Abstract
Published studies of Hh (Hedgehog) signaling in the developing prostate have reported varying and discrepant effects on epithelial proliferation, ductal morphogenesis and growth. We report here that these differing observations accrue from stage-specific effects of Hh signaling in the developing prostate. Using in vitro organ culture of the E16 UGS and P1 prostate, we show that ectopic Hh pathway activation stimulates epithelial proliferation prenatally, but inhibits epithelial proliferation postanatally. Extrapolating from previously published observations that Hh target gene expression is altered in the reactive stroma of prostate cancer, we examined and found discordant regulation of a subset of target genes by Hh signaling in the prenatal and postnatal prostate. Cell based studies and recombination assays show that these changes are not simply attributable to the age of the mesenchyme or the epithelium, but more likely reflect a complex regulation by the cellular microenvironment. To determine the in vivo relevance of these observations, we examined the effect of transgenic activation of Hh signaling on epithelial proliferation in the prenatal and postnatal prostate and confirmed the operation of stage-specific effects. These observations demonstrate stage-specific differences in the effect of Hh signaling on epithelial proliferation in the developing prostate and suggest that these are a product of complex interactions determined by the cellular microenvironment.
Keywords: Prostate, Development, Hedgehog signaling, Proliferation
INTRODUCTION
The male sex accessory gland prostate develops from the urogenital sinus (UGS) and is located between the base of bladder and urethra. Its development has been widely studied using rodents as models. Prostate development is initiated at E16 /E18 (E16 in mouse and E18 in rat), when solid prostatic buds emerge from the UGE and invade into the surrounding mesenchyme to form the prostatic ductal buds. These buds undergo elongation ,branching , cell differentiation and ductal canalization and form the prostatic ducts of the ventral, dorsolateral and anterior lobes of the adult prostate. The complex process of prostate development is androgen dependent and involves mesenchymal-epithelial interactions mediated by multiple signaling pathways.
Hedgehog (Hh) signaling controls multiple cellular events including patterning, proliferation and differentiation in a variety of embryonic developmental processes. There are three Hh ligands in mammals: Sonic hedgehog (shh), Indian Hedgehog (Ihh) and Desert Hedgehog (dhh). All three ligands bind to the transmembrane receptor, Patched (Ptc). Binding of Hh ligand to Ptc relieves repression of another transmembrane protein, Smoothened (Krstic et al.), activates the Hh signal transduction pathway and results in activation of the Gli zinc-finger family of transcription factors and transcription of Hh target genes. Gli1 and Ptc1 are two conserved target genes and increase in their expression is considered a reliable indicator of the pathway activation.
Of the two Hh ligands (Shh and Ihh) both expressed in the developing prostate; Shh is the more abundant. Localization studies showed that Shh is expressed in the prostate epithelial ducts, concentrated in the ductal tips, while its functional genes Gli1 and Ptc1 are expressed in the surrounding mesenchyme. This pattern indicates paracrine signaling from the epithelium to the mesenchyme. Gene analysis studies revealed that the expression of Shh is most abundant from E16 to P1, which is a crucial window for early prostatic duct formation, and then gradually decreases to a very low level in the adult stage. This expression pattern suggests a potential role of Hh signaling in regulation of early prostate development. The very first study using a polyclonal neutralizing Shh antibody showed that treatment of the grafted E15.5 UGS with neutralizing Shh antibody prevented formation of prostatic ducts (Podlasek et al., 1999). This observation indicated Hh signaling is required for prostatic ducts induction. Consistently, chemical inhibition of Hh signaling by cyclopamine in E14 explants reduced the ductal tips number and decreased cell proliferation in both epithelium and mesenchyme (Lamm et al., 2002). Those observations support a stimulatory role of Hh signaling in controlling the prostate morphogenesis, In contrast, Hh signaling was showed to inhibit both ductal branching and cell proliferation in P2 rat ventral prostate (VP) explants, suggest a negative regulation of Hh signaling on prostate morphogenesis (Wang et al., 2003). These authors found decreased epithelial proliferation and duct tips when explants were cultured in the presence of exogenous Shh, and a corresponding increase in cell proliferation and duct tips when cultured in the presence of the Hh signaling inhibitor cyclopamine. Further, when P2 VP was co-cultured with UGM cells that overexpressed activated Gli1 or Smo, the effects on ductal branching and proliferation were found to mimic the results obtained with exogenous Shh ligand. Another study using rat P0 VP explants found a suppressive effect of Hh signaling on ductal branching, but yielded inconsistent observations on epithelial cell proliferation (Freestone et al., 2003). A later study found no effect of either cyclopamine or Shh on duct tip number in cultured E16.5 mouse UGS explants (Berman et al., 2004).
We revisited the role of Hh signaling on early prostate development, focusing on its influences on epithelial proliferation and ductal branching. We utilized chemical inhibition of cultured tissues, genetic activation of the Hh pathway in stromal cells cultured together with tissues in vitro tissue and transgenic mice with conditional activation of the Hh pathways to compare the effects of paracrine signaling pre- and postnatal. These studies showed that Hh signaling exerts unique stage-specific effects on epithelial proliferation and prostatic ductal branching morphogenesis that correlated with stage-dependent changes in Hh target gene regulation by Hh signaling.
MATERIALS AND METHODS
Explant culture, primary mesenchymal cell culture and tissue recombination
E16 and P1 UGS tissues were collected from C57Bl/6 mice (Charles River) and then placed on Millicell-CM filters (Millipore) suspended on serum-free medium with 10−8 M DHT and other supplements as described before(Doles et al., 2006). The medium was changed every other day. For the co-culture experiment, the E16 and P1 UGS tissues were cultured together with the UGSM-2 cells infected with retrovirus that expressed GFP (Green fluorescent protein) and activated SmoM2 or Gli2 at a ratio of 1×105 UGSM-2 cells per UGS tissue. Primary UGM cells were freshly isolated from the mouse E16/P1 UGS mesenchyme as described. Primary UGM cells were placed in cell culture dishes with Dulbecco’s modified Eagles’ medium/F12 containing 10% fetal bovine serum, allowing 1-week culture to reach the confluence before exposure to Shh. For the tissue recombination studies, the mesenchymal-epithelium separations were done on the E18/P1 UGS of Sprague Dawley rats (Charles River) and E16/P1 UGS of C57bl mice as described. Basically, the UGS tissue were incubated with 1% trypsin at 4°C for 75min, followed by mechanical dissociation. The recombinants were constructed by wrapping the mouse E16/P1 UGM around the rat E18/P1 UGE tube, placed together on the tissue culture inserts, followed by 7 days incubation in the serum-free defined media supplemented with 10−8 M DHT.
AZ75 (AstraZeneca) (a cyclopamine derivative that binds to Smo as a specific inhibitor of Hh signaling) was dissolved in 95% EtOH and used in concentration at 0.5uM; Exogenous Shh (Curis) was dissolved in PBS/0.1% BSA and diluted in the culture media to 5nM as final concentration.
Explants were photographed under the dissection microscope (Zeiss, Diagnostic) to quantitate the number of ductal tips(Lamm et al., 2001).
Real-time RT-PCR gene analysis
RNA was harvested from cultured tissues and UGM cells with RNeasy Mini kit (Qiagen, Valencia, CA) with optional DNase digestion to eliminate DNA contamination, followed by reverse transcription and real-time PCR as described before(Doles et al., 2006). PCR primers used are the same as listed in the previous study(Yu et al., 2009). Results were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels. All experiments were performed in triplicate and reported differences were statistically significant (p < 0.05; Student’s t test).
Transgenic Mice and genotyping
Smo. YFP mice were obtained from the Jackson Laboratory. Fsp1.cre mice were obtained from Dr. Bhowmick (University of Vanderbilt, Nashville, TN). Rosa26 reporter mice were provided by Dr.Sun (University of Wisconsin-Madison, Madison,WI). DNA was isolatd from mouse tail and genotyped by PCR analysis using the following primers:
Fsp1,cre: AGGTGTAGAGAAGGCACTTAGC (forward),
CTAATCGCCATCTTCCAGCAGG (reverse), 411bp;
SmoM2.YFP: AAGTTCATCTGC ACCACCG (mutant forward),
TCCTTGAAGAAGATGGTGCG (mutant reverse),173bp;
CGTGATCTGCAACTCCAGTC (wild type forward),
GGAGCGGGAGAAATGGATATG (wild type reverse), 410bp.
The SmoM2.YFP; Fsp1.cre mutants are showing positive bands with both cre and SmoM2.YFP mutant; the rest of the littermates are considered as controls.
BrdU incorperation and Immunohistochemistry
To visualize the proliferation cells in the explants cultured tissues, 5-bromo -2′-deoxyuridine (BrdU) labeling medium (1:1000, Roche Applied Science) was added to the culture media 2 hours before fixing the tissues. In the transgenic mice model, BrdU was injected into the dam (prenatal E18) or P10 mouse (10ul undiluted per gram body weight, i.p.) 1hr before euthanasia. Explants or UGS tissues were collected and fixed in 10% formalin, followed by paraffin embedding and sectioning (5um/section) to perform immunofluorescence staining as described(Cook et al., 2007). The following primary antibodies were applied: mouse anti-BrdU (1:10, Roche Applied Science), Rabbit anti-PanCk (1;50) Rabbit anti-P63 ( 1:100 ,Santa Cruz), Sections were counterstained with Hoechst, cover slipped and imaged by Olympus model BX51 fluorescent microscope. To calculate the proliferation index, 10-15 digital images were randomly selected from each group. BrdU positive cells, P63 or PanCk positive cell were counted in each image and the proliferation index in the epithelium were obtained by calculating the ratio of the BrdU labeled epithelial cells to the total epithelial cells. For statistical comparisons, BrdU labeling index was analyzed by two-tailed Student’s t-test.
X-gal Staining
Prostate tissues were collected and fixed (37% formaldehyde, 50% glutaraldehyde (Sigma), 10% NP40 (Calbiochem) in PBS) for 30min, followed by cyroprotection step with 30% sucrose overnight immerse. The processed tissues were then embedded in the optimum cutting temperature (OCT) compound for sectioning, Tissue sections were then incubated with X-gal reaction buffer (2 mmol/L magnesium chloride (Sigma), 0.2% IGEPAL CA-630 (Sigma), 0.1% sodium deoxycholic acid (Calbiochem), 5uM potassium ferrocyandine, 5uM potassium ferricynaide, 1mg/ml X-gal substrate (Fisher Scientific) in PBS) overnight in 37 °C water bath protected from light. The X-gal stained tissue section was washed in PBS, counterstained with fast-red nucleus staining, cover slipped and imaged (Olympus, BX-41).
Tissue microdissection
Ventral and dorsalateral lobes were removed from the P21 mutant and control littermates. Microdissection were performed as previously described(Sugimura et al., 1986). Briefly, each prostate lobe was incubated in 1% collagenase for 5-10min, and then was microdissected into 2-dimension with fine forceps under the microscope. Quantitative of dutal tips were performed as previously described.(Podlasek et al., 1997; Sugimura et al., 1986)
RESULTS
Differential effects of Hh signaling inhibition on prostate ductal branching in explant culture
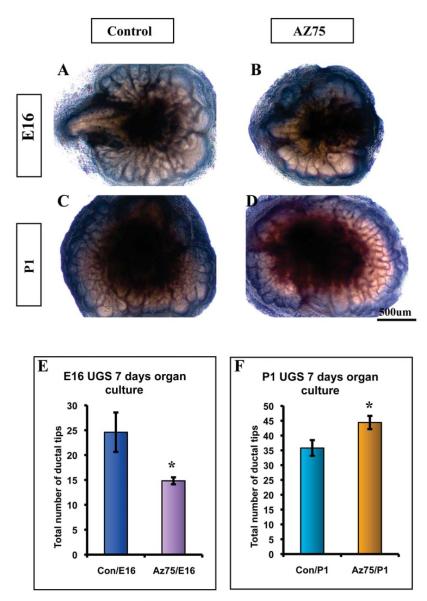
To compare the effect of Hh signaling on ductal budding and branching morphogenesis of the prenatal and postnatal prostate, we cultured the E16 UGS and P1 prostate in vitro with testosterone supplemented serum free media in the presence and absence of a Hh inhibitor. The cultured tissues were examined daily and the total number of duct tips counted by a blinded observer after seven days in culture. We observed nothing to suggest a toxic effect of AZ75. Blockade of Hh signaling by AZ75 was confirmed by RT-PCR analysis of Gli1 expression in the cultured tissues at the conclusion of the experiment. Comparison of the cultured E16 UGS revealed a significantly decreased number of duct tips in tissues cultured in the presence of AZ75. This finding replicates our previously published data that ductal budding in the E16 UGS was inhibited by the plant-derived Hh inhibitor cyclopamine. By contrast, AZ75 significantly increased the number of duct tips in the cultured P1 explants (Fig 1). This finding is consistent with the reported stimulation of branching morphogenesis in the postnatal rat prostate reported by Wang et al(Wang et al., 2003).
Figure 1. Chemical inhibition of Hh signaling exerts differential effects on ductal morphogenesis in prenatal and postnatal explants.
E16 UGS (A-B) and P1 prostate (C-D) tissues were cultured +/− Hh inhibitor AZ75 (0.5uM). Images were taken on the 7th day of culture. (E-F) Quantitative comparison of total duct tips. Duct tips were counted from images taken on the 7th day of culture. This revealed that culture in the presence of AZ75 decreased the number of tips in E16 UGS (E) while increasing the number of tips in P1 Prostate tissues (F). n=4, P<0.05. Scale Bar: 500uM.
Differential effect of activated paracrine Hh signaling on prostate ductal proliferation in explant co-culture
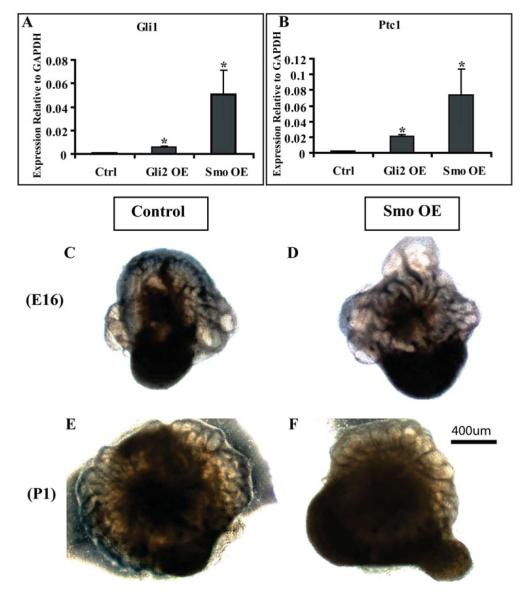
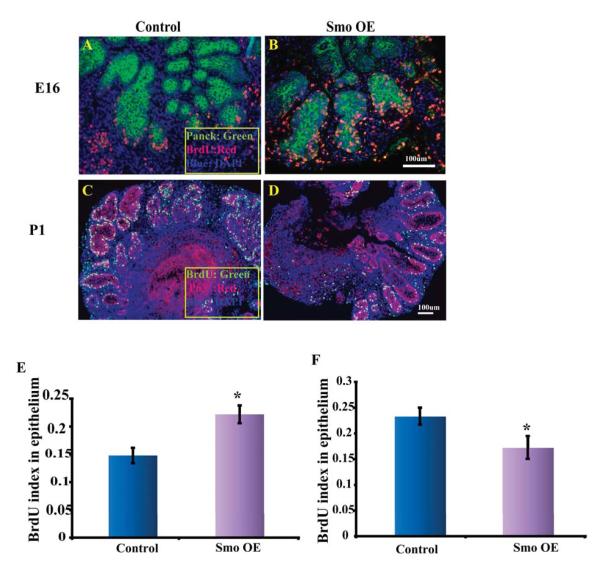
In our previous study of Hh signaling in prostate development, we observed that inhibition of ductal budding in the E16 UGS was associated with an inhibition of epithelial proliferation (Lamm et al., 2002). In contrast, the studies by Freestone and Wang suggested that inhibition of Hh signaling actually increased epithelial proliferation in the postnatal prostate (Freestone et al., 2003; Wang et al., 2003). These observations suggest stage-specific effects on epithelial proliferation that parallel the differential effects on ductal morphogenesis. Chemical inhibition of Hh signaling could affect epithelial proliferation by blocking autocrine Hh signaling, blocking paracrine signaling or blocking both. To specifically examine the effect of paracrine Hh signaling on epithelial proliferation in the prenatal and postnatal prostate we performed co-culturing of the E16 UGS and P1 prostate with mesenchymal cells genetically engineered to have constitutive Hh pathway activation. The UGSM-2 cell line is an immortalized mesenchymal cell line derived from the E16 urogenital sinus mesenchyme (Shaw et al., 2006). These cells were transfected with either activated Smo or Gli2 to produce constitutive ligand independent Hh pathway activation as indicated by elevated expression of Gli1 and Ptc1 as compared to cells transfected with an empty vector (Fig 2A-B). E16 UGS or P1 prostatic rudiments were co-cultured with the transfected UGSM-2 cells for seven days in serum free media supplemented with testosterone (Fig 2C-F). BrdU labeling was performed 2 hours before tissues were harvested and prepared for histological examination. Serial sections were co-stained for BrdU with epithelial marker PanCk/P63 and the epithelial mitotic index calculated as previously described(Cook et al., 2007). The presence of UGSM-2 cells transfected with activated Smo increased epithelial proliferation in the E16 UGS tissues as compared to UGSM-2 cells transfected with an empty vector (Fig 3). This stimulatory effect of increased Hh signaling is consistent with the previously reported decrease in epithelial proliferation when the E14 UGS was cultured in the presence of cyclopamine to inhibit Hh signaling. (Lamm et al., 2002). The effect of activated paracrine Hh signaling was strikingly different on the cultured P1 prostatic rudiments. There the presence of UGSM-2 cells transfected with activated Smo decreased epithelial proliferation (Fig 3). This echoes similar findings by Wang and colleagues in their studies of the P2 rat UGS(Wang et al., 2003). These gain of function studies, together with our studies of chemical inhibition, suggest stage-specific effects of Hh signaling - stimulating ductal budding and proliferation in the prenatal prostate and decreasing ductal branching and proliferation in the postnatal prostate.
Figure 2. Co-Culture of E16/P1 tissues with UGSM-2 cells transfected with activated Gli2 (Gli2 OE) or Smo (Smo OE).
(A-B) RT-PCR analysis demonstrates increased expression of Gli1 and Ptc1 in UGSM-2 cells transfected with Gli2 or Smo compared to cells transfected with GFP alone. * P<0.05, n=3. This confirmed that transfection with Gli2 or Smo induced ligand-independent Hh pathway activation. (C-D) UGSM-2 cells (1×105) transfected with GFP alone (control) or activated Smo (Smo OE) were co-cultured with E16 or (E-F) P1 tissues (n=3). Representative images were taken on the 7th day of culture. Scale bar: 400um.
Figure 3. Activation of paracrine Hh signaling exerts differential effects on epithelial proliferation in cultured prenatal and postnatal tissues.
E16 and P1 tissues were co-cultured with UGSM-2 cells (Control or Smo OE) for 7 days. BrdU labeling was performed prior to harvest and sections were immunostained for BrdU and either PanCk or P63. (A-B) BrdU (red) and Panck (green) co-staining on 7 day co-cultured E16 UGS tissue with UGSM-2 cells (Control and Smo OE); (C-D) BrdU (green) and P63 (red) co-staining on 7 day cultured P1 Prostatic rudiments with UGSM-2 cells (Control and Smo OE). The images are representative of n=5 tissues in each group. (E-F) Co-culture with Smo OE cells increased epithelial proliferation in E16 UGS tissues but decreased proliferation in P1 prostatic rudiments. *P<0.05.
Stage-specific regulation of Hh target genes
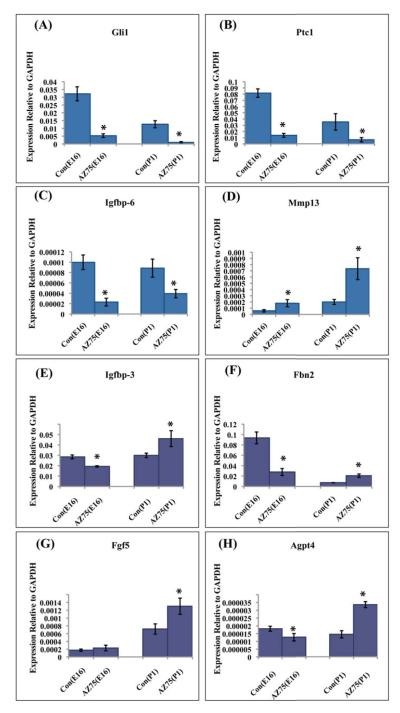
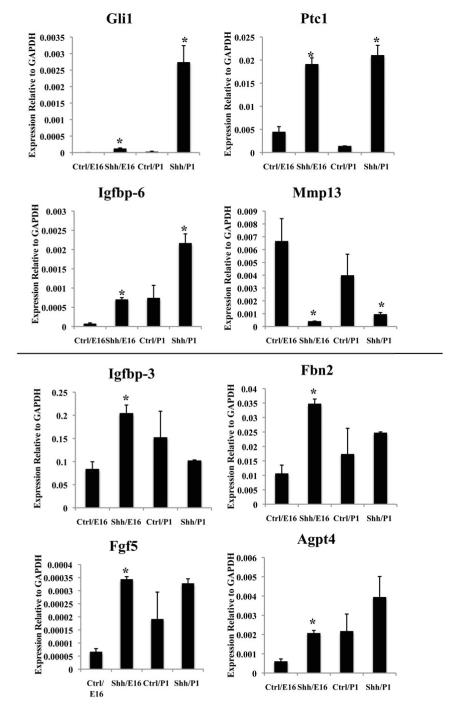
The transcriptional response to Hh signaling includes the canonical target genes Ptc1, Gli1 and HIP as well as tissue and cell-specific target genes. We have previously characterized Hh target gene expression in the E16 urogenital sinus mesenchyme (Yu et al., 2009). Recognizing that target gene regulation may be influenced by the developmental stage and/or tissue microenvironment, we compared the regulation of selected target genes in the E16 UGS and P1 prostate. Isolated E16 UGS and P1 prostatic rudiments were cultured in testosterone supplemented, serum free media in the presence or absence of AZ75 for seven days and RNA was prepared for RT-PCR analysis of canonical and non-canonical target gene expression. AZ75 uniformly inhibited expression of the canonical target genes Gli1 and Ptc1 in the cultured explants (Fig 4A-B), validating effective blockade of Hh-induced target gene activation. In the cultured E16 UGS, AZ75 inhibited expression of Igfbp-6, Igfbp-3, Fbn2 and Agpt4 (Fig 4C, E, F, H); increased expression of Mmp13 and did not significantly alter Fgf 5 gene expressio (Fig 4D,G). These observations recapitulate the observed effect of cyclopamine on gene expression in the cultured E16 UGS (Yu et al., 2009). The results in the cultured P1 prostate were quite different. AZ75 treatment decreased Igfbp-6 and increased expression of Mmp13 (Fig 4C-D) as it did in early E16 explants but paradoxically increased expression of Igfbp-3, Fbn2, Agpt4 and Fgf 5 (Fig 4E-H).
Figure 4. Discordant regulation of Hh target genes patterns in the cultured prenatal UGS and postnatal prostate.
E16/ P1 tissues were cultured in vitro +/− AZ75 (0.5uM) for 7 days. Expression of Hh target genes was quantitated by RT-PCR . (A-D) Four target genes (Gli1, Ptc1, Igfbp-6 and MMP13) displayed the same response to AZ75 treatment in both E16 UGS and P1 Prostatic rudiments. AZ75 decreased expression of Gli1, Ptc1 and Igfbp-6 and increased expression of Mmp13). (E-H) Four genes exhibited discordant responses to AZ75 in E16 UGS and P1 Prostatic rudiments. AZ75 decreased expression of Igfbp-3, Fbn2 and Agpt4 in E16 UGS tissues and did not alter expression of Fgf5. In contrast, AZ75 increased expression of all four genes in P1 prostatic rudiments. *P<0.05, n=3.
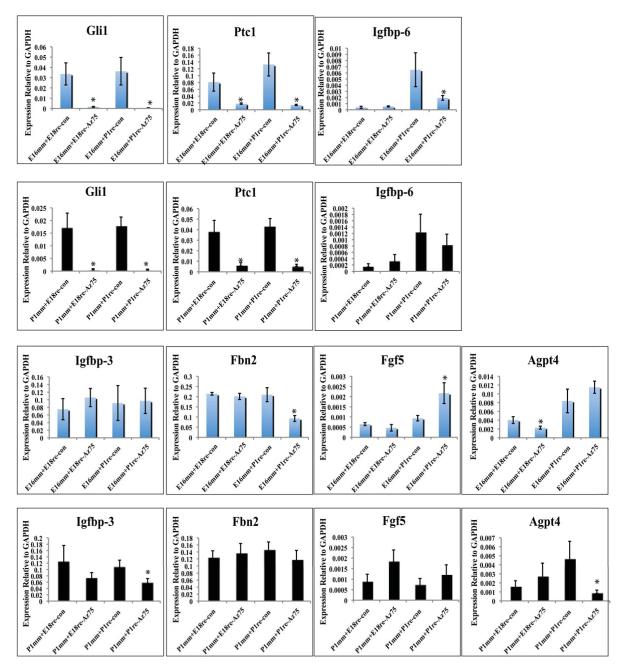
The mesenchymal and epithelial compartments of the developing prostate undergo extensive morphogenetic changes and change in differentiation during the perinatal period. To determine if the developmental stage of the mesenchyme determines the profile of Hh target gene expression, we compared Hh target gene expression in freshly prepared primary mesenchymal cells from the E16 UGS and P1 prostate tissues cultured with or without exogenous Shh for 24 hours. Shh peptide increased expression of Gli1, Ptc1 and Igfbp-6 and decreased expression of Mmp13 in both E16 and P1 mesenchymal cells(Fig 5). These effects mirror the equivalent Hh-dependence of these target genes in the intact E16 UGS and P1 prostate (above). The remaining four genes assayed showed disparate responses (Fig 5). Expression of Igfbp-3, Fbn2 and Agpt4 was induced by Shh in E16 mesenchyme cells, a finding that mirrors their positive regulation by Hh signaling in the intact E16 UGS. This inductive effect was not observed in P1 mesenchymal cells and this suggests that the transcriptional response to Hh ligand may vary simply with the developmental stage or state of differentiation of the mesenchyme. However, this cannot fully explain the disparate responses at different developmental stages since Shh treatment of P1 mesenchymal cells did not recapitulate the negative regulation of Igfbp-3, Fbn2 and Agpt4 observed in the intact P1 prostate. Other factors such as interaction with the epithelium or the stromal microenvironment play a role in determining the net response to Hh signaling. This is further suggested by the effects of Hh signaling on FGF5 expression. Identification of FGF5 came from microarray studies performed on the UGSM-2 cell line(Yu et al., 2009). FGF5 expression is significantly up-regulated by Shh in mesenchymal cells isolated from the E16 UGS; it is increased but not significantly in mesechymal cells from the P1 prostate. By contrast, FGF5 is not Hh regulated in the cultured E16 UGS but is down-regulated by Hh signaling in the P1 prostate.
Figure 5. Differential regulation of Hh target genes in cultured primary mesenchymal cells from E16 and P1 tissues.
Primary cells were cultured with or without exogenous Shh (5nM) for 24 hr and expression of Hh target genes analyzed by RT-PCR. Shh increased expression of Gli1, Ptc1 and Igfbp-6, and decreased expression of Mmp13 in both E16 and P1 mesenchymal cells. Shh increased expression of Igfbp-3, Fbn2, Fgf-5 and Agpt4 in E16 mesenchymal cells but did not significantly alter expression in P1 mesenchymal cells. *P<0.05, n=4.
To examine the influence of dynamic interaction with the epithelium on the mesenchymal target gene response to Hh signaling, we investigated the target gene response in recombinant tissues composed of epithelium and mesenchyme from different stages of development and cultured in the presence or absence of AZ75. In order to selectively measure mesenchymal gene expression, we utilized the mesenchyme from the mouse and epithelium from the rat. The developmental stages used were mouse E16 UGS and the developmental stage equivalent rat E18 UGS and both mouse and rat P1 prostate. The expectation that recombinants of mouse mesenchyme and rat epithelium would recapitulate the molecular interactions of the developing mouse prostate is based upon the finding that such recombinants grafted under the renal capsule generate normal appearing prostate tissue with rat-derived prostate ductal epithelium and mouse-derived stromal elements(Chung and Cunha, 1983). Freshly separated mesenchyme and epithelium were recombined and cultured for seven days in testosterone supplemented media in the presence or absence of AZ75. The four tissue recombinants were: E16 mouse mesenchyme with either E18 or P1 rat epithelium and P1 mouse mesenchyme with either E18 or P1 rat epithelium. Hh target gene expression was assayed by RT-PCR using mouse target gene primers (Fig 6). Expression of the canonical target genes Gli1 and Ptc1 were strongly inhibited by AZ75 in all cultured tissue. This finding indicates both that robust Hh signaling was re-established in the tissue recombinants and was effectively inhibited by addition of AZ75 to the media. All of the five non-canonical Hh target genes assayed (Igfbp6, Igfbp3, Fbn2, Fgf5 and Agpt4) showed significantly regulated expression by Hh signaling in at least one of the recombinants. However, the canonical patterns of Hh regulated target gene expression in the cultured E16 UGS and P1 prostate was not recapitulated in the cultured (M)E16m/(R)E18e and (M)P1m/(R)P1e recombinants, respectively. Only Agpt4 was significantly regulated by Hh signaling in the cultured (M)E16m/(R)E18e recombinant. Both Igfbp3 and Agpt4 were positively regulated by Hh signaling in the (M)P1m/(R)P1e recombinant but this contrasts with their negative regulation in the mouse P1 prostate. Hh regulated expression of several target genes was observed in the heterochronic recombinants (M) E16m/(R) P1e and (M) P1m/(R) E18e but this was inconsistent. These findings indicate that even though paracrine Hh signaling is re-established in the tissue recombinants, canonical patterns of positive and negative target gene regulation are only sporadically recapitulated in the temporally homotypic M)E16m/(R)E18e and (M)P1m/(R)P1e recombinants. While we cannot discount the influence of species differences, these data suggest that the physical disruption associated with mesenchymal-epithelial separation and recombination perturbs stage-specific patterns of Hh target gene regulation determined by the architecture of the developing tissue and the mesenchymal cell microenvironment.
Figure 6. Hh target gene analysis results in cultured mouse-rat tissue recombinants with AZ75 (0.5uM) treatment for 7 days.
Mouse mesenchyme isolated at either E16 (E16mM) or P1 (P1mM) was recombined with epithelium isolated from the rat at either E18 (E18rE) or P1 (P1rE) and cultured for 7 days in testosterone-supplemented media +/− AZ75 (0.5uM). Hh target gene expression was assayed by RT-PCR using mouse target gene primers. *P<0.05,n=3.
Differential effect of activated paracrine Hh signaling on prenatal and postnatal prostate growth in vivo
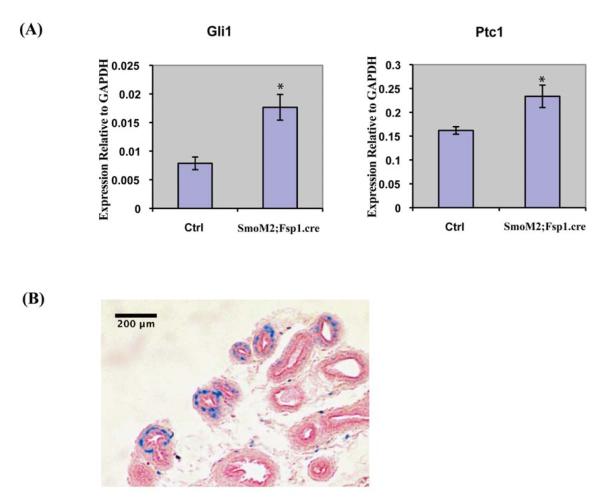
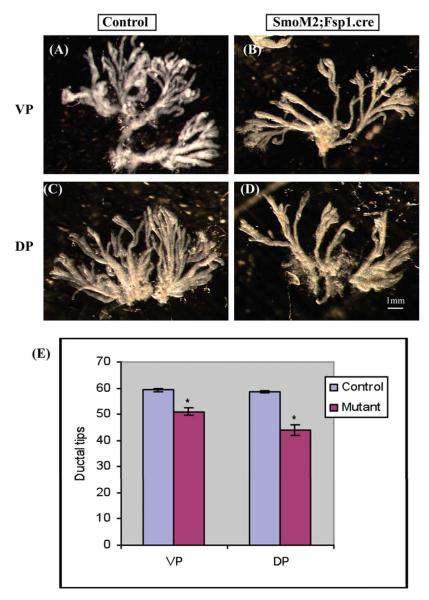
To examine the role of Hh signaling in regulating prostate growth in vivo, we utilized transgenic mice in which Hh signaling was conditionally activated in the UGS mesenchyme/prostate stroma. We utilized transgenic mice expressing cre from a Fibroblast specific protein -1 (Fsp1) promoter, Fsp1.cre mice, in which, cre is selectively expressed in fibroblast cells as early as embryonic day 9.5(Bhowmick et al., 2004; Iwano et al., 2002). When the Fsp1.cre mouse was mated with the Rosa26 reporter mouse, there was efficient recombination in the developing prostate as shown by X-gal staining (Fig7B). The cre mice were mated with the SmoM2.YFP transgenic mouse to selectively express an activated form of Smo in the mesenchyme of the developing prostate. The progeny were viable and activated Hh signaling was confirmed by RT-PCR analysis of Gli1 and Ptc1 expression in the UGS (Fig 7A). BrdU labeling of the E18 UGS demonstrated increased proliferation in the epithelium of the SmoM2; Fsp1.cre recombinant as compared to littermate controls (Fig 8A,B, E). In contrast, activated Hh signaling decreased the BrdU labeling index in the ductal epithelium of the P10 prostate (Fig 8C,D, F) and the P21 SmoM2; Fsp1.cre recombinant prostate was grossly smaller. To examine the possibility that activated Hh signaling decreased branching morphogenesis in the postnatal prostate, we performed quantitative microdissection of the prostate lobes as previously described (Sugimura et al., 1986). This revealed significantly decreased number of ductal tips in both ventral and dorsal lobes of the adult prostate in the P21 SmoM2; Fsp1.cre recombinant as compared to controls (Fig. 9). This observation indicates that activated Hh signaling inhibits epithelial proliferation during postnatal prostate development and that this is associated with an impairment of branching ductal morphogenesis.
Figure 7. Conditional activation of Hh signling in stromal cells of SmoM2; Fsp1.cre transgenic mice.
(A) RT-PCR analysis of Gli1 and Ptc1 expression in the E18 UGS from SmoM2; Fsp1.cre transgenic mice and control littermates. Increased expression of Gli1 and Ptc1 in SmoM2; Fsp1.cre mice indicates constitutive activation of Hh signaling in mutants. *P<0.05, n=3. (B) X-gal staining of the P10 prostate obtained from the progeny from crossing Fsp1.cre and Rosa26 LacZ reporter mice reveals selective staining of stromal cells (blue color). Section was counterstained with fast-red. n=3.
Figure 8. Conditional transgenic activation of paracrine Hh signaling exerts stage-specific effects on epithelial proliferation.
Immunostaining for BrdU and P63/Panck were performed on paraffin-sectioned tissues from E18 UGS and P10 prostate removed from SmoM2; Fsp1.cre mutant and the control littermates one hour after BrdU administration. (A-B) Representative image of E18 control UGS immunostained for Brdu (red) and P63 (green). (C-D) Representative image of control P10 prostate immunostained for BrdU (red) and Panck (green). (E-F) Quantitative comparison of epithelial BrdU labeling index reveals increased proliferation at E18 and a decreased proliferation at P10 in SmoM2; Fsp1.cre mutants. *P<0.05, n=3
Figure 9. The activated paracrine Hh signaling in transgenic mice model decreased ductal tips number in both ventral prostate (VP) and dorsal prostate (DP) at postnatal P21 stage.
Prostate lobes were removed from the mutant SmoM2; Fsp1.cre mice and the control littermates at P21, followed by microdissection to count the number of ductal tips. (A,C): Control; (B,D) Mutant. (E) The quantitative data of the total ductal tips number revealed a decreased ductal tips number in both VP and DP in SmoM2; Fsp1.cre mutant mice. *P<0.05, n=3, Scale bar=1mm.
Discussion
The role of Hh signaling in prostate development has been studied by several different laboratories using a both mouse and rat as the experimental subject and a variety of experimental conditions and approaches. The result has been a puzzling array of apparently inconsistent conclusions. We utilized in vitro culture of the prenatal and postnatal mouse prostate complemented by studies of transgenic mouse with activated mesenchymal Hh signaling to resolve these long-standing discrepancies by showing that Hh signaling exerts a temporal stage-specific effect on prostate growth.
In our study, chemical inhibition of Hh signaling in cultured E16 explants decreased the number of ductal tips while increasing the number of ductal tips in the cultured P1 explants. This finding suggested a stage-specific role of Hh signaling in regulation of prostatic budding and branching. Our experiments revealed similarly discordant effects on prostatic epithelial proliferation. Co-culture with UGSM-2 cells transfected with activated Smo in the cells stimulated epithelial proliferation in the E16 UGS tissues while inhibiting proliferation in the P1 UGS tissues. In addition to corroborating stage-specific effects on growth, this experiment demonstrated that these differences are attributable specifically to indirect paracrine Hh signaling. This observation is all the more remarkable because the activated cell line, UGSM2, is the same in both E16 and P1 cultured tissues. The altered growth effects suggest either that the epithelial target of paracrine Hh signaling changes with developmental stage or that the transcriptional readout and growth effects of UGSM-2 cells is influenced by the age of the co-cultured tissues.
Our studies of target gene expression at the different stages of development suggest that differential regulation of target genes in the prenatal and postnatal stages may be an important factor. Specifically, several genes positively regulated by Hh signaling in E16 explants displayed negative regulation in cultured P1 explants. Stage-specific differences in target gene regulation were also evident in primary cultured mesenchymal cells from E16 and P1. However, these cells could not fully recapitulate the differences seen in the cultured explants – a finding which suggests either further factors other than the mesenchymal character at different development stages exerts an influence. To determine the relative contributions of the epithelium and mesenchyme to stage-specific patterns of target gene regulation, we performed heterotypic recombination between rat and mouse. The failure to recapitulate the canonical patterns of regulation in the E16/E18 and P1/P1 recombinants made it impossible to interpret the cross-stage recombinants. At the same time, this finding suggested that physical dissociation of the tightly organized mesenchyme and epithelium perturbs – at least temporarily - the canonical patterns of stage-specific target gene regulation and implicated the tissue microenvironment as a critical feature of the response to Hh signaling.
Our transgenic mice model experiment complemented the in vitro studies. This is the first time, to our knowledge, that transgenic activation of Hh signaling has been used to examine the effect on prostate development, and corroborated our in vitro observations by demonstrating changes in growth and morphogenesis consistent with stage-specific effects of activated Hh signaling on growth. Theses studies used the fibroblast specific promoter FSP1.cre to drive a conditional activated Smo in the prostate mesenchyme/stroma. The UGS mesenchyme and prostate stoma are both heterogeneous in composition, X-gal staining on prostate tissue from FSP1.cre; Rosa26 mice indicated that cre is expressed in only a subpopulation of the stromal cells. A limitation of this approach is that conditional activation of the Hh pathway in a mesenchymal/stromal sub-population may not faithfully recapitulate the canonical pattern of Hh pathway activity among the heterogeneous cell population in this tissue layer. Future studies using conditional and inducible promoters to activate Hh signaling in selected sub-populations will be able to parse the specific effects of these sub-populations in the Hh-regulated growth of the prostate.
Hh signaling has been shown to exert a variety of function in different tissues. In a study of Hh signaling function during pancreas development, Lau provided show that before E12.5, low level of Hh signaling promotes the expansion of pancreatic epithelium. In contrast, Hh signaling blocks the proliferation of pancreatic epithelium at midgestation (Lau and Hebrok). In tongue development, inhibition of the Hh signaling from early development (E12-E14) can eliminate tongue development (E12) or alter tongue shape (E13) or increased number of fungiform papillae, but disruption of Hh signaling after E16 has no effect on the patterning of fungiform paillae(Liu et al., 2004). Temporal regulation of Hh signaling has also been reported in forebrain and hair follicle development as well(Oro and Higgins, 2003; Sousa and Fishell). Our data adds to the growing evidence that Hh signaling exerts pleiomorphic actions that are different not only between sites in the developing embryo but also within the same organ at different times.
The Hh target genes that were discordantly regulated pre- and postnatally included Igfbp3, Fgf5, Agpt4 and Fbn2. These are genes reported to be involved in events of cell proliferation, vascular angiogenesis, and extracellular matrix assembly. Noting that these genes were positively regulated by Hh signaling prenatally and negatively regulated postnatally, we speculate that these genes collaborate in stimulating epithelial proliferation. Fgf5 belongs to the fibroblast growth factor family and it is expressed in a complex spatiotemporal pattern during embryogenesis(Goldfarb et al., 1991). Its growth promoting activities were evidenced in pancreatic cancer and brain cancer(Allerstorfer et al., 2008; Kornmann et al., 2001). It also has been identified as an overexpressed antigen in multiple adenocarcinomas including breast carcinoma and prostate carcinoma(Hanada et al., 2001). Another function of Fgf5 is its potential role as an angiogenic factor(Giordano et al., 1996). Similarly, Agpt4, which belongs to angiopoietin family, promotes angiogenesis process by targeting specifically at endothelial cells(Lee et al., 2004). Fbn2, a major structural component of extracellular microfibrils, has been implicated in regulation of TGF-beta/BMP signaling (Ramirez et al., 2008; Ramirez and Dietz, 2007). Igfbp3 has exhibited a complex array of activities depending on the context(Granata et al., 2004; Massoner et al., 2009; Silha et al., 2006). Functional studies of these genes in the developing prostate may elucidate how their evolution in responsiveness to Hh signaling is linked to regulation of growth and morphogenesis. Interestingly, FGF5, Agpt4 and Fbn2 all exhibit positive regulation in prostate cancer with a reactive stroma (Shaw et al., 2009). Correlating this finding with our differential regulation results, we postulate that during embryonic development Hh stimulates expression of these genes to promote growth and morphogenesis, inhibits expression of these genes postnatal to down-regulate growth and maintain tissue homeostasis, and that reiteration of positive regulation in the tumor stroma stimulates prostate cancer cell growth(Shaw et al., 2009).
In conclusion, our study demonstrates a stage-specific effect of paracrine Hh signaling on prostate epithelial ductal growth, a mechanism possibly mediated by discordant regulation of the mesenchymal target genes determined by the complex interactions in the tissue microenvironment of the developing prostate. With the emerging evidence of the paracrine Hh signaling mechanism in directing the tumorigenesis of the prostate, we believe that better understanding of the pararcine Hh signaling in normal prostate development will help elucidate its role in prostate cancer and facilitate therapeutic targeting of the Hh pathway.
Highlights.
Hedgehog signaling is growth promoting pre natally and growth inhibitory post natally during prostate development
There is a discordant regulation of a subset of hedgehog target genes in the pre and post natal prostate
Cellular microenvironment regulates stage specific growth effects
Acknowledgements
This work was supported by funding from the NIH (P50 DK065303, RO1 DK056238), and the Department of Defense prostate cancer research predoctoral training grant (GRANT00263515).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, Czech T, Marosi C, Buchroithner J, Pichler J, Silye R, Mohr T, Holzmann K, Grasl-Kraupp B, Marian B, Grusch M, Fischer J, Micksche M, Berger W. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–90. doi: 10.1038/onc.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, Beachy PA, Shen MM. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267:387–98. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Chung LW, Cunha GR. Stromal-epithelial interactions: II. Regulation of prostatic growth by embryonic urogenital sinus mesenchyme. Prostate. 1983;4:503–11. doi: 10.1002/pros.2990040509. [DOI] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–30. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Dev Biol. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264:352–62. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, Mathieu-Costello O, Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Goldfarb M, Bates B, Drucker B, Hardin J, Haub O. Expression and possible functions of the FGF-5 gene. Ann N Y Acad Sci. 1991;638:38–52. doi: 10.1111/j.1749-6632.1991.tb49016.x. [DOI] [PubMed] [Google Scholar]
- Granata R, Trovato L, Garbarino G, Taliano M, Ponti R, Sala G, Ghidoni R, Ghigo E. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 2004;18:1456–8. doi: 10.1096/fj.04-1618fje. [DOI] [PubMed] [Google Scholar]
- Hanada K, Perry-Lalley DM, Ohnmacht GA, Bettinotti MP, Yang JC. Identification of fibroblast growth factor-5 as an overexpressed antigen in multiple human adenocarcinomas. Cancer Res. 2001;61:5511–6. [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann M, Lopez M, Beger H, Korc M. Expression of the IIIc Variant of FGF Receptor-1 Confers Mitogenic Responsiveness to Heparin and FGF-5 in TAKA-1 Pancreatic Ductal Cells. Int J Gastrointest Cancer. 2001;29:85–92. [PubMed] [Google Scholar]
- Krstic Z, Perovic S, Radmanovic S, Necic S, Smoljanic Z, Jevtic P. Surgical treatment of intersex disorders. J Pediatr Surg. 1995;30:1273–81. doi: 10.1016/0022-3468(95)90484-0. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–66. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232:301–14. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes. 59:1211–21. doi: 10.2337/db09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT, Ahn SY, Kim JH, Oh JL, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–8. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- Liu HX, Maccallum DK, Edwards C, Gaffield W, Mistretta CM. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev Biol. 2004;276:280–300. doi: 10.1016/j.ydbio.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Massoner P, Colleselli D, Matscheski A, Pircher H, Geley S, Jansen Durr P, Klocker H. Novel mechanism of IGF-binding protein-3 action on prostate cancer cells: inhibition of proliferation, adhesion, and motility. Endocr Relat Cancer. 2009;16:795–808. doi: 10.1677/ERC-08-0175. [DOI] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–48. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997;208:454–65. doi: 10.1002/(SICI)1097-0177(199704)208:4<454::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Carta L, Lee-Arteaga S, Liu C, Nistala H, Smaldone S. Fibrillin-rich microfibrils - structural and instructive determinants of mammalian development and physiology. Connect Tissue Res. 2008;49:1–6. doi: 10.1080/03008200701820708. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Dietz HC. Fibrillin-rich microfibrils: Structural determinants of morphogenetic and homeostatic events. J Cell Physiol. 2007;213:326–30. doi: 10.1002/jcp.21189. [DOI] [PubMed] [Google Scholar]
- Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28:4480–90. doi: 10.1038/onc.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. Prostate. 2006;66:1347–58. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silha JV, Sheppard PC, Mishra S, Gui Y, Schwartz J, Dodd JG, Murphy LJ. Insulin-like growth factor (IGF) binding protein-3 attenuates prostate tumor growth by IGF-dependent and IGF-independent mechanisms. Endocrinology. 2006;147:2112–21. doi: 10.1210/en.2005-1270. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Fishell G. Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Curr Opin Genet Dev. doi: 10.1016/j.gde.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–71. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Wang BE, Shou J, Ross S, Koeppen H, De Sauvage FJ, Gao WQ. Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. J Biol Chem. 2003;278:18506–13. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, Bushman W. Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem. 2009;284:5620–9. doi: 10.1074/jbc.M809172200. [DOI] [PMC free article] [PubMed] [Google Scholar]