation of women in BLA late-phase clinical trials
A study conducted by the CBER showed that women's representation in biologic vaccine, blood, and therapeutic clinical trials between 1995 and 1999 was 45.3%.17 The CBER study reported that the population enrolled in the clinical trials was reflective of the population expected to receive the biologic. However, the study did not differentiate participation by clinical trial phase or category of biologic therapeutics, while the current study focused only on LPCTs of biologic therapeutic products. In this current study, the LPCTs of biologics appear to enroll women in numbers similar to disease prevalence as evidenced by the fact that 10 of the 11 enrolled female populations closely resembled the proportion of women in the indicated population with a ratio of at least 0.80 (Fig. 3). However, nine of the biologics were approved for conditions that affect more women than men or affect both sexes equally (Table 2 and Table A1). This may have contributed to the high representation of women in BLA LPCTs. Furthermore, 73% (8 of 11) of the biologics were approved with orphan status to treat diseases that affect fewer than 200,000 people according to the Orphan Drug Act, 1983.23 Sex analyses on the small numbers of patients in the LPCTs of these biologics may not have been statistically powered to draw sex-based efficacy and safety conclusions. This could explain the low percentage of conclusive sex analyses by safety (5 out of 7, 71%) and efficacy (5 out of 11, 45%) in biologic LPCTs (Table 5).
Presentation of sex analyses in FDA reviews
The 1992 GAO report showed less than 50% of the approved drugs had sex-based analyses on the efficacy and safety outcomes of the drug.13 After the 1998 IND and NDA regulations requiring submission of participation data and analyses by sex,24 both the 2001 GAO report14and Yang et al.'s 2009 study16 concluded that sex analyses in FDA reviews from 1998–2002 had increased to more than 70%. The current study revealed that presentation of sex-based analysis in the FDA reviews of drugs between 2007 and 2009 has remained consistent in the last 10 years at around 74% (Table 5). The previous CBER biologic study of therapeutic biologics approved between 1995 and 1999 had shown a 37% sex-based analyses of phase 3 BLA clinical trial data.17 The presentation of sex analyses in approved BLAs has increased according to the findings of the present study. Of the 11 therapeutic biologics included in this study, 100% of the biologics had sex-based efficacy analyses, and 64% of the biologics had both safety and efficacy analyses by sex in their FDA reviews (Table 5).
Limitations and implications
It should be noted that our study was limited only to late-phase clinical studies that supported the approval of the NMEs. Early-phase studies, studies that supported the approval of new indications for existing approved drugs, and studies for NDAs and BLAs not approved were not included in our evaluation. It is therefore not representative of all the studies that were submitted to FDA from January 2007 to December 2009.
There are some limitations in deriving the prevalence or incidence data for certain diseases for the calculations reported in Table 2 and Table A1. These limitations include the following:
The population used to derive the prevalence or incidence data may not be similar to the population included in the LPCTs for a particular NME. For example, the age distribution for the NME LPCTs may be different from that used to derive the prevalence or incidence rate data.
ome of the available data were reported as actual number of men and women in the diseased population, while other data were reported as incidence rates or ratios, and these numbers were used to estimate the women's proportion in the disease populations.
A limitation in this study was the coding criteria used to track sex-based analyses on efficacy and/or safety presented in FDA reviews. Since the data were accessed as a third party, coding was dependent on the data available in the reviewer's comments on sex analyses. The types of sex analyses observed in the reviews varied from summary data tables displaying statistical analyses concluding sex differences in clinical outcomes to summary statements describing observational data that indicated presence or absence of sex differences in safety and efficacy of a drug or biologic product.
Conclusions
This study has shown that women's participation in clinical drugs and biologics trials are in the range of 40% to 50%, with certain disease areas representing higher or lower participation. As examples, overactive bladder and depression reflect higher participation, while HIV shows lower participation of women despite the fact that women make up 25% of HIV patients living in the United States.20 The latter observation of lower participation in HIV trials warrants further attention by clinical investigators. Our study also shows an increase in the sex-based analysis for safety and efficacy in clinical trials, which is a reflection of the impact of guidances and regulations implemented by the FDA as well as the recognition that patient demographics are important considerations in individualized dosing strategy. Overall, this study provides the current trends in the participation and analysis of women and men in clinical trials and adds to the previous findings from GAO and other authors. The FDA continues to encourage pharmaceutical sponsors to look for innovative ways to overcome the barriers to the recruitment and retention of women in clinical trials. The issue was the focus of a 2011 symposium, Dialogues on Diversifying Clinical Trials, organized by the FDA Office of Women's Health and the Society for Women's Health Research (SWHR) that brought patients, patient advocacy groups, clinicians, regulators, and industry participants together for a 2-day discussion.25 Another initiative by the FDA that would enable efficient estimation of women's participation in clinical trials and help understand adverse outcomes in women and other subgroup populations is the FDA's data standardization initiative.26,27 Through this initiative, clinical trial data submitted to the FDA are being harmonized for consistency by adopting the standards from the Clinical Data Interchange Consortium,28 which will enable pooling of data from several studies for efficient queries of women's participation in clinical trials and assessment of sex differences in the safety and efficacy outcomes of drug products which is a difficult task if each dataset from the clinical trials were to be analyzed separately.
Table A1.
Additional Examples of Estimation of Women's Proportion in the Disease Population
| Disease | Unit | Male | Female | Sum | %P | Source | Notes |
|---|---|---|---|---|---|---|---|
| Neuroblastoma | Cases | 891 | 773 | 1664 | 46.5 | Navalkele P, O'Dorisio MS, Zamba GK, Lynch CF. Incidence, survival, and prevalence of neuroendocrine tumors versus neuroblastoma in children and young adults: Nine standard SEER registries, 1975–2006. Pediatr Blood Cancer 2011;56:50–57. | Using SEER Registry |
| Idiopathic thrombocytopenic purpura | Incidence (per 100,000) | 6.1 | 11.3 | 17.4 | 64.9 | Segal JR, Powe NR. Prevalence of immune thrombocytopenia: Analyses of adminstrative data. J Thromb Haemos 2006;4: 2377–2383. | Maryland Health Care Commission data |
| Peripheral T-cell lymphoma | Cases | 58 | 38 | 2.5 | 40.0 | Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): Results from the non-Hodgkin's lymphoma classification project. Ann Oncol 2002;13:140–149. | Cohort study (rare disease) |
| Atrial flutter/fibrillation | Cases | 133552 | 109351 | 242903 | 45.0 | Lloyd-Jones D, Adams RJ, Brown TM, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215 | Using CDC and NIH data for prevalence |
| Gout | Cases | 4975 | 1158 | 6133 | 18.9 | Harrold LR. Yood R A, Mikuls TR, et al. Sex differences in gout epidemiology: Evaluation and treatment. Ann Rheum Dis 2006;65:1368–1372. | Data from seven managed care plans |
| Fibromyalgia | Prevalence (%) | 0.5% | 3.4% | 0.039 | 87.2 | Centers for Disease Control and Prevention. Fibromyalgia. Retrieved from: http://www.cdc.gov/arthritis/basics/fibromyalgia.htm Accessed December 20, 2012. | CDC data |
| Paroxysmal nocturna hemoglobinuria | Ratio | 1 | 1 | 2 | 50.0 | FDA Pharmacology Review, BLA 125166 (Eculizumab), “Equal sex distribution”, http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/125166s0000_PharmR.pdf | Rare disease |
| Chronic kidney disease | Prevalence (%) | 17.6 | 16.7 | 34.3 | 48.7 | Centers for Disease Control and Prevention. Prevalence of chronic kidney disease and associated risk factors—United States, 1999–2004. (2007). MMWR Morb Mortal Wkly Rep 2007;56:161–165. | from NHANES |
| Crohn's disease | Cases | 3480.42 | 5912 | 58.9 | Loftus EV, Schoenfeld P, Sandborn WJ. The epidemiology and natural histroy of Crohn's disease in population-based patient cohorts from North America: A systematic review. Aliment Pharmacol Ther 2002;16:51–60. | From 10 cohort studies | |
| Chronic lymphocytic leukemia | Incidence | 5.7 | 3 | 8.7 | 34.5 | National Cancer Institute. Chronic lymphocytic leukemia. Retrieved from SurveillanceEpidemiology and End Results database at: http://seer.cancer.gov/statfacts/html/clyl.html/ Accessed December 20, 2012. | SEER database |
| Impetigo | Average incidence (per 100,000) | 10.5 | 13.1 | 23.6 | 55.5 | Elliot AJ, Cross KW, Smith GE, Burgess IF, Fleming DM. The association between impetigo, insect bites, and air temperature: A retrospective 5-year study (1999–2003) using morbidity data collected from a sentinel general practice network database. Family Practice 2006;23:490–496. | from Sentinel General Practice Network |
| Cutaneous T-cell lymphoma | Cases | 2449 | 1580 | 4029 | 39.2 | Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dematol 2007;143:854–859. | from SEER registries |
| Cryopyrin-associated periodic syndromes | Ratio | 1 | 1 | 2 | 50.0 | Shinawi M, Scaglia F. Hereditary periodic fever syndrome. Retrieved from Medscape Reference website: http://emedicine.medscape.com/article/952254-overview#a1 Accessed July 16, 2012. | Rare disease (males and females equally affected) |
| Psoriasis | cases | 244 | 357 | 601 | 59.4 | Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004;9:136–139. | Population sample survey |
| Cervical dystonia | Cases | 52 | 196 | 248 | 79.0 | Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism Relat Disord 2007;13:411–416. | Census weighted sample of 60,062. |
| Rheumatoid arthritis | Prevalence (%) | 0.61 | 1.06 | 1.67 | 63.5 | Helmick C, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum 2008;58:15–25. | from NHANES data |
%P, Estimated proportion of women in the overall disease population; CDC, Centers for Disease Control and Prevention; NCI, National Cancer Institute; NHANES, National Health and Nutrition Examination Survey; NIH, National Institutes of Health.
Acknowledgments
The authors thank Dr. Leslie Chinn, Ms. Onyekachi Otugo and Ms. Noha Eshera for assistance in the preparation of the manuscript. The manuscript reflects the views of the authors and should not be construed to represent the FDA's views or policies.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Anderson GD. Sex and Racial differences in pharmacological response: Where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 2005;14:19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- 2.Benton R. Sale M. Flockhart D. Woosley R. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67:413–418. doi: 10.1067/mcp.2000.105761. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan V. Krahn AD. Walker B. Klein GJ. Skanes A. Yee R. Sex differences in QTc interval and QT dispersion: Dynamics during exercise and recovery in healthy subjects. Am Heart J. 2002;114:858–864. doi: 10.1067/mhj.2002.125619. [DOI] [PubMed] [Google Scholar]
- 4.Rautaharju PM. Zhou SH. Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 5.Kahn SE. Zinman B. Lachin JM, et al. Diabetes Outcome Progression Trial (ADOPT) Study Group. Rosiglitazone-associated fractures in type 2 diabetes: An analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 6.Alexander K. Chen A. Roe M, et al. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 7.Campbell N. Hull R. Brant R, et al. Different effects of heparin in males and females. Clin Invest Med. 1998;l21:71–78. [PubMed] [Google Scholar]
- 8.U.S. Government Accountability Office (GAO) General accounting report, 2001 . Drug safety: Most drugs withdrawn in recent years had greater health risks for women. http://www.gao.gov/new.items/d01286r.pdf. [Dec 20;2011 ]. http://www.gao.gov/new.items/d01286r.pdf
- 9.UNSW Embryology. Abnormal development: Thalidomide. http://embryology.med.unsw.edu.au/Defect/page5i.htm. [Dec 20;2011 ]. http://embryology.med.unsw.edu.au/Defect/page5i.htm
- 10.U.S. Food and Drug Administration (FDA) General considerations for the clinical evaluation of drugs, 1977. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071682.pdf. [Dec 23;2011 ]. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071682.pdf HEW Publication No. (FDA) 77-3040.
- 11.Pinn V. The role of NIH Office of Research on Women's Health. Acad Med. 1994;69:698–702. doi: 10.1097/00001888-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. Guideline for the study and evaluation of gender differences in clinical trials. 1993. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072044.pdf. [Aug 20;2012 ]. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072044.pdf
- 13.U.S. Government Accountability Office (GAO) Women's health: FDA needs to ensure more study of gender differences in prescription drug testing. HRD-93-17. 1992. http://archive.gao.gov/d35t11/147861.pdf. [Dec 28;2011 ]. http://archive.gao.gov/d35t11/147861.pdf
- 14.U.S. Government Accountability Office (GAO) Women's health: Women sufficiently represented in new drug testing, but FDA oversight needs improvement. 2001. www.gao.gov/new.items/d01754.pdf. [Dec 28;2011 ]. www.gao.gov/new.items/d01754.pdf GAO-01-754.
- 15.Evelyn B. Toigo T. Banks D, et al. Women's participation in clinical trials and sex-related labeling: A review of new molecular entities approved 1995–1999. 2001. www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ParticipatinginClinicalTrials/ucm197788.htm. [Dec 28;2011 ]. www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ParticipatinginClinicalTrials/ucm197788.htm [PMC free article] [PubMed]
- 16.Yang Y. Carlin A. Faustino P. Pagán-Motta P. Hamad M. He R, et al. Participation of women in clinical trials for new drugs approved by the Food and Drug Administration between 2000 and 2002. J Womens Health (Larchmt) 2009;18:303–310. doi: 10.1089/jwh.2008.0971. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration. Participation of females in clinical trials and gender analysis of data in biologic product applications. 2009. www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/InvestigationalNewDrugINDorDeviceExemptionIDEProcess/ucm094300.htm. [Dec 28;2011 ]. www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/InvestigationalNewDrugINDorDeviceExemptionIDEProcess/ucm094300.htm
- 18.Falagas ME. Matthaiou DK. Rafailidis PI. Panos G. Pappas G. Worldwide prevalence of head lice [letter] Emerg Infect Dis. 2008. www.cdc.gov/EID/content/14/9/1493.htm. [Dec 28;2011 ]. pp. 1493–1494.www.cdc.gov/EID/content/14/9/1493.htm [DOI] [PMC free article] [PubMed]
- 19.Soon G. Min M. Struble KA, et al. Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008) AIDS Patient Care STDs. 2012;8:444–453. doi: 10.1089/apc.2011.0278. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. HIV among women. Aug, 2011. [Jul 30;2012 ]. www.cdc.gov/hiv/topics/women/index.htm www.cdc.gov/hiv/topics/women/index.htm
- 21.Wallace KL. Riedel AA. Joseph-Ridge N. Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–1587. [PubMed] [Google Scholar]
- 22.FDA Clinical review of Febuxostat (Uloric®) www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s 000_MedR_P1.pdf. [Dec 20;2012 ]. www.accessdata.fda.gov/drugsatfda_docs/nda/2009/021856s 000_MedR_P1.pdf
- 23.U.S. Food and Drug Administration. Orphan Drug Act. 1983. www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/orphandrugact/default.htm. [Dec 28;2012 ]. www.fda.gov/regulatoryinformation/legislation/federalfooddrugandcosmeticactfdcact/significantamendmentstothefdcact/orphandrugact/default.htm
- 24.U.S. Department of Health and Human Services, Food and Drug Administration. Investigational new drug applications and new drug applications, final rule. Fed Regist. 1998;63(8):6854–6862. [PubMed] [Google Scholar]
- 25.Coakley M. Fadiran EO. Parrish LJ, et al. Dialogues on diversifying clinical trials: Successful strategies for engaging women and minorities in clinical trials. J Womens Health. 2012;2012;21:713–716. doi: 10.1089/jwh.2012.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slides for the November 15, 2010 meeting of the Science Board to the FDA. www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/ScienceBoardtotheFoodandDrugAdministration/ucm233252.htm. [Aug 20;2012 ]. www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/ScienceBoardtotheFoodandDrugAdministration/ucm233252.htm
- 27.Food and Drug Administration. CDER data standards plan version 1.0—Draft. www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/UCM214120.pdf. [Aug 20;2012 ]. www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/UCM214120.pdf
- 28.Written notice of participation by the Clinical Data Interchange Standards Consortium (CDISC) and written statement for discussion topics to be addressed in the FDA public hearing: Electronic submission of regulatory information and creating an electronic platform for enhanced information management. www.fda.gov/ohrms/dockets/dockets/06n0464/06N-0464-EC10-Attach-1.pdf. [Aug 20;2012 ]. www.fda.gov/ohrms/dockets/dockets/06n0464/06N-0464-EC10-Attach-1.pdf