One sentence summary
Women who indicate DMPA use have a significantly increased risk of prevalence of periodontal conditions as compared to women who have never used DMPA.
INTRODUCTION
Hormonal contraceptives have been reported to impact a woman's oral health. 1, 2Oral contraceptives, primarily containing estrogen have been associated with increased gingival inflammation and periodontitis although a consensus has not been reached in the literature. 3-6 Although, less studied, progestin only contraceptives which contain a synthetic version of the sex hormone, progesterone, may also impact periodontal tissues. 1, 7, 8 Progesterone has been associate with changes to gingival and other inter-oral tissues in females especially during life periods such pregnancy. 9-11 Furthermore, progesterone has been shown to reduce corpuscular flow rate allowing for accumulation of inflammatory cells, increased vascular permeability 1, 12and increased vascular proliferation. 13, 14
DMPA
Depot medroxyprogesterone acetate (DMPA) injectable contraception (Depo-Provera; Pfizer Pharmaceutical Group, New York, NY) is a highly efficacious long lasting progestin-only injectable contraceptive. DMPA is administered by intermuscular injection every 3 months. A National study reports that between 3.0% and 12.0% of women in the United States between the ages of 15-44 use DMPA. 15 The contraceptive action of DMPA results from its suppression of gonadotropin secretion which in turn inhibits ovarian estradiol production and prevents ovarian follicular maturation and ovulation. In addition to prescribing DMPA for contraception, DMPA is used in the management of abnormal uterine menstrual bleeding through the prevention of the overgrowth of the uterine endometrium. 16, 17After one year of DMPA use (four injections), 50% of women experience amenorrhea. 16
Women using this contraceptive method have high levels of synthetic progestins and low circulating endogenous estradiol levels comparable to those seen in the early follicular phase of a menstrual cycle or postmenopause. 18With the suppression of ovarian estradiol production, declines in bone mineral density (BMD) at the hip and spine of DMPA users has been shown to decrease by 0.5–3.5% after 1 year and 5.7–7.5% after 2 years of use as compared to non-users. 19-22 Due to the skeletal health concerns a black box warning was issued by the FDA stating use should be no longer than 2 years. 23
Progestins and Periodontal health
Progestin only contraceptive use has been associated with periodontal changes in adult women. 1, 7, 24 Tilakaratne et al. 8 observed that women who used a progestin-only injectable contraceptive (DMPA) for greater than 2 years had significantly higher levels of gingival inflammation and periodontal attachment loss as compared to non-users. In a clinical study by Seck-Diallo et al.7 women using injectable progestin-only contraceptives demonstrated more gingival inflammation, periodontal pocketing and clinical attachment loss than non users. More recently, in a prospective 6 month clinical study examining the effect of the levonorgestrel implant on the periodontium,1 women using the progestin implant contraceptives exhibited a statistically significant increase in gingival pocket depths over the study period as compared to non users. However, these studies have important limitations, such as low number of DMPA users or reporting DMPA use and oral contraceptive use together and lack of control for important periodontal disease associated confounders.
A suggested mechanism for DMPA's effect on periodontal tissues is that progestins, in it's active form, may stimulate the synthesis of prostaglandins, thereby contributing to increased vascular permeability within the chronically inflamed periodontium. Other possibility is that progestins may promote tissue catabolism possibly resulting in increased periodontal attachment loss. 25, 26 Because DMPA, suppresses estradiol concentrations, and estrogen deprivation has been associated with tooth loss, alveolar bone loss, and periodontal attachment loss, there is a possibility that the drug could adversely affect the periodontal structures.
Many women of all social economic backgrounds and ages use DMPA due to the method's convenience and contraceptive efficacy. 15, 27-29 However, roughly twice as many blacks and one-third Hispanics and Latinas use DMPA as compared to whites. 27In addition, the majority of DMPA users are women of low social economic status who are already at risk for increased levels of gingival disease. 30 Given that DMPA use is common among high risk women, it is important to learn more about potential deleterious effects on periodontal tissues. The objective of this analysis was to determine if a progestin-only contraceptive, Depo-Provera, was associated with an increased periodontal conditions among women 15-44 years in the U.S. population.
MATERIALS AND METHODS
Data Source
Data for this study were obtained from NHANES 1999-2004 public use datasets. The NHANES surveys are cross-sectional studies designed to obtain information on the health and nutritional status of the non-institutionalized population of the United States conducted by the National Center for Health Statistics (NCHS). The sampling plan of each of the NHANES surveys followed a highly stratified multistage probability design in which a sample of the U.S. civilian, non-institutionalized population was selected to provide national estimates. Methods for the standardized interviews, dental examinations, and procedures for human protection and consent have been described in detail elsewhere. 31 The number of records available for analysis varied depending on the variables used.
Population
From the initial sample of 4,988 non-pregnant, premenopausal women ages 15 to 44 years, 4,462 received periodontal examinations and had complete DMPA use data. This age range was used for comparability of data to existing National Survey of Family Growth surveys. Data were excluded for women who indicated the current use of oral contraceptives (n=2). Menopausal status was ascertained based on a respondent's report that her menstrual periods had not occurred within the last 12 months or stopped entirely (excluding women who were reported to be breastfeeding or pregnant. In addition, 1812 participants had missing data in relation to other variables used in the analysis; therefore regression analyses models were based on 2,648 women.
DMPA Use (Exposure to Sex Steroids)
The main exposure variable, DMPA use, was determined from two questions from the Reproductive Health section of the Examination Interview conducted at the MEC. 31 The questions were as follows: “Have you or respondent ever used Depo-Provera or injectables to prevent pregnancy?” and “Are you/or the respondent now using Depo-Provera or injectables to prevent pregnancy?” The NHANES survey did not ask respondents about duration of Depo-Provera use or age of initiation of the contraceptive.
Measurement of Periodontal Conditions
All dental examinations were conducted by trained and standardized examiners in dental units located in mobile examination centers (MEC). The periodontal status of individuals in NHANES surveys was assessed using randomly assigned half-mouths (one upper and one lower quadrant) for each individual using a NIDCR periodontal probe. There were slight differences in the periodontal examinations data between the NHANES surveys. The data from the 1999-2000 survey included clinical attachment (CA) loss, periodontal pockets (PD) assessments at two sites per tooth and the gingival sweep was used to assess gingival bleeding at the quadrant level. For the 2001-2004 survey, periodontal assessments were taken at 3 sites per tooth were assessed for periodontal pocket depth, clinical attachment loss and periodontal bleeding 32, 33 We defined gingival bleeding as the presence or absence of gingival bleeding in one or more quadrants or one or more sites. Periodontal disease was defined as at least two sites with 4 mm of clinical attachment loss and a probing depth ≥4 mm following previously published reports.6, 34
Sociodemographic Covariates
Sociodemographic and behavioral factors which have been shown to be associated with DMPA use were evaluated for confounding and effect modification. Variables obtained from the face-to-face interview included age, which was specified as both continuous and categorical, with six age categories. Race/ethnicity was defined as Non Hispanic Black, Hispanic and Non-Hispanic White to allow comparisons with the National Survey of Family Growth.15 Other race/ethnicities were excluded from the analysis. Marital status was defined as married (married or living together as married) or not married. Poverty income ratio is the ratio of reported family income category divided by the poverty income threshold. Using the suggested cutpoints from the NHANES III Analytic Guidelines, 35 three categories, low, medium, and high, were created for poverty index level in both data sets – 0.00 to 1.350, 1.351 to 3.500, and ≥ 3.501. Parity was collected from the question “How many live births have you had?” Parity was coded as a categorical variable with categories being 0, 1 to 2, ≥ 3 live births. Smoking status was defined as never smoked (< 100 cigarettes in lifetime), former smoker (a positive answer to ever smoked but do not smoke cigarettes now), and current smoker (a positive answer to smoke now and have smoked ≥100 cigarettes in a lifetime). Education level was reported as < 12 years of education, 12 years of education, or > 12 years of education.
Statistical Analysis
The NHANES surveys involve complex sampling designs; therefore, all statistical analyses were performed taking into account the effect of the study design as well as incorporating the examination sampling weights. The dependent variables for this analysis were gingival bleeding and periodontal disease.
Univariate statistics were calculated for all variables to describe the variables and their distributions along with measures of unadjusted association between the periodontal outcomes (gingival bleeding/periodontitis) and other covariates of interest for the total sample. The bivariate relationships between categorical variables were assessed with the Pearson Chi-square test. The relationships between continuous and categorical variables were assessed with simple (unadjusted) linear regression models. Multiple logistic regression analysis utilizing the manual backward selection method was used to assess the relationship between DMPA use and periodontal outcomes while controlling for other covariates. Because level of education and poverty index were highly correlated, only poverty index was used when generating the regression models.
Potential interactions between DMPA use and smoking history were also examined. Due to the complex interpretation associated with a three level variable (current, past, and never use) for both DMPA exposure and smoking status, the interaction term was re-categorized into a cross product of a dichotomous variable of smoking history (ever vs. never) and DMPA use (ever vs. never). The interaction term was not significant for the gingival bleeding model, therefore only the main effects model is reported.
All analyses were conducted using a software package∥ which can account for complex sampling design and which gives adjusted variance estimations. Therefore, in all tables the number of participants per category ∥is unweighted, while all means, percentages, and ORs are weighted to reflect the target population and standard errors and 95% confidence intervals (CI) are adjusted for sampling design.
Results
The study sample included 4,460 non-pregnant, premenopausal women ages 15 to 44 years as shown in Table 1. The distribution of the subpopulation was estimated to be 65.6% white, 51.4% well-educated (more than a high school degree), well-represented in each of the poverty index levels, more than 57% were not married and 27.2% have never had a child. Approximately 4% were current DMPA users while 12.1% indicated a past history of DMPA use. Nearly one third of the respondents indicated current smoking, 59.5% indicated a dental visit within the last two years. Using the study definitions of periodontal disease, 53% had gingival bleeding where as 12% had periodontitis.
Table 1.
Demographic Characteristics of Women Aged 15 to 44 Years, by DMPA use: National Health and Nutrition Examination Survey, 1999–2004
| DMPA Use* | ||||
|---|---|---|---|---|
| Variable | Current n=157 Weighted % (SE) | Past n=553 Weighted% (SE) | Never n=3,750 Weighted% (SE) | Total n=4,460 Weighted %‡ (SE) |
| Gingival Bleeding | ||||
| Yes | 3.6 (0.4) | 14.0 (0.9) | 80.4 (1.0) | 53.9 (1.9) |
| No | 2.5 (0.4) | 10.0 (0.8) | 87.5 (1.1) | 46.1 (1.9) |
| Periodontitis | ||||
| Yes | 2.3 (0.4) | 12.3 (1.0) | 85.4 (1.1) | 10.6 (1.7) |
| No | 3.3 (0.5) | 8.1 (0.9) | 88.6 (1.0) | 89.4 (1.7) |
| Age | ||||
| 15-19 | 4.2 (0.8) | 5.0 (0.7) | 90.8 (1.0) | 16.3 (0.4) |
| 20-29 | 4.7 (0.7) | 17.6 (1.4) | 77.7 (1.5) | 30.9 (0.9) |
| 30-39 | 1.8 (0.4) | 11.8 (1.3) | 86.4 (1.5) | 34.2 (0.8) |
| 40-44 | 1.4 (0.4) | 5.6 (1.3) | 92.0 (1.3) | 18.5 (0.6) |
| Race | ||||
| Non-Hispanic White | 2.7 (0.5) | 8.7 (0.7) | 88.6 (0.9) | 65.6 (1.6) |
| Hispanic | 3.0 (0.5) | 15.4 (2.0) | 81.6 (1.9) | 17.1 (1.5) |
| Non-Hispanic Black | 4.0 (0.7) | 18.0 (1.8) | 78.0 (1.9) | 17.3 (1.2) |
| Education | ||||
| Less than high school | 4.3 (0.6) | 13.2 (1.2) | 82.5 (1.4) | 26.1 (0.7) |
| High school | 3.5 (0.8) | 14.9 (1.6) | 81.6 (1.8) | 22.5 (0.8) |
| More than high school | 2.1 (0.5) | 8.9 (0.8) | 89.0 (0.9) | 51.4 (1.0) |
| Family Poverty Level | ||||
| 0 - 1.3 | 4.9 (0.8) | 18.0 (1.7) | 76.1 (1.9) | 26.3 (1.2) |
| 1.31 - 3.49 | 3.3 (0.5) | 11.0 (1.1) | 85.7 (1.2) | 36.9 (1.1) |
| 3.5 > | 1.2 (0.3) | 5.8 (0.8) | 93.0 (0.8) | 36.8 (1.4) |
| Marital Status | ||||
| Not married | 3.7 (0.4) | 11.5 (0.9) | 84.8 (0.9) | 57.2 (1.1) |
| Married/co-habitating | 1.9 (0.3) | 11.4 (0.8) | 86.7 (1.1) | 42.8 (1.1) |
| Parity (live births) | ||||
| 0 | 3.1 (0.9) | 4.5 (1.0) | 92.4 (1.2) | 27.2 (1.2) |
| 1 | 6.1 (1.0) | 18.4 (1.6) | 75.5 (2.1) | 21.8 (0.9) |
| 2 | 2.2 (0.4) | 14.0 (1.1) | 83.8 (1.2) | 43.6 (1.4) |
| 3 > | 1.7 (0.6) | 18.0 (2.2) | 80.3 (2.3) | 7.4 (0.6) |
| Dental visit < two years | ||||
| Yes | 2.5 (0.3) | 9.0 (0.6) | 88.3 (0.7) | 59.5 (0.9) |
| No | 3.9 (0.5) | 15.9 (1.3) | 80.2 (1.5) | 40.5 (0.9) |
| Smoking status | ||||
| Current | 3.6 (0.9) | 17.4 (1.3) | 79.0 (1.7) | 30.2 (1.0) |
| Past | 2.7 (0.8) | 12.9 (2.3) | 84.4 (2.4) | 15.4 (0.7) |
| Never | 2.4 (0.3) | 10.6 (0.9) | 87.0 (0.9) | 54.4 (1.2) |
Note. All demographic factors differed significantly between the 3 DMPA user groups using a Chi-Square test for association (P≤.003).
Total sample weighted percents in column format.
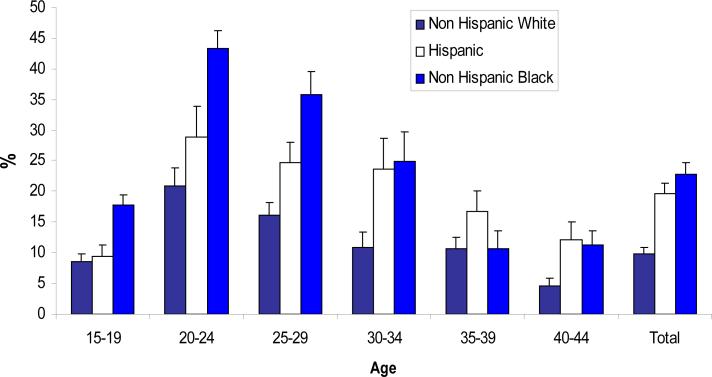
Figure one shows the prevalence of DMPA use by age and ethnicity. Of the three ethnicity groups, non Hispanic Black women demonstrate the highest use at 22.9%. When examining the ethnicity by age groups, young Non Hispanic Black women ages 18-35 years reported that highest use of the DMPA. For women older than 35, a higher percentage of Hispanic women indicated use of DMPA especially among the oldest age group 40-44 years.
Figure 1.
Women ages 15-44 years of age who reported ever use of DMPA by age and race/ethnicity: NHANES, 1999-2004
Table 1 shows a summary of characteristics of the sample by DMPA use. DMPA users were significantly more likely to be young, non-white, among lower education and poverty levels, to have at least one child, and be less likely to have a dental visit within the last two years. The prevalence of gingivitis was significantly associated with DMPA use. Current DMPA users were more likely to have gingivitis (4.3% vs 2.5 %) as well as past DMPA users (15% vs. 10%). Past users of DMPA were more likely to have periodontitis (12.0% vs. 8.0%) whereas current users were less likely to have periodontitis.
Table 2 outlines the results from simple linear regression analyses for periodontal conditions among premenopausal adult U.S. women, 15 to 44 years of age, stratified by DMPA use. DMPA users were significantly younger than non DMPA users (25.1 yrs (0.58) vs. 30.0 yrs (0.23) P=0.001). Gingival bleeding and periodontal pocket depths were significantly increased among current and past DMPA users as compared to never users. There was no significant difference in the mean number of teeth among the contraceptive groups.
Table 2.
Periodontal Characteristics of Women Aged 15 to 44 Years, by DMPA Exposure (N=4,460)
| Periodontal Measure | Current Use | Past Use | Never Use | P-Value* |
|---|---|---|---|---|
| Characteristic | N=157 %(SE)† | N=553 % (SE)† | N=3,750 %(SE)† | |
| Mean Age | 25.1(0.58) | 28.6(0.29) | 30.0 (0.23) | 0.001 |
| Gingival Bleeding | 5.2(0.97) | 3.54(0.38) | 2.9 (0.23) | 0.01 |
| PD§ 4mm | 33.6(3.0) | 36.8(1.7) | 27.2(0.7) | 0.01 |
| Max CA Loss‡ | 1.6 (0.06) | 1.7 (0.04) | 1.3 (0.02) | 0.04 |
| Mean CA Loss‡ | 0.40 (0.04) | 0.46(0.02) | 0.44 (0.02) | 0.35 |
| Max PD§ | 2.2 (0.09) | 2.4(0.06) | 2.0 (0.04) | 0.04 |
| Mean PD§ | 1.0(0.06) | 0.96(0.03) | 0.90 (0.02) | 0.04 |
| Mean # Teeth | 27.7 | 27.8 | 27.3 | 0.43 |
Comparisons were carried out using simple unadjusted linear regression
Standard error of the mean
Clinical attachment loss
PD=Periodontal pocketing
The results of the logistic regression analyses presented in Table 3 indicate that, in general, women using DMPA have increased odds of poor gingival health. In the unadjusted analysis, compared with non-DMPA users, current users (OR=1.91; 95% CI 1.20, 1.83), and past users (OR=1.43; 95% CI 1.19, 3.02), respondents had greater odds of gingivitis. After adjusting for covariates including race, age, dental utilization, these associations remained significant for current DMPA use was 1.73 (95% CI, 1.09-1.67). Comparing past users and non users, the prevalence was higher (logistic OR, 1.34; 95% CI, 0.98-2.67) but did not reach statistical significance. Hispanic and non Hispanic Black women were estimated to have between 39% and 50% higher odds of having any periodontal disease compared with non-Hispanic white women. In addition, both lower poverty index levels and not having a dental visit within the last 2 years resulted in an increased odds of having gingivitis. Smoking history was not significantly associated with a higher odds of having gingivitis. The interaction between smoking status and DMPA did not have a significant relationship with gingivitis (p=0.46). Therefore, we presented the main effects model without the dichotomous re-categorization of smoking and DMPA use as the collapsed variables did not show differences between current and past DMPA users with respect to increase risk of gingival inflammation.
Table 3.
Logistic Regression Model for Gingival Bleeding – Odds of Having Gingival Bleeding Among U.S. Women Ages 15-44 NHANES, 1999-2004 (N=2,648)
| Variable | Unadjusted OR | 95% CI | Adjusted* OR | 95% CI |
|---|---|---|---|---|
| DMPA Use | ||||
| Current | 1.91 | (1.20– 1.83) | 1.73 | (1.09– 1.67) |
| Past | 1.43 | (1.19– 3.02) | 1.34 | (0.98– 2.67) |
| Never | 1 | Ref | 1 | Ref |
| Age | ||||
| 15-19 | 0.95 | (0.83 – 1.13) | 1.02 | (0.69 – 1.50) |
| 20-29 | 0.87 | (0.73 – 1.10) | 0.95 | (0.66 – 1.37) |
| 30-39 | 0.96 | (0.66 – 0.96) | 0.89 | (0.61 – 1.30) |
| 40 -44 | 1 | Ref | 1 | Ref |
| Race | ||||
| Non-Hispanic Black | 1.85 | (1.29 – 2.02) | 1.50 | (1.08 – 2.07) |
| Hispanic | 1.61 | (1.45 – 2.35) | 1.39 | (1.04 – 2.19) |
| Non-Hispanic White | 1 | Ref | 1 | Ref |
| Poverty Index Level | ||||
| 0 - 1.3 | 1.81 | (1.45 -2.26) | 1.50 | (1.10 -2.08) |
| 1.31- 3.49 | 1.25 | (1.07 -1.45) | 1.01 | (0.59 -1.73) |
| >3.5 | 1 | Ref | 1 | Ref |
| Dental Visit < two years | ||||
| Yes | 1.90 | (1.63 – 2.20) | 1.80 | (1.59 – 2.11) |
| No | 1 | Ref | 1 | Ref |
| Smoking status | ||||
| Current | 0.90 | (0.69 – 1.18) | 1.00 | (0.76 – 1.32) |
| Past | 1.06 | (0.90 – 1.25) | 1.15 | (0.77 – 1.71) |
| Never | 1 | Ref | 1 | Ref |
Model adjusted for age, race, poverty income level, dental visit and smoking status
The results of the logistic regression model examining the association of DMPA use with periodontal disease is presented in Table 4. A significant interaction between smoking status and DMPA use was found using the collapsed categorization of the two variables discussed above. There was a significant association between periodontal disease and race (specifically, non-Hispanic Blacks compared to non-Hispanic Whites), poverty index levels, age, and not having a dental visit within the last two years.
Table 4.
Logistic Regression Model for Periodontitis – Odds of Having Periodontitis Among U.S. Women Ages 15-44 NHANES, 1999-2004 (N=2,648)
| Variable | Unadjusted OR | 95% CI | Adjusted* OR | 95% CI |
|---|---|---|---|---|
| DMPA Use | ||||
| Ever | 1.62 | (1.27– 1.92) | 1.49 | (1.01– 2.22) |
| Never | 1 | Ref | 1 | Ref |
| Race | ||||
| Non-Hispanic Black | 1.51 | (1.17 – 1.82) | 1.45 | (1.00 – 2.11) |
| Hispanic | 1.34 | (1.06 – 1.71) | 1.39 | (0.96 – 2.01) |
| Non-Hispanic White | 1 | Ref | 1 | Ref |
| Age | ||||
| 20-29 | 1.71 | (1.36 – 2.15) | 1.65 | (1.00 – 2.62) |
| 30-39 | 3.52 | (2.81 – 4.54) | 2.43 | (1.89 – 3.18) |
| 40 -44 | 5.82 | (4.61 – 7.52) | 4.38 | (3.20 – 6.01) |
| 15-19 | Ref | 1 | Ref | |
| Poverty Index Level | ||||
| 0 - 1.3 | 2.32 | (1.33 -3.20) | 1.70 | (1.16- 2.47) |
| 1.31- 3.49 | 1.86 | (1.66- 2.55) | 1.51 | (1.19 -1.91) |
| >3.5 | 1 | Ref | 1 | Ref |
| Dental Visit < two years | ||||
| Yes | 1.59 | (1.38 – 1.83) | 1.44 | (1.18 – 1.76) |
| No | 1 | Ref | 1 | Ref |
| Smoking status | ||||
| Ever | 1.67 | (1.43 – 1.93) | 1.71 | (1.26 – 2.38) |
| Never | 1 | Ref | 1 | Ref |
| DMPA use * Smoking interaction† | 0.49 | (0.30-0.79) | 0.55 | (0.32-0.93) |
Model controls for age race, poverty index level, smoking status and dental visits.
Interaction term model for DMPA use (Ever/Never) and Smoking (Ever/Never)
The interaction between smoking status and DMPA use is shown in Table 5. For those women who indicated never smoking, ever (current/past) use of DMPA was associated with an increased odds of having periodontal disease as compared to women who indicated never use of DMPA (OR=1.49; 95% CI, 1.01-2.22). For women that never used DMPA, respondents who indicate ever (current/past) smoking have an increase odds of having periodontal diseases compared to those that never smoked (OR=1.71; 95% CI, 1.26-2.38). For those women with a history of ever smoking and a history of ever DMPA use, the odds of having periodontal diseases decreased (OR=0.55; 95% CI 0.32, 0.93).
Table 5.
Odds of Periodontal Disease Associated with Smoking History According to DMPA Use Among U.S. Women Ages 15-44 NHANES, 1999-2004
| Variable | Adjusted* OR | 95% CI |
|---|---|---|
| Never DMPA Use & No Smoking | 1 | Ref |
| Ever DMPA Use & No Smoking | 1.49 | (1.01– 2.22) |
| Never DMPA Use & Smoking† | 1.71 | (1.26 – 2.38) |
| Ever DMPA Use & Smoking† | 0.55 | (0.32 – 0.93) |
Model controls for age, race, poverty index level, smoking status and dental visits.
Smoking is defined as ever (current/past).
Discussion
In the year 2004, the USFDA added a “Black Box” into the package labeling for the injectable contraceptive DMPA warning about bone health indicating that use of DMPA for more than 2 years may increase bone loss and put the women at risk for osteoporotic fractures 36. Furthermore there is evidence that progestin only contraceptives may affect periodontal health 1, 7, 8. Hence, the aim of this study was to investigate the association between DMPA and periodontal diseases using a representative sample of U.S. women ages 15-44. This study suggests that DMPA use may be associated with an increase in adverse periodontal changes: gingival bleeding and periodontitis.
A significant association between current DMPA use and gingival bleeding was observed after controlling for potential confounding variables (OR=1.73; 95% CI: 1.09 to 1.67). A similar trend was observed for past DMPA users but failed to reach the significance level of P <0.05 (P = 0.057). To our knowledge this is the first study to examine DMPA use and periodontal conditions using multivariable modeling to control for potential confounders providing further evidence of an association between DMPA use and gingival changes.
Using clinical periodontal measures, we found significant differences in pocket depths, gingival bleeding and CA loss between DMPA users and non users. The increased pocket depths among DMPA users is similar to previous small clinical studies 1, 7 which found women using the progestin implant contraceptives exhibited a statistically significant increase in gingival pocket depths as compared to non–users. Like the Tilakaratne and colleagues study 8 we found statistically increased clinical attachment loss in DMPA users compared to non users. Interestingly, for our population, the mean pocket depths were much lower as compared to the above mentioned studies. Users of DMPA in this sample may be younger, however, as the above studies did not disclose the age of their population it is difficult to make comparisons.
As our adjusted logistic regression model from Table 4 indicates, DMPA use has a modest association with the increased odds of periodontal disease. High systemic progestin levels associated with the use of DMPA have been shown to reduce skeletal BMD levels.22 Recent reports suggest that the BMD deficits are completely reversed within 1 to 3 years following discontinuation of the contraceptive. 37, 38 As the majority of DMPA users are very young, this may allow for bone recovery without deleterious effects occurring in the periodontium.
Our analysis suggested that there is a strong interaction between smoking status and DMPA use on the prevalence of periodontal disease among women. Surprisingly, among DMPA users, smoking appears to decrease the risk for periodontitis. As smoking is considered a risk factor for periodontal diseases, these results are puzzling. DMPA use and smoking may not synergistically increase the risk of periodontitis; and it can be speculated that smoking and DMPA may mask each other's effect on periodontitis. Accurate clinical diagnosis of periodontal disease has been shown to be difficult in smokers because of decreased gingival inflammation, BOP and obstruction of periodontal probe penetration at the pocket base during examination. 39 Further studies are needed to clarify this relationship and the possible effect modification of smoking on DMPA use and periodontal disease.
The socio-demographic composition of DMPA users in our study are similar to the National Survey of Family Growth estimates for contraceptive choices in women ages 15-44 in the U.S.28 The majority of DMPA users are young, non-white women of low socio-economic status, who are more likely to smoke. Our prevalence estimates are consistent with those of Brunner-Huber and colleagues which showed that Hispanic women have a higher prevalence of DMPA use among those aged 35-44. 40 Furthermore, we found that women who use DMPA were more likely to smoke than women who had never used DMPA contraception. Women who smoke have been shown to be more likely to use implants, injectable contraceptives and hormonal patches as compared to non-smokers. 40 These same groups are disproportionately at higher risk for gingival bleeding and other periodontal diseases 30, 41 Significantly fewer current DMPA users reported visiting a dentist within the last 2 years as compared to non users (2.5% vs 4.0%) thus potentially increasing the risk of poor oral health among these women.
Compared to prior studies of this association, strengths of the current study include a large nationally representative sample which provides greater generalizability of findings across race-ethnicity and age groups as compared to periodontal based samples and detailed covariate information Furthermore the use of multiple logistic regression models allowed us to control for potential confounders increasing the validity of our outcomes.
Although the logistic model examining the increase in the prevalence of periodontitis with DMPA use demonstrated only a modest association, an increased risk due to the use of an injectable contraceptive requires further evaluation. Eke and colleagues recently reported that the partial mouth periodontal examinations used in the NHANES produce underestimation of the prevalence of periodontal disease resulting in disease misclassification. 42 The small number of DMPA users coupled with the young age of the population and the ensuing small number of periodontal cases may have affected our ability to effectively capture the association between DMPA use and periodontal disease. Furthermore, progestins, synthetic versions of the hormone progesterone, are used for both contraception and to treat dysfunctional uterine bleeding. Therefore, women using DMPA for uterine bleeding may have different population characteristics than younger women who use DMPA for contraceptive purposes. These factors could lead to non-differential misclassification among those who use DMPA and those who do not, thus attenuating the strength of the association identified in the analysis and therefore suggesting that the associations identified in this study may be even stronger than reported here.
This study was subject to another limitation. NHANES did not ask questions about the duration of DMPA use or age of initiation of the contraceptive. Consequently, we cannot determine the dose response between DMPA duration and periodontal diseases. Another limitation was the cross-sectional nature of the data. Because DMPA use and periodontal status were measured at one point in time, it is impossible to know whether the use of DMPA causes adverse periodontal changes. Furthermore, unmeasured variables related to oral health (oral hygiene measures, time since last pregnancy) or other non contraceptive use of DMPA use may have influenced the results. Despite these limitations, this study demonstrates that poor gingival health is associated with a progestin-only contraceptive.
Conclusions
Our study confirms and expands on the findings of previous research to suggest that DMPA use influences on periodontal health. In addition, women who use DMPA may be at increased risk for poor oral health due to socio-demographic and lifestyle behavior factors. Future clinical studies which include oral health behaviors and duration of DMPA use are required to evaluate the relationship between DMPA use and the incidence periodontal health.
Acknowledgements
This paper is dedicated to the memory of Dr. MaryFran Sowers, a great mentor and colleague who was a pioneer in the field of women's health. This paper was supported with funding from the Michigan Institute for Clinical Health Research/ CTSA pilot grant UL1RR024986 and the National Institute on Dental and Craniofacial Research (NIDCR) grant 1K23DEO21779.
Footnotes
STATA Statistics and Data Analysis, Version 11, STATA Corporation
The authors report no conflicts of interest related to this study.
References
- 1.Kazerooni T, Ghaffarpasand F, Rastegar N, Kazerooni Y. Effect of levonorgestrel implants on the periodontium. Int J Gynaecol Obstet. 2008;103:255–256. doi: 10.1016/j.ijgo.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Sooriyamoorthy M, Gower DB. Hormonal influences on gingival tissue: relationship to periodontal disease. J Clin Periodontol. 1989;16:201–208. doi: 10.1111/j.1600-051x.1989.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Haerian-Ardakani A, Moeintaghavi A, Talebi-Ardakani MR, Sohrabi K, Bahmani S, Dargahi M. The association between current low-dose oral contraceptive pills and periodontal health: a matched-case-control study. J Contemp Dent Pract. 2011;11:033–40. [PubMed] [Google Scholar]
- 4.Mullally BH, Coulter WA, Hutchinson JD, Clarke HA. Current oral contraceptive status and periodontitis in young adults. J Periodontol. 2007;78:1031–1036. doi: 10.1902/jop.2007.060163. [DOI] [PubMed] [Google Scholar]
- 5.Preshaw PM, Knutsen MA, Mariotti A. Experimental gingivitis in women using oral contraceptives. J Dent Res. 2001;80:2011–2015. doi: 10.1177/00220345010800111201. [DOI] [PubMed] [Google Scholar]
- 6.Taichman LS, Eklund SA. Oral contraceptives and periodontal diseases: rethinking the association based upon analysis of National Health and Nutrition Examination Survey data. J Periodontol. 2005;76:1374–1385. doi: 10.1902/jop.2005.76.8.1374. [DOI] [PubMed] [Google Scholar]
- 7.Seck-Diallo A, Cisse ML, Benoist HM, et al. Periodontal status in a sample of Senegalese women using hormonal contraception. Odontostomatol Trop. 2008;31:36–42. [PubMed] [Google Scholar]
- 8.Tilakaratne A, Soory M, Ranasinghe AW, Corea SM, Ekanayake SL, de Silva M. Effects of hormonal contraceptives on the periodontium, in a population of rural Sri-Lankan women. J Clin Periodontol. 2000;27:753–757. doi: 10.1034/j.1600-051x.2000.027010753.x. [DOI] [PubMed] [Google Scholar]
- 9.Lapp CA, Thomas ME, Lewis JB. Modulation by progesterone of interleukin-6 production by gingival fibroblasts. J Periodontol. 1995;66:279–284. doi: 10.1902/jop.1995.66.4.279. [DOI] [PubMed] [Google Scholar]
- 10.Miyagi M, Morishita M, Iwamoto Y. Effects of sex hormones on production of prostaglandin E2 by human peripheral monocytes. J Periodontol. 1993;64:1075–1078. doi: 10.1902/jop.1993.64.11.1075. [DOI] [PubMed] [Google Scholar]
- 11.Ojanotko-Harri AO, Harri MP, Hurttia HM, Sewon LA. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J Clin Periodontol. 1991;18:262–266. doi: 10.1111/j.1600-051x.1991.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 12.Abraham-Inpijn L, Polsacheva OV, Raber-Durlacher JE. The significance of endocrine factors and microorganisms in the development of gingivitis in pregnant women. Stomatologiia. 1996;75:15–18. [PubMed] [Google Scholar]
- 13.Lindhe J, Attstrom R, Bjorn AL. Influence of sex hormones on gingival exudation in gingivitis-free female dogs. J Periodontal Res. 1968;3:273–278. doi: 10.1111/j.1600-0765.1968.tb01930.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindhe J, Branemark PI, Lundskog J. Changes in vascular proliferation after local application of sex hormones. J Periodontal Res. 1967;2:266–272. doi: 10.1111/j.1600-0765.1967.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 15.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23:1–160. [PubMed] [Google Scholar]
- 16.Belsey EM. Menstrual bleeding patterns in untreated women and with long-acting methods of contraception. Task Force on Long-Acting Systemic Agents for Fertility Regulation. Adv Contracept. 1991;7:257–270. doi: 10.1007/BF01849416. [DOI] [PubMed] [Google Scholar]
- 17.Kaunitz AM, Portman DJ, Hait H, Reape KZ. Adding low-dose estrogen to the hormone-free interval: impact on bleeding patterns in users of a 91-day extended regimen oral contraceptive. Contraception. 2009;79:350–355. doi: 10.1016/j.contraception.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Jeppsson S, Gershagen S, Johansson ED, Rannevik G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol. 1982;99:339–343. doi: 10.1530/acta.0.0990339. [DOI] [PubMed] [Google Scholar]
- 19.Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466–1474. doi: 10.1016/j.fertnstert.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580–1586. doi: 10.1016/j.fertnstert.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 21.Kaunitz AM, Arias R, McClung M. Bone density recovery after depot medroxyprogesterone acetate injectable contraception use. Contraception. 2008;77:67–76. doi: 10.1016/j.contraception.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Renner RM, Edelman AB, Kaunitz AM. Depot medroxyprogesterone acetate contraceptive injections and skeletal health. Womens Health (Lond Engl) 2010;6:339–342. doi: 10.2217/whe.10.17. [DOI] [PubMed] [Google Scholar]
- 23.Cromer BA, Scholes D, Berenson A, Cundy T, Clark MK, Kaunitz AM. Depot medroxyprogesterone acetate and bone mineral density in adolescents--the Black Box Warning: a Position Paper of the Society for Adolescent Medicine. J Adolesc Health. 2006;39:296–301. doi: 10.1016/j.jadohealth.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Tilakaratne A, Soory M, Ranasinghe AW, Corea SM, Ekanayake SL, de Silva M. Periodontal disease status during pregnancy and 3 months post-partum, in a rural population of Sri-Lankan women. J Clin Periodontol. 2000;27:787–792. doi: 10.1034/j.1600-051x.2000.027010787.x. [DOI] [PubMed] [Google Scholar]
- 25.Tilakaratne A, Soory M. Androgen metabolism in response to oestradiol-17beta and progesterone in human gingival fibroblasts (HGF) in culture. J Clin Periodontol. 1999;26:723–731. doi: 10.1034/j.1600-051x.1999.t01-4-261101.x. [DOI] [PubMed] [Google Scholar]
- 26.Tilakaratne A, Soory M. Modulation of androgen metabolism by estradiol-17beta and progesterone, alone and in combination, in human gingival fibroblasts in culture. J Periodontol. 1999;70:1017–1025. doi: 10.1902/jop.1999.70.9.1017. [DOI] [PubMed] [Google Scholar]
- 27.Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat. 2004;23:1–48. [PubMed] [Google Scholar]
- 28.Abma JC, Sonenstein FL. Sexual activity and contraceptive practices among teenagers in the United States, 1988 and 1995. Vital Health Stat. 2001;23:1–79. doi: 10.1037/e304002003-001. [DOI] [PubMed] [Google Scholar]
- 29.Pinkston Koenigs LM, Miller NH. The contraceptive use of Depo-Provera in U.S. adolescents. J Adolesc Health. 1995;16:347–349. doi: 10.1016/S1054-139X(94)00047-I. [DOI] [PubMed] [Google Scholar]
- 30.Brown LJ, Loe H. Prevalence, extent, severity and progression of periodontal disease. Periodontol 2000. 1993;2:57–71. doi: 10.1111/j.1600-0757.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination operations. Survey Questionnaires, Examination Components and Laboratory Components 1999-2000. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2011. [November 5th, 2011]. Available http://www.cdc.gov/nchs/data/nhanes/crhq.pdf. [Google Scholar]
- 32.Dye BA, Barker LK, Selwitz RH, et al. Overview and quality assurance for the National Health and Nutrition Examination Survey (NHANES) oral health component, 1999-2002. Community Dent Oral Epidemiol. 2007;35:140–151. doi: 10.1111/j.1600-0528.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 33.Dye BA, Nowjack-Raymer R, Barker LK, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES) 2003-04. Public Health Dent J. 2008;68:218–226. doi: 10.1111/j.1752-7325.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 34.Tsakos G, Sabbah W, Hingorani AD, et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J Hypertens. 2010;28:2386–2393. doi: 10.1097/HJH.0b013e32833e0fe1. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services . In: National Center for Health Statistics. Third National Health and Nutrition Examination Survey, 1988-94, Plan and Operations Procedures Manuals (CD-ROM) Centers for Disease Control and Prevention, editor. Hyattsville, MD: 1996. [Google Scholar]
- 36.United States Food and Drug Administration Black box warning on Depo-Provera contraceptive injection. 2004 Nov 17; [Google Scholar]
- 37.Viola AS, Castro S, Bahamondes MV, Fernandes A, Viola CF, Bahamondes L. A cross-sectional study of the forearm bone mineral density in long-term current users of the injectable contraceptive depot medroxyprogesterone acetate. Contraception. 2011;84:31–37. doi: 10.1016/j.contraception.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Harel Z, Johnson CC, Gold MA, Cromer B, et al. Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception. 81:281–291. doi: 10.1016/j.contraception.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 39.van der Weijden GA, de Slegte C, Timmerman MF, van der Velden U. Periodontitis in smokers and non-smokers: intra-oral distribution of pockets. J Clin Periodontol. 2001;28:955–960. doi: 10.1034/j.1600-051x.2001.028010955.x. [DOI] [PubMed] [Google Scholar]
- 40.Brunner Huber LR, Huber KR. Contraceptive Choices of Women 35-44 Years of Age: Findings From the Behavioral Risk Factor Surveillance System. Annals of Epidemiology. 2009;19:823–833. doi: 10.1016/j.annepidem.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Bhat M. Periodontal health of 14-17-year-old US schoolchildren. J Public Health Dent. 1991;51:5–11. doi: 10.1111/j.1752-7325.1991.tb02168.x. [DOI] [PubMed] [Google Scholar]
- 42.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2011;89:1208–1213. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]