Abstract
Our approach to study multi-pollutant aerosols isolates a single emissions source, evaluates the toxicity of primary and secondary particles derived from this source, and simulates chemical reactions that occur in the atmosphere after emission. Three U.S. coal-fired power plants utilizing different coals and with different emission controls were evaluated. Secondary organic aerosol (SOA) derived from α-pinene and/or ammonia was added in some experiments. Male Sprague-Dawley rats were exposed for 6 h to filtered air or different atmospheric mixtures. Scenarios studied at each plant included the following: primary particles (P); secondary (oxidized) particles (PO); oxidized particles + SOA (POS); and oxidized and neutralized particles + SOA (PONS); additional control scenarios were also studied. Continuous respiratory data were obtained during exposures using whole body plethysmography chambers. Of the 12 respiratory outcomes assessed, each had statistically significant changes at some plant and with some of the 4 scenarios. The most robust outcomes were found with exposure to the PO scenario (increased respiratory frequency with decreases in inspiratory and expiratory time); and the PONS scenario (decreased peak expiratory flow and expiratory flow at 50%). PONS findings were most strongly associated with ammonium, neutralized sulfate, and elemental carbon (EC) in univariate analyses, but only with EC in multivariate analyses. Control scenario O (oxidized without primary particles) had similar changes to PO. Adjusted R2 analyses showed that scenario was a better predictor of respiratory responses than individual components, suggesting that the complex atmospheric mixture was responsible for respiratory effects.
Keywords: Respiratory function, particulate matter, ambient particles, power plant emissions, aerosol sources, pollution sources, breathing pattern, respiratory mechanics
Introduction
Particulate air pollution consists of primary particles emitted from sources of pollution and secondary particles formed by chemical reactions of gases in the atmosphere (Derwent, 1999). Sources contributing to air pollution include power plants, vehicles, home heating and combustibles, industrial plants and natural sources (Holman, 1999). The TERESA approach isolates a single source of pollution and studies both primary and secondary particles derived from this source. For these studies, three U.S. coal-fired power plants with different types of coal and with different pollution control equipment were used. The emissions from the power plant stacks were delivered to a photolytic chamber to simulate in a compressed period of time the chemical reactions that occur in the atmosphere, aging the emissions and delivering the fine particulate fraction to experimental animals (Ruiz et al., 2007b). Four different experimental scenarios were used to develop a step-wise increase in complexity of the reaction, and an additional 3 control scenarios were used to further delineate effects of these exposures. An array of pulmonary and cardiovascular response outcomes were studied to determine whether adverse health effects result from these exposures. This paper reports effects on breathing pattern.
Previous studies from our group (Godleski et al., 2000; Nikolov et al., 2008; Clarke et al., 1999) have shown changes in respiratory patterns in animals exposed to concentrated ambient particles (CAPs) from Boston, and the association of these changes with exposure components considered markers for major air pollution sources. These studies have also illustrated the complexity of isolating sources of pollution solely based on the characterization of the exposure. The TERESA approach attempts to address this problem by isolating specific sources. Measurements of breathing pattern are accepted indicators of physiological effects and have been used in numerous studies (Clarke et al., 1999; Glaab et al., 2006; Glaab et al., 2002; Heinrich et al., 1989; Saldiva et al., 1985). In many of these studies, baseline and post-exposure measurements of parameters are the basis of evaluation of the respiratory effects of the exposure. Continuous respiratory monitoring (during the exposure) allows us to have a more detailed understanding of the physiological responses to any given exposure and correlate how these responses change over the length of the exposure (Clougherty et al., 2010).
Materials and methods
Exposure system
The exposure system was designed to simulate chemical reactions that power plant emissions undergo in a plume during transport from the stack to receptor areas (e.g. urban areas), the main features of the exposure system and facilities used have been described in detail (Ruiz et al., 2007a; Kang et al., 2011; Godleski et al., 2011b).
Mobile lab
A mobile laboratory was designed and constructed specifically for this project. The mobile lab has two parts: (i) Bus with the photochemical chambers, pre-exposure sampling equipment and devices to remove excess pollutants gases (Ruiz et al., 2007a; Ruiz et al., 2006; Ruiz et al., 2007b); and (ii) The toxicological laboratory, a Wells-Cargo trailer custom built as a laboratory and conditioned to meet NIH standards for the care and housing of animals for research (temperature and humidity control, air changes per hour, light cycles, security and remote monitoring). More details on the mobile laboratory are provided by Godleski et al. (2011a) and Kang et al. (2011).
Individual exposure/plethysmography chambers were designed and built for these experiments. A clear cylinder of polycarbonate (10 cm in diameter × 18 cm in length) was fitted with connectors for air delivery and connections for airflow transducers (Buxco Electronics, Sharon, CT). An anodized table was constructed to hold these chambers and serve as the exposure area for the animals. The table had panels that shield the space between animals providing a private space for the animals during the exposures. This table had spaces for 12 exposure unit chambers, individual flow meters for each chamber with a range of up to 4 LPM, and a system of filtration for the exhaust air from the chambers.
Animals
Male Sprague-Dawley CD rats 250–300 g were obtained from Charles River Laboratories (Portage, MI), delivered to the power plant, housed, and managed according to the NIH guidelines for the care and use of laboratory animals. Upon arrival, animals were assigned a unique identification number, which determined the exposure date and exposure group (aerosol or filtered air) for the animal. Veterinary care and husbandry of the animals was contracted with local universities at each site. These universities also served as overseers of our animal procedures and reported directly to the compliance office for the Harvard Medical Area Standing Committee on the use of animals in research.
Experimental design and breathing pattern analyses
For any given day of exposure, five animals were exposed to aerosol and five to filtered room air in the individual exposure chambers for 6 h. These chambers were clear polycarbonate chambers to allow visualization of the animals during the exposure. The animals usually took 10–20 min to adapt to these exposure chambers, and after this acclimation time, they were asleep during most of the exposure.
These chambers also served as whole body plethysmographs for assessment of breathing patterns throughout the exposure.
Flow through each chamber was maintained at 1.5 LPM and Buxco airflow transducers (TRD5700) (Buxco Electronics Inc. Wilmington, NC) were connected to the chambers and to a reference chamber to compensate for changes in pressure in the system. Each chamber was calibrated to its respective transducer using a 1.5-LPM flow at the beginning of each scenario (week), and a daily check of the accuracy of these calibrations was performed before each exposure. Continuous breathing cycle data were collected from each animal. These data were reduced to 10-min averages of the following parameters for each animal: Frequency (f), Tidal Volume (TV), Inspiratory Time (Ti), Expiratory time (Te), Enhanced Pause (Penh), Accumulated Volume (AV), Minute Volume (MV), Peak of Inspiratory Flow (PIF), Peak of Expiratory Flow (PEF), Relaxation Time (RT), End Inspiratory Pause (EIP), End Expiratory Pause (EEP), Delta Inspiratory–Expiratory Flow (dV), Expiratory Flow at 50% (EF50) and Pause (PAU). Units of measure for these parameters are as follow: Times are in seconds, volumes in mL, flows in mL/s, and frequency is given in breaths/minute. A rejection algorithm was automatically included in the breath-by-breath analysis to eliminate noise resulting from animal movement within the chamber.
Power plants
Three US coal-fired power plants using different types of coal and with different emissions controls were included in this study; plant-specific characteristics are presented in detail by Godleski et al. (2011a) and Kang et al. (2011). Power plant 1 (PP1) was located in the Upper Midwest, power plant 2 (PP2) was located in the Southeast, and power plant 3 (PP3) was located in the Midwest. Briefly, PP1 burned a low sulfur (0.2%) sub-bituminous coal and was equipped with an electrostatic precipitator (ESP) for primary PM control. PP2 burned a low-to-medium sulfur (~1%) bituminous coal and was equipped with an ESP and a selective catalytic reduction (SCR) system for control of nitrogen oxides (NOx). Finally, PP3 burned a high sulfur (~3%) bituminous coal and had an ESP, SCR, and a wet flue gas desulfurization (FGD) scrubber for the removal of sulfur dioxide (SO2).
Scenarios
In order to simulate atmospheric transformations that coal power plant emissions undergo in a plume, the following scenarios were chosen (Kang et al., 2011): (i) primary emissions only (“P”); (ii) the oxidation of SO2 to form H2SO4 aerosol, along with primary particles (“PO”); (iii) the oxidation of SO2 plus the reaction of α-pinene with ozone to form secondary organic aerosol (SOA), along with primary particles (“POS”); (iv) the neutralization of H2SO4 aerosol by NH3, along with primary particles and SOA (“PONS”); (v) a control scenario including oxidation of SO2 scenario that included primary gases but excluded primary particles (“O”); (vi) the O control scenario with added SOA (“OS”), and; (7) a control scenario with no primary particles or gases and only SOA produced using particle-free ambient air (“S”). The control scenarios (“O”, “OS”, and “S”) were conducted only at plant 3. Thus, for any given scenario, at least 4 days of 6-h exposures with 5 rats exposed to the aerosol of the scenario and 5 control animals on each day were assessed. The number of animals per scenario was not always the same because some scenarios were repeated; for example, the PONS scenario at plant 1 was repeated 3 times so that 12 exposure days were analyzed. Since this was the first plant studied, we repeated scenarios in some cases to be certain outcomes were optimized and not influenced by the field setting. Since we had no reason to reject these collected data, they were included in the final analyses. Because filtered air exposures were conducted on each exposure day, this imbalance in sample size does not bias our scenario-specific estimates of exposure, but does cause tests of an effect to be more powerful for some scenarios than for others. Therefore, in interpreting the results of our statistical analyses, we focus on not only the strength (statistical significance) of the effects but also on the magnitude of these estimated effects.
The total number of animals used and analyzed for respiratory function per scenario in most instances was 20 exposed and 20 controls. Each animal was exposed to either the scenario aerosol or filtered air once, and then sacrificed after exposure for other assessments. These included pulmonary inflammation reported by Godleski et al. in this series and in vivo chemiluminescence reported in Lemos et al. in this series. Continuous respiratory data were collected during 6 h of exposure, analyzed individually as primary outcomes, and then combined for all plants and scenarios to assess the effects of individual measured exposure components. A total of 78 exposure days were included in the study.
Statistical assessment
Detailed explanation of the statistical analysis is included in Coull et al. (2011). Briefly, exploratory analyses of the respiratory outcomes indicated some outcomes were normally distributed and some were log-normally distributed. However, because of the large amount of data for the respiratory outcomes, the central limit theorem protects against misspecification of the normal assumption in regression analyses for these outcomes. It is likely that inferences from regression models fit to the untransformed outcomes are valid. Therefore, for consistency of interpretation across respiratory outcomes, all models were applied to the data on the original scale. Sensitivity analyses were then run on the log-transformed values to confirm that these inferences were qualitatively similar, and this was indeed the case. The statistical analyses used multi-layered approaches, whereby multiple analyses that assess exposure metrics of increasing sensitivity are conducted. First, ANOVA techniques that treat exposure as a categorical variable were used. In these ANOVA analyses, we used additive mixed models (Coull et al., 2001; Ruppert et al., 2003) to assess whether there was evidence that differences between exposure groups vary across the period of exposure. This model specified distinct mean trends over the exposure period for the exposed and filtered air animals, including random animal effects to account for the repeated measurements taken on each animal. The difference between these estimated trends represents the time-varying effect of the experimental exposure over the exposure period. We fitted this model to each day’s data separately. Seeing little evidence of time-varying effects of exposure within an exposure period, we fitted a simplified ANOVA model that specified a constant effect of exposure over time. These models were fitted to data from each plant separately as well as to data from all three plants together. As mentioned above, our interpretation of the results focuses on both the estimated magnitude of the estimated health effects, as well as the estimated strength of these associations. Based on a Bonferroni correction for multiple comparisons (Coull et al., 2011), we consider p-values less than 0.007 as strong evidence of an exposure effect for a given scenario, and a p-value satisfying 0.007 < p < 0.05 as marginal evidence of an effect.
Next, to assess univariate associations between mass levels or exposure composition and health, we conducted single-component analyses in which a separate regression model was fitted using differences between exposed and filtered air responses as the outcome and either mass, particle number, or a measured component concentration as the exposure metric. We used the resulting p-values from these models to rank the strength of associations between each component and health. To support univariate findings, we used the concept of variable importance in a random forest analysis to investigate joint effects of multiple pollution components. The reader is directed to Coull et al. (2011) for a full description of the models that correspond to each level of the analysis hierarchy. As a final analysis, adjusted R2 values were used to compare the relative importance of scenario vs. individual components.
Results
Exposure data
The three power plants were all roughly the same size, but had differences in emissions controls and types of coal used. Table 1 summarizes the concentrations of particulate species for the exposures by scenario and power plant. More detailed information on the characterization and exposure can be found in Kang et al. (2011).
Table 1.
Summary of aged particulate species derived from the three coal-fired power plants for the TERESA study.
| Power plant | Exposure scenario | Aged particle mass (μg/m3) | Particle number (#/cm3) | Total sulfate (μg/m3) | Acidic sulfatea (μg/m3) | Neutralized sulfateb (μg/m3) | Organic carbon (μg/m3) |
|---|---|---|---|---|---|---|---|
| PP 1 | P (n = 4)c | 1.0 ± 0.9 | 1726 ± 1277 | 0.2 ± 0.3 | 2.3 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PO (n = 3) | 46.0 ± 12.6 | 6723 ± 3550 | 36.1 ± 7.7 | 27.6 ± 9.5 | 8.4 ± 2.6 | 2.6 ± 4.5 | |
| POS (n = 4) | 123.3 ± 28.4 | 16924 ± 4495 | 55.8 ± 22.8 | 50.2 ± 21.6 | 5.6 ± 3.4 | 51.6 ± 8.6 | |
| PONS (n = 12) | 154.9 ± 41.7 | 52109 ± 11951 | 68.2 ± 28.8 | 14.7 ± 13.6 | 53.6 ± 16.8 | 30.2 ± 16.4 | |
| PP 2 | P (n = 4) | 1.7 ± 1.8 | 910 ± 964 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PO (n = 4) | 115.5 ± 18.5 | 4281 ± 1911 | 100.3 ± 16.3 | 71.6 ± 17.0 | 28.8 ± 1.3 | 0.0 ± 0.0 | |
| POS (n = 8) | 212.1 ± 39.7 | 11473 ± 3774 | 146.0 ± 36.7 | 107.9 ± 31.7 | 38.1 ± 12.0 | 59.0 ± 20.2 | |
| PONS (n = 4) | 257.1 ± 10.0 | 40811 ± 2179 | 154.8 ± 12.4 | 15.7 ± 3.8 | 139.1 ± 15.5 | 35.1 ± 10.1 | |
| PP 3 | P (n = 4) | 43.2 ± 14.6 | 55947 ± 11769 | 34.0 ± 13.3 | 12.8 ± 7.1 | 21.2 ± 9.2 | 1.9 ± 3.8 |
| PO (n = 4) | 82.3 ± 15.6 | 69372 ± 8523 | 77.9 ± 14.5 | 66.6 ± 16.8 | 10.3 ± 2.8 | 0.0 ± 0.0 | |
| POS (n = 8) | 144.4 ± 31.6 | 40446 ± 6657 | 83.3 ± 21.3 | 68.9 ± 18.2 | 14.5 ± 7.1 | 54.7 ± 27.5 | |
| PONS (n = 4) | 173.5 ± 20.9 | 38483 ± 3651 | 85.0 ± 12.9 | 2.5 ± 2.0 | 82.5 ± 13.5 | 52.0 ± 23.0 | |
| OS (n = 4) | 137.8 ± 9.3 | 35959 ± 6290 | 47.2 ± 14.6 | 30.3 ± 11.6 | 16.9 ± 11.6 | 83.6 ± 9.6 | |
| O (n = 4) | 43.8 ± 3.5 | 29294 ± 2392 | 40.6 ± 3.8 | 31.7 ± 5.8 | 8.9 ± 2.3 | 0.0 ± 0.0 | |
| S (n = 4) | 61.4 ± 6.6 | 7574 ± 1598 | 1.3 ± 0.4 | 1.0 ± 1.3 | 0.7 ± 0.5 | 59.7 ± 6.1 |
Acidic sulfate was calculated from strong acidity (pH) measurements as the equivalent concentration of H2SO4 aerosol.
Neutralized sulfate = Total sulfate - Acidic sulfate.
Number of days.
All values are average ± standard deviation.
The differences between the plants and scenarios resulted in differences in the PM mass and composition of exposures. Exposures at PP1 and PP2 had the lowest primary particle concentrations in the P scenario (1.0 and 1.7 μg/m3, respectively), whereas PP3 had a substantially higher concentration (43.2 μg/m3). The P scenario particles at PP3 tended to be primarily comprised of sulfate (79%), both in its acidic and neutralized forms, due to the operation of the FGD system. PP2 had the highest mass concentrations in the aged particle scenarios (PO, POS, and PONS), and the highest sulfate concentrations. Total mass concentrations in these scenarios ranged from 46.0 to 154.9 μg/m3 at PP1, 115.5–257.1 μg/m3 at PP2, and 82.3–173.5 μg/m3 at PP3). The POS and PONS scenarios, which included added SOA, resulted in high organic carbon (OC) concentrations at all three plants, ranging from 35.1 to 54.7 μg/m3. Neutralized sulfate was highest in the PONS scenario (53.6–139.1 μg/m3). Acidic sulfate was not completely neutralized in the PONS scenarios at PP1 and PP2, with residual acidic sulfate remaining of 14.7 and 15.7 μg/m3, respectively.
Breathing pattern
All respiratory data were analyzed as 10 min averages over the 6-h exposure time. Figure 1A and 1B illustrate this time-based approach showing EF50 in the PO scenario at PP2. Figure 1A illustrates the average of all exposed animals (dark line)compared to the average of the control animals (light line) over the 6 h of exposure. After the initial period of acclimation in which EF50 decreases in both the exposed and filtered air groups, there was lesser flow in the exposed group compared to control; Figure 1B illustrates the average difference between individual exposed animals and the daily average for the corresponding control animals over time (solid line) with the standard errors (dotted line). For subsequent analyses, all respiratory data were assessed as differences between specific parameters in individual exposed animals and the average control (filtered air sham exposures) for any given day. Thus, all biological parameter outcomes were assessed statistically as differences from control. These differences from control were assessed by power plant by scenario, and then using pooled data from all plants (for a given scenario) to provide an overall picture of the response. Individual exposure measurements using univariate analyses and random forest approaches used combined data, but specify plant and scenario.
Figure 1.
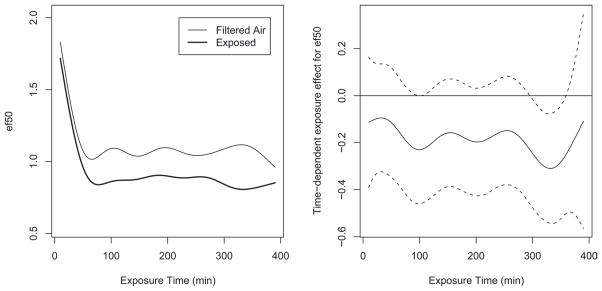
Trend over time of exposure for EF50 in the PO scenario in PP2. Figure 1A illustrates the average of all exposed animals (dark line) compared to the average of the control animals (light line) over time; this difference was marginally significant by ANOVA analyses. Figure 1B illustrates the difference between individual exposed animals and the daily average for the corresponding control animals over time (solid line) with the standard errors (dotted line).
Results by power plant/scenario
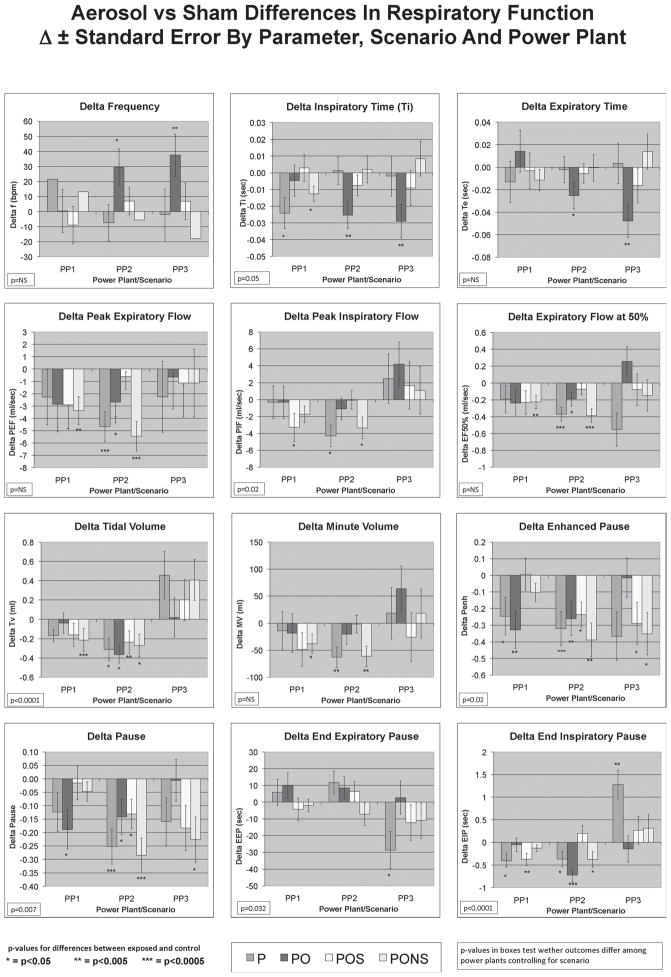
Figure 2 shows the difference between exposed and control animals in respiratory parameters at the individual power plants. Changes from control with exposure having p-values <0.007 are considered robust based on the Bonferoni correction, whereas those with p < 0.05 are considered marginally significant (see Coull et al 2011 and the description above). Significant increases in frequency and decreases in Ti and Te were observed at PP3, with less significant changes at PP2 and no significant changes at PP1. Since there were no instances where p-values fell between 0.007 and 0.005, we show all robust p-values as p < 0.005. Decreases in PEF, PIF, and EF50 were exhibited most strongly at PP2, and were supported to a lesser extent in PP1, whereas at PP3 there were no significant changes in any flow parameters. Decreases in TV were observed at PP1 and PP2, but not in PP3 where it increased. Statistically significant decreases in Enhanced Pause (Penh) were observed at all power plants. There was little change in End Expiratory Pause; however, End Inspiratory Pause was significantly reduced at PP1 and PP2, but not at PP3 (increased). There were decreases in respiratory pause in all power plants, but they only reached a significant level in PP2, the PO scenario in PP1 and the PONS scenario in PP3.
Figure 2.
Differences in respiratory parameters by scenario and power plant. Changes in frequency and times of inspiration and expiration were observed at PP2 and PP3, while changes in expiratory flow parameters in the PONS scenario were robust at PP2 and in the P scenario at PP1. PP2 showed the most respiratory effects overall. Based on the Bonferroni corrections for multiple comparisons, p < 0.007 was considered as strongly significant, and all p-values at p < 0.005 exceed this criteria. The p-values for plant-to-plant differences controlling for scenario are indicated in the box on each respiratory parameter graph. (See colour version of this figure online at www.informahealthcare.com/iht)
Since there are plant-to-plant differences in responses, we assessed the differences in outcomes between plants controlling for scenario. Of the 12 respiratory parameters, 3 have strongly significant differences when comparing among plants. These are TV, EIP and Pause. Ti, PIF, EEP and Penh are marginally significant, and the remainder are not significant. The p-value for these plant-to-plant differences are noted on each respiratory parameter graph in Figure 2.
Results by scenario (all power plants combined)
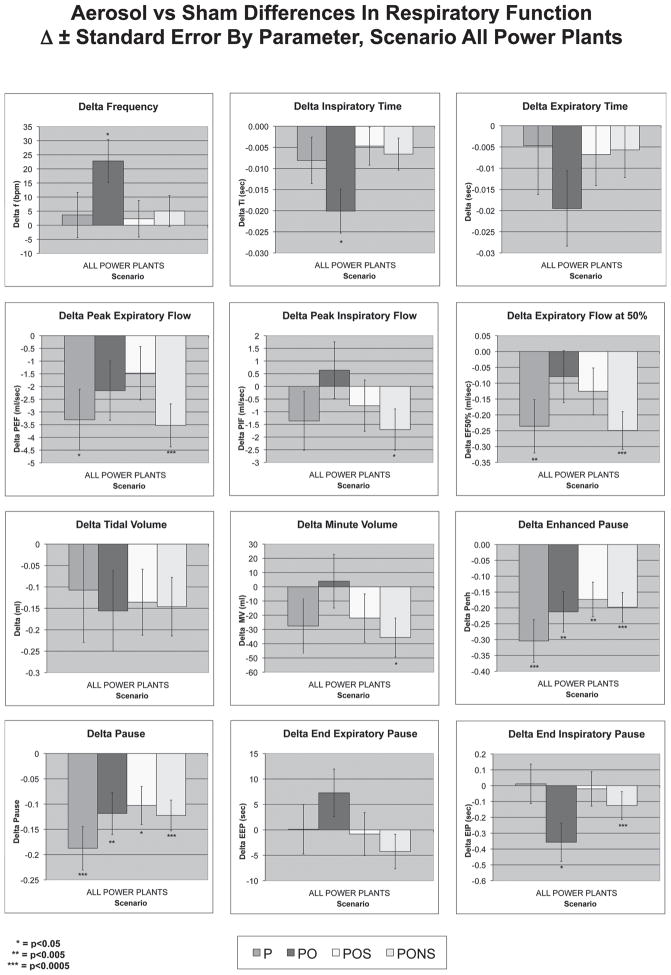
Since there were only 3 parameters exhibiting robust plant-to-plant differences, combining data from all plants for ANOVA analyses as well as to explore univariate and multivariate analysis in relationship to compositional components was done. Figure 3 shows the data for all plants combined by scenario. Robust decreases in PEF and EF50 were observed with the PONS scenario. Reduced EIP was also observed in response to the PONS scenario (p < 0.0005). Pause and Penh had significant reductions with all scenarios. Marginally significant changes in frequency, Ti, Te, and PIF were also observed with exposure to various scenarios.
Figure 3.
Differences in respiratory parameters by scenario. Data from all power plants combined, graphics show the difference between filtered air control and TERESA aerosol-exposed animals by scenario in all power plants combined. Robustly significant findings included reduced PEF and EF50 with the PONS scenario, and reduced Penh in all scenarios. (See colour version of this figure online at www.informahealthcare.com/iht)
Control scenarios
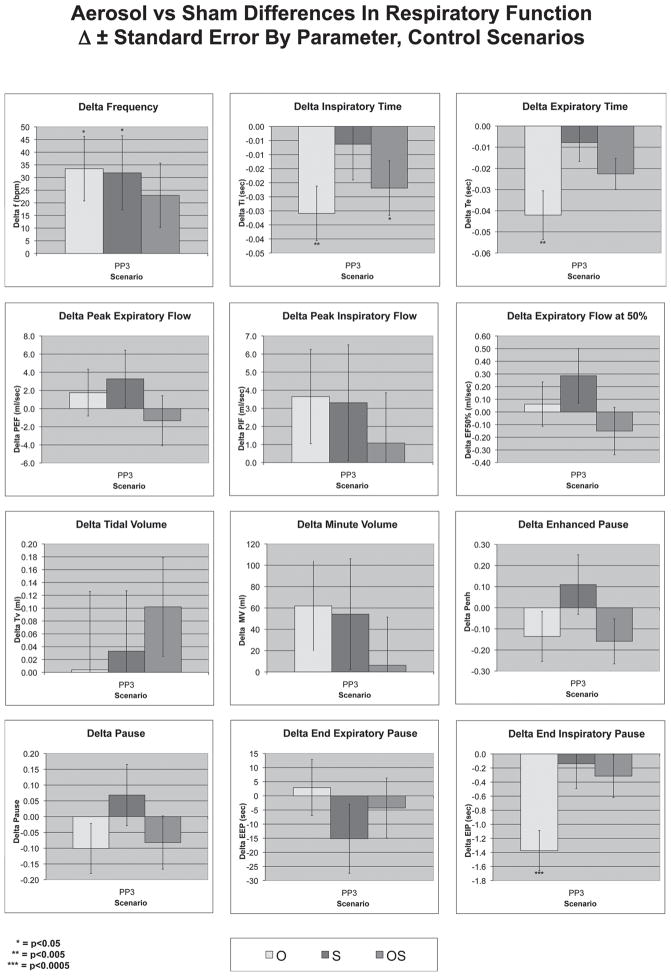
Figure 4 shows control scenarios (without primary particles) conducted at PP3. The only strongly significant findings were reductions in Ti, Te, and EIP in response to the O scenario. There were marginally significant increases in f with the O and S scenarios.
Figure 4.
Differences in respiratory parameters in control scenarios in PP3. The O scenario produced a strongly significant reduction in Ti, Te, and EIP. No flow parameters were affected in the control scenarios. (See colour version of this figure online at www.informahealthcare.com/iht)
Univariate analyses
Univariate analyses assessed dose response relationships for various exposure metrics and specific respiratory parameter outcomes. Table 2 shows the standardized respiratory parameter changes vs. components of the aerosol using all data from all power plants. There were 33 relationships with p < 0.01 out of 336 assessments. Of these, 13 had p < 0.001. Increases in frequency were associated with NO (p < 0.01), while decreases were associated with Al (p < 0.01). Decreases in f were associated with Fe, Zn, and NH4 (p < 0.05). NO was also associated with reductions in Ti (p < 0.05) and Te (p < 0.01) that typically accompany increases in f. An increase in Ti was observed for pinene (p < 0.05). Statistically significant decreases in TV were associated with OC, TC, Si, and Pb, whereas significant increases were observed with Ni and Mg. Only elemental carbon (EC) was associated with a robust decrease in MV, while Na was associated with a significant increase in this parameter. Decreases (p < 0.05) in MV were observed with NH4, Pb, NO2, and NO3, whereas increases were associated with Mg. A significant reduction in PIF was associated with EC, with marginally significant decreases observed with NH4, NO2, and NO3. Marginally significant increases in PIF were observed with Mg, Na, and NO.
Table 2.
Univariate analyses: Standardized respiratory parameter changes vs. aerosol composition.
| F | Ti | Te | Tv | Mv | PIF | PEF | EF50 | PAU | EIP | EEP | Penh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | Coef ± SE | |
| Mass | −3.20 ± 2.59 | 0.002 ± 0.002 | 0.000 ± 0.003 | −0.04 ± 0.04 | −10.71 ± 6.45 | −0.60 ± 0.40 | −0.41 ± 0.33 | −0.04 ± 0.03 | −0.01 ± 0.02 | 0.07 ± 0.10 | −0.81 ± 1.91 | −0.02 ± 0.02 |
| PN | 0.10 ± 2.66 | −0.001 ± 0.002 | −0.003 ± 0.003 | 0.07 ± 0.04 | 4.48 ± 6.66 | 0.46 ± 0.40 | −0.28 ± 0.34 | 0.02 ± 0.03 | 0.02 ± 0.02 | 0.15 ± 0.10 | −4.08 ± 1.86b | 0.02 ± 0.02 |
| Total SO4 | −0.40 ± 2.62 | 0.000 ± 0.002 | −0.004 ± 0.003 | −0.03 ± 0.04 | −6.25 ± 6.54 | −0.12 ± 0.40 | −0.32 ± 0.33 | −0.02 ± 0.03 | −0.03 ± 0.01 | 0.06 ± 0.10 | −0.41 ± 1.92 | −0.04 ± 0.02 |
| Acidic SO4 | 4.48 ± 2.58 | −0.004 ± 0.002 | −0.006 ± 0.003 | −0.03 ± 0.04 | 5.10 ± 6.58 | 0.59 ± 0.40 | 0.58 ± 0.33 | 0.05 ± 0.03b | 0.00 ± 0.02 | 0.02 ± 0.10 | 2.65 ± 1.92 | 0.00 ± 0.02 |
| Neutralized SO4 | −4.39 ± 2.58 | 0.003 ± 0.002 | 0.001 ± 0.003 | −0.01 ± 0.04 | −12.29 ± 6.43 | −0.66 ± 0.40 | −0.88 ± 0.32* | −0.06 ± 0.03* | −0.03 ± 0.01b | 0.06 ± 0.10 | −2.72 ± 1.92 | −0.04 ± 0.02b |
| NH4 | −5.00 ± 2.55b | 0.004 ± 0.002 | 0.002 ± 0.003 | −0.02 ± 0.04 | −13.99 ± 6.35b | −0.80 ± 0.39b | −0.94 ± 0.32* | −0.07 ± 0.03* | −0.03 ± 0.01b | 0.06 ± 0.10 | −2.39 ± 1.89 | −0.04 ± 0.02 |
| S | −0.40 ± 2.62 | 0.000 ± 0.002 | −0.004 ± 0.003 | −0.03 ± 0.04 | −6.25 ± 6.54 | −0.12 ± 0.40 | −0.32 ± 0.33 | −0.02 ± 0.03 | −0.03 ± 0.01 | 0.06 ± 0.10 | −0.41 ± 1.92 | −0.04 ± 0.02 |
| OC | −2.95 ± 2.60 | 0.003 ± 0.002 | 0.004 ± 0.003 | −0.01 ± 0.04** | −2.43 ± 6.58 | −0.37 ± 0.40 | 0.21 ± 0.33 | −0.01 ± 0.03 | 0.01 ± 0.02 | 0.06 ± 0.10 | −0.55 ± 1.92 | 0.02 ± 0.02 |
| EC | −4.42 ± 2.57 | 0.003 ± 0.002 | 0.001 ± 0.003 | −0.12 ± 0.04 | −18.25 ± 6.19* | −1.27 ± 0.37** | −0.77 ± 0.32b | −0.07 ± 0.03* | 0.00 ± 0.02 | −0.05 ± 0.10 | 2.78 ± 1.89 | −0.01 ± 0.02 |
| TC | −3.36 ± 2.59 | 0.003 ± 0.002 | 0.004 ± 0.003 | −0.03 ± 0.04** | −4.61 ± 6.56 | −0.51 ± 0.40 | 0.11 ± 0.34 | −0.02 ± 0.03 | 0.01 ± 0.02 | 0.05 ± 0.10 | −0.18 ± 1.92 | 0.02 ± 0.02 |
| Al | −6.76 ± 2.56* | 0.004 ± 0.002 | 0.006 ± 0.003 | 0.12 ± 0.04 | 7.54 ± 6.70 | 0.44 ± 0.41 | −0.26 ± 0.34 | 0.00 ± 0.03 | −0.02 ± 0.02 | 0.31 ± 0.10** | −4.03 ± 1.90b | −0.04 ± 0.02 |
| Si | −3.89 ± 2.66 | 0.004 ± 0.002 | 0.003 ± 0.003 | −0.02 ± 0.04* | −7.69 ± 6.70 | −0.54 ± 0.41 | −0.53 ± 0.34 | −0.03 ± 0.03 | 0.00 ± 0.02 | 0.04 ± 0.10 | 1.97 ± 1.95 | 0.00 ± 0.02 |
| Fe | −5.84 ± 2.60b | 0.004 ± 0.002 | 0.003 ± 0.003 | 0.10 ± 0.04 | 4.48 ± 6.74 | 0.31 ± 0.41 | −0.08 ± 0.34 | 0.00 ± 0.03 | −0.03 ± 0.01b | 0.18 ± 0.10 | −3.35 ± 1.92 | −0.05 ± 0.02b |
| Ni | −4.76 ± 2.63 | 0.003 ± 0.002 | 0.004 ± 0.003 | 0.07 ± 0.04** | 1.65 ± 6.76 | 0.09 ± 0.41 | −0.10 ± 0.34 | −0.01 ± 0.03 | −0.03 ± 0.01b | 0.09 ± 0.10 | −1.94 ± 1.95 | −0.05 ± 0.02b |
| Zn | −6.2 ± 2.63b | 0.004 ± 0.002 | 0.004 ± 0.003 | 0.14 ± 0.04 | 8.03 ± 6.79 | 0.64 ± 0.41 | 0.38 ± 0.35 | 0.02 ± 0.03 | −0.01 ± 0.02 | 0.18 ± 0.10 | −3.53 ± 1.94 | −0.03 ± 0.02 |
| Pb | −0.52 ± 2.70 | 0.000 ± 0.002 | −0.001 ± 0.003 | −0.02 ± 0.04** | −13.5 ± 6.6b | −0.12 ± 0.41 | −0.24 ± 0.34 | −0.01 ± 0.03 | −0.01 ± 0.02 | −0.04 ± 0.10 | 0.28 ± 1.97 | −0.03 ± 0.02 |
| Mg | −3.67 ± 2.66 | 0.002 ± 0.002 | −0.004 ± 0.003 | 0.14 ± 0.04** | 15.15 ± 6.49b | 0.87 ± 0.40b | −0.02 ± 0.34 | 0.03 ± 0.03 | −0.02 ± 0.02 | 0.30 ± 0.10* | −3.92 ± 1.91b | −0.04 ± 0.02b |
| Na | −3.73 ± 2.66 | 0.001 ± 0.002 | −0.005 ± 0.003 | 0.13 ± 0.04 | 17.71 ± 6.39* | 0.81 ± 0.40b | −0.06 ± 0.34 | 0.02 ± 0.03 | −0.03 ± 0.02b | 0.30 ± 0.10* | −4.29 ± 1.89b | −0.06 ± 0.02* |
| O3 | 3.10 ± 2.60 | −0.002 ± 0.002 | −0.004 ± 0.003 | −0.01 ± 0.04 | 9.48 ± 6.48 | 0.39 ± 0.40 | 0.37 ± 0.33 | 0.04 ± 0.03 | 0.03 ± 0.01b | 0.10 ± 0.10 | 0.34 ± 1.92 | 0.04 ± 0.02 |
| NO | 6.44 ± 2.50* | −0.005 ± 0.002b | −0.005 ± 0.003* | 0.04 ± 0.04 | 11.81 ± 6.42 | 0.76 ± 0.39b | 0.34 ± 0.33 | 0.05 ± 0.03 | 0.00 ± 0.02 | −0.07 ± 0.10 | −1.51 ± 1.91 | 0.01 ± 0.02 |
| NO2 | 0.60 ± 2.62 | 0.000 ± 0.002 | −0.004 ± 0.003 | −0.04 ± 0.04 | −12.57 ± 6.40b | −0.77 ± 0.39b | −0.50 ± 0.33 | −0.03 ± 0.03 | 0.05 ± 0.01** | 0.02 ± 0.10 | −1.02 ± 1.91 | 0.07 ± 0.02** |
| NO3 | −2.08 ± 2.61 | 0.001 ± 0.002 | 0.001 ± 0.003 | −0.05 ± 0.04 | −14.13 ± 6.35b | −0.82 ± 0.39b | −0.75 ± 0.32b | −0.06 ± 0.03b | 0.02 ± 0.01 | 0.02 ± 0.10 | −0.46 ± 1.92 | 0.03 ± 0.02 |
| SO2 | 0.00 ± 2.62 | 0.000 ± 0.002 | −0.005 ± 0.003 | 0.03 ± 0.04 | −0.12 ± 6.58 | 0.20 ± 0.40 | 0.25 ± 0.33 | 0.02 ± 0.03 | 0.01 ± 0.02 | 0.02 ± 0.10 | −3.52 ± 1.87 | 0.01 ± 0.02 |
| Pinene | −4.41 ± 2.61 | 0.004 ± 0.002b | −0.005 ± 0.003 | −0.02 ± 0.04 | −4.50 ± 6.56 | −0.46 ± 0.40 | −0.05 ± 0.34 | −0.02 ± 0.03 | −0.02 ± 0.02 | 0.06 ± 0.10 | −1.51 ± 1.94 | −0.02 ± 0.02 |
| Formaldehyde | 1.59 ± 3.02 | 0.000 ± 0.003 | −0.005 ± 0.003 | −0.03 ± 0.03 | −3.37 ± 5.31 | −0.02 ± 0.38 | 0.06 ± 0.32 | 0.03 ± 0.02 | 0.07 ± 0.01** | 0.14 ± 0.07b | −4.35 ± 1.64* | 0.09 ± 0.02** |
| Acetaldehyde | −0.69 ± 3.02 | 0.002 ± 0.003 | −0.005 ± 0.003 | −0.01 ± 0.03 | −2.73 ± 5.32 | −0.15 ± 0.38 | 0.10 ± 0.32 | 0.02 ± 0.02 | 0.04 ± 0.02* | 0.14 ± 0.07b | −4.03 ± 1.66b | 0.06 ± 0.02* |
| Acetone | −1.51 ± 3.02 | 0.002 ± 0.003 | −0.005 ± 0.003 | 0.00 ± 0.03 | −2.71 ± 5.32 | −0.16 ± 0.38 | 0.11 ± 0.32 | 0.02 ± 0.02 | 0.04 ± 0.02* | 0.13 ± 0.07 | −3.36 ± 1.70b | 0.07 ± 0.02* |
| Total Aldehyde | −0.18 ± 3.03 | 0.001 ± 0.003 | 0.004 ± 0.003 | −0.01 ± 0.03 | −3.26 ± 5.31 | −0.11 ± 0.38 | 0.10 ± 0.32 | 0.02 ± 0.02 | 0.06 ± 0.01** | 0.15 ± 0.07b | −4.20 ± 1.65* | 0.08 ± 0.02** |
p < 0.05.
p < 0.01.
p < 0.001.
Coef: Change in Δ per SD of the concentration.
PEF and EF50 are important flow parameters, reductions in which may be indicative of bronchoconstrictive effects. Significant reductions in PEF and/or EF50 were associated with neutralized sulfate and NH4 (both p < 0.01). Reductions with EC, were PEF(p < 0.05) and EF50 (p < 0.01), whereas marginally significant associations were observed for NO3 (decrease) and acidic sulfate (increase). Significant increases in Enhanced Pause (Penh), which can also be indicative of airway restriction, were associated with NO2, formaldehyde, acetaldehyde, acetone, and total aldehydes. Reductions in Penh were associated with Na, neutralized SO4, Fe, and Ni. EIP, an increase of which can be indicative of a sensory irritant effect if coupled with a reduction in frequency, significantly increased in relation to Al, Mg, and Na, and marginally increased in relationship to formaldehyde, acetaldehyde, and total aldehydes. Increases in EEP, which can be indicative of pulmonary irritation, were not observed in association with any pollutants; however, decreases in EEP were strongly associated with formaldehyde and total aldehydes.
Multivariate analysis (random forest)
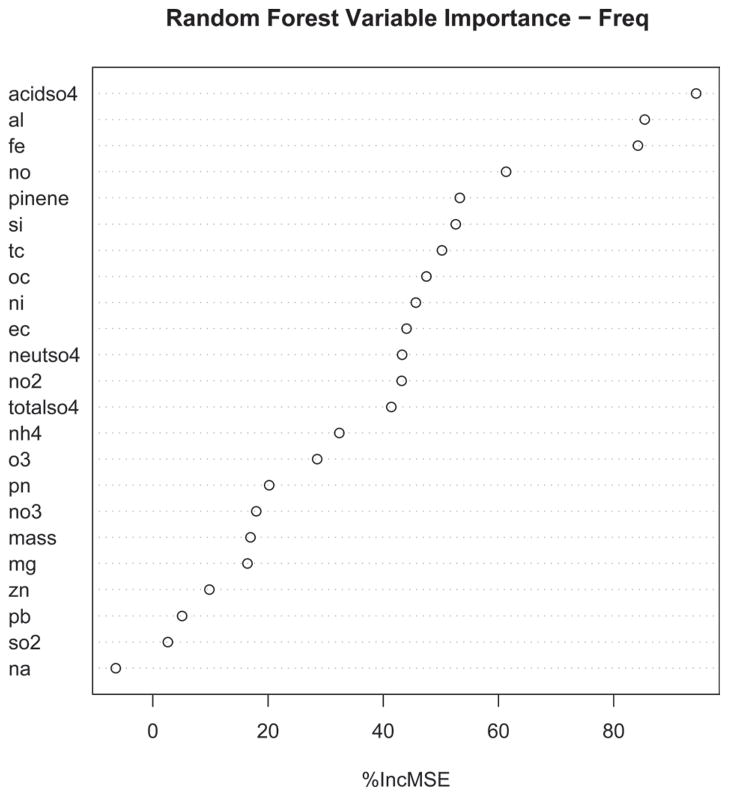
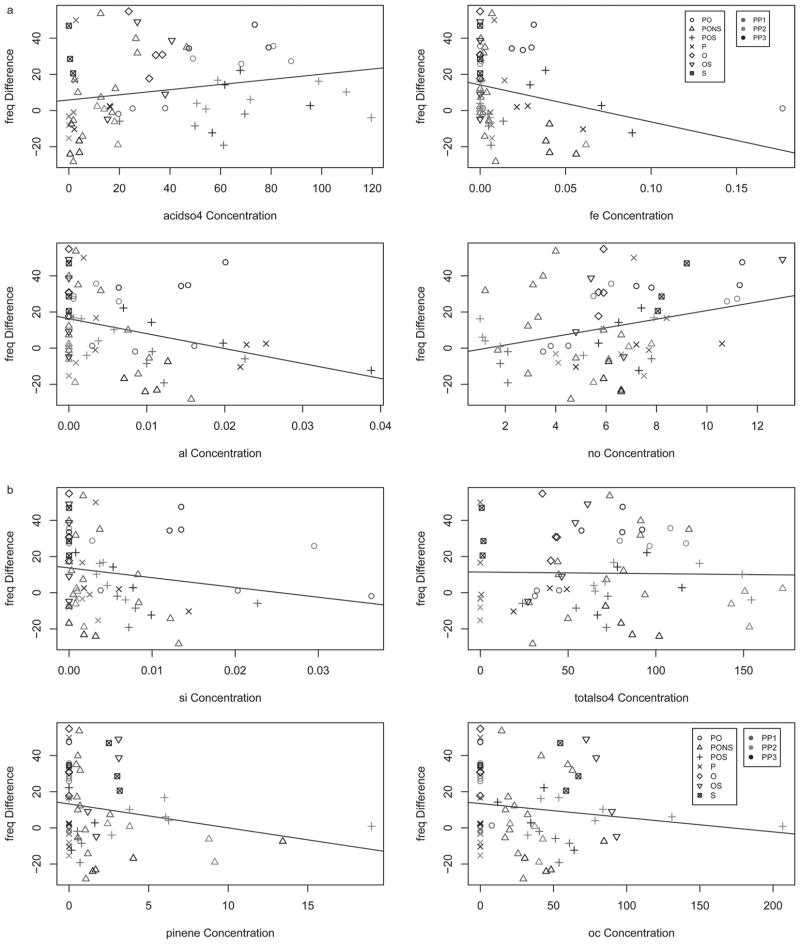
The Random Forest statistical approach was used as a form of multivariate analysis to rank the importance of each measured exposure component in predicting differences between exposed and control animals. Table 3 shows all the random forest ratings for all components and all outcomes. A typical random forest plot is shown in Figure 5. This analysis was supplemented by plotting raw mean differences for each adverse outcome vs. the components identified in the univariate and random forest analyses. Figure 5 shows the random forest for frequency; acidic sulfate was first on this list and aluminum and iron were second and third, respectively. These three pollutants are the most important variables, as evidenced by their location at the far right-hand side of the plot. Univariate analyses (Table 2) showed a positive (but not significant association with acidic sulfate), a marginally significant positive association with Fe, and a strongly significant negative association with aluminum. Figure 6A and 6B shows scatter plots for the top eight random forest ranked components with identification of data points in terms of scenario and power plant. Acid sulfate was first in the random forest ranking; however, as mentioned, the univariate analysis was not significant, as illustrated in the scatter plot, which shows a wide distribution for both outcome and concentration data. Aluminum, ranked second, had a negative slope driven by the P and POS scenarios. Fe ranked third and its negative slope appeared to be driven largely by the POS and PONS scenarios at PP3. NO ranked fourth, had a wide distribution for both outcome and concentration data, and had a positive slope with the PO and OS scenarios mostly above the slope. Pinene (Figure 6B), ranked 5th, and Si, ranked 6th were associated with a decrease in frequency. OC, ranked 8th, and total sulfate, ranked 13th, are shown to illustrate their lack of significant associations in both the univariate and random forest analyses.
Table 3.
Random forest ranking of exposure components for each respiratory function outcome. Relative importance ranking of each measured component in predicting differences between exposed and control animals.
| F
|
Ti
|
Te
|
Tv
|
Mv
|
PIF
|
PEF
|
EF50
|
PAU
|
EIP
|
EEP
|
Penh
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass | 18 | 17 | 16 | 16 | 6 | 15 | 6 | 4 | 4 | 5 | 10 | 3 |
| PN | 16 | 18 | 10 | 9 | 9 | 12 | 5 | 7 | 6 | 4 | 2 | 5 |
| Total SO4 | 13 | 11 | 5 | 12 | 12 | 20 | 15 | 11 | 2 | 6 | 14 | 2 |
| Acidic SO4 | 1 | 1 | 1 | 13 | 14 | 18 | 8 | 12 | 22 | 7 | 16 | 20 |
| Neutralized SO4 | 11 | 12 | 6 | 10 | 7 | 13 | 9 | 8 | 11 | 8 | 4 | 11 |
| NH4 | 14 | 13 | 4 | 7 | 8 | 10 | 3 | 6 | 10 | 9 | 6 | 6 |
| OC | 8 | 7 | 17 | 11 | 11 | 8 | 20 | 18 | 13 | 17 | 7 | 16 |
| EC | 10 | 10 | 9 | 2 | 1 | 1 | 1 | 1 | 9 | 13 | 20 | 14 |
| TC | 7 | 6 | 12 | 14 | 2 | 2 | 12 | 2 | 14 | 15 | 8 | 13 |
| Al | 2 | 4 | 2 | 18 | 21 | 19 | 17 | 19 | 16 | 2 | 9 | 18 |
| Si | 6 | 5 | 11 | 23 | 17 | 17 | 21 | 21 | 20 | 23 | 18 | 21 |
| Fe | 3 | 2 | 14 | 8 | 20 | 6 | 19 | 15 | 3 | 16 | 12 | 12 |
| Ni | 9 | 9 | 18 | 5 | 22 | 11 | 14 | 13 | 8 | 18 | 13 | 8 |
| Zn | 20 | 20 | 22 | 3 | 16 | 16 | 16 | 16 | 19 | 11 | 17 | 9 |
| Pb | 21 | 21 | 19 | 22 | 18 | 22 | 2 | 22 | 23 | 21 | 23 | 22 |
| Mg | 19 | 19 | 20 | 6 | 19 | 4 | 11 | 10 | 18 | 1 | 21 | 19 |
| Na | 23 | 23 | 21 | 1 | 5 | 3 | 13 | 17 | 17 | 3 | 5 | 15 |
| O3 | 15 | 8 | 7 | 4 | 3 | 5 | 2 | 3 | 12 | 12 | 1 | 4 |
| NO | 4 | 15 | 3 | 15 | 10 | 9 | 18 | 20 | 5 | 22 | 22 | 7 |
| NO2 | 12 | 16 | 13 | 20 | 4 | 7 | 4 | 5 | 1 | 14 | 11 | 1 |
| NO3 | 17 | 22 | 15 | 19 | 15 | 14 | 10 | 14 | 15 | 20 | 15 | 17 |
| SO2 | 22 | 14 | 23 | 21 | 23 | 23 | 23 | 23 | 21 | 19 | 19 | 23 |
| Pinene | 5 | 3 | 8 | 17 | 13 | 21 | 7 | 9 | 7 | 10 | 3 | 10 |
Figure 5.
Random forest variable importance for frequency. Changes in f were best predicted by acid sulfate, Al, and Fe.
Figure 6.
(A and B) Scatter plots for top 8 ranked elements in random forest for frequency. Increases in frequency were most strongly related to NO, while decreases were related to Al. (See colour version of this figure online at www.informahealthcare.com/iht)
In Table 3, acidic sulfate is ranked first for F, Ti and Te. Changes in TV and MV rank Na and EC first, respectively. PIF, PEF, and EF50 rank EC first, and TC, ozone and TC second, respectively. Pause, EIP, EEP, and Penh have NO2, Mg, ozone and NO2 ranked first, respectively. Overall, the correlations between univariate results of Table 2 and the multivariate results of Table 3 are variable.
R2 analysis
In an attempt to determine the relative importance of plant/scenario vs. individual measured component impact on the respiratory outcomes, we calculated the adjusted R2 for plant/scenario ANOVA models and the adjusted R2 for the component regression models for all outcomes (Table 4). While plant/scenario models were able to explain 0.2–0.5 (median = 0.4) of the variance in the outcomes, the single components in univariate analysis were only able to explain 0.001–0.14(median = 0.015) of the variance in the outcomes. We also calculated the predicted R2 for the random forest as a multivariate analysis, and found that for this approach individual components explained less than 0.1 of the variance. There were no major differences between plant/scenario ANOVA and random forest analyses. As an example, predicted R2 values for frequency were 0.36 and 0.32; for EF50 0.20 and 0.18 and for PEF 0.1 and 0.09, for ANOVA and random forest analyses, respectively.
Table 4.
Comparison of adjusted R2 for Plant/Scenario ANOVA models and various exposure metrics identified in univariate regression models.
| Parameter | Scenario/Plant | Mass | Neutral sulfate | Amonium ion | Total sulfate | Acidic sulfate | Elemental carbon |
|---|---|---|---|---|---|---|---|
| F | 0.4580 | 0.0080 | 0.0280 | 0.0410 | −0.0150 | 0.0300 | 0.0290 |
| Ti | 0.3283 | 0.0050 | 0.0210 | 0.0310 | −0.0150 | 0.0340 | 0.0130 |
| Te | 0.2566 | −0.0060 | −0.0020 | 0.0080 | −0.0110 | 0.0320 | 0.0160 |
| PEF | 0.4301 | 0.0080 | 0.0880 | 0.1060 | −0.0010 | 0.0290 | 0.0660 |
| PIF | 0.4339 | 0.0190 | 0.0260 | 0.0460 | −0.0140 | 0.0180 | 0.1380 |
| EF50 | 0.4979 | 0.0190 | 0.0730 | 0.0950 | −0.0100 | 0.0390 | 0.0840 |
| Tv | 0.4132 | 0.0040 | −0.0140 | −0.0120 | −0.0060 | −0.0070 | 0.1450 |
| MV | 0.3948 | 0.0260 | 0.0390 | 0.0540 | −0.0010 | −0.0060 | 0.1030 |
| Penh | 0.4297 | −0.0070 | 0.0440 | 0.0320 | 0.0280 | −0.0150 | −0.0140 |
| PAU | 0.3999 | −0.0070 | 0.0660 | 0.0520 | 0.0310 | −0.0150 | −0.0140 |
| EIP | 0.2210 | −0.0070 | −0.0100 | −0.0100 | −0.0100 | −0.0150 | −0.0110 |
| EEP | 0.2383 | −0.0120 | 0.0150 | 0.0090 | −0.0140 | 0.0140 | 0.0170 |
Adjusted R2 may have negative values because adjustment for model size.
Comparison of adjusted R2: The plant/scenario approach vastly outperforms any single component in predicting any given outcome of respiratory function.
Discussion
Measurements of change in breathing pattern, air flow in the breathing cycle, and changes in pause parameters have been used in many studies to assess respiratory toxicology (Glaab et al., 2006; Glaab et al., 2002; Hoymann, 2006; Hoymann, 2007; Lundblad et al., 2002; Mitzner & Tankersley, 2003; Saldiva et al., 1985). In this study, we assessed large numbers of animals during exposure to determine these changes, their magnitude, and correlation with parameters of exposure. We used step-wise exposures of increasing complexity to simulate changes that might occur in a power plant plume as it mixes with other atmospheric constituents and travels to distant sites. We used step-wise statistical analyses of increasing complexity to evaluate exposures at individual plants with specific combinations of coal types and emissions controls. After the plant-specific evaluation, we combined data from all plants in an attempt to delineate any specific patterns or components of the exposure linked with respiratory changes. In the final analyses, we showed that the plant/scenario associations are much stronger than individual component associations. The primary objective of this study was to evaluate in a more realistic way the effects of power plant-derived particles, both in the form of primary particles and as secondary particles having undergone chemical transformations in the atmosphere with the addition of other atmospheric constituents.
P scenario
PP1 and PP2 had the lowest primary particle concentrations in the P scenario (1.0 and 1.7 μg/m3, respectively), whereas PP3 had a substantially higher concentration (43.2 μg/m3). This difference was due to the operation of the FGD scrubber at PP3, which is well known to both remove and contribute particles to plant emissions (Kang et al., 2011). The contributed particles at PP3 tended to be primarily comprised of acidic and neutralized sulfate. PP1 showed no robustly significant changes in outcomes with the P scenario, which would be expected with the very low concentration. PP3, which had the highest P scenario concentration, showed one robust finding in EIP (decrease); however, a reduction in EIP without other respiratory or breathing pattern changes is not indicative of any particular pathophysiological response. Finding robustly significant outcomes (decreases in MV, PEF, and EF50, and an increase in Penh) with the P scenario at PP2, with its very low particle concentration, was surprising and not easily explained. When all plant data were combined, the strength of the association for EF50 was strongly significant (p < 0.005) since this parameter in the P scenario at PP1 and PP3 also decreased although not individually significant. These findings with the P scenario are difficult to explain based on exposure because this scenario at PP2 did not have any measured gaseous or particulate component substantially higher than or outside the range of PP1 or PP3 (Kang et al., 2011). Although there are inconsistencies in the measurements of continuous, summed, and gravimetric mass (13.9 ± 9.5 μg/m3 vs. 1.7 ± 1.8 μg/m3 vs. 1.9 ± 1.3 μg/m3), all of these measurements are very low for toxicological studies of ambient particle health effects. Particle number was also lower at PP2 than at PP1 or PP3 (910 ± 964 vs. 1726 ± 1277 vs. 55,947 ± 11,769 particles/cm3, respectively, (Kang et al., 2011)). Similarly, there was no consistency between these respiratory findings in the P scenario and the in vivo chemiluminescence studies of the (Lemos et al., 2011), or the studies of inflammation (Godleski et al., 2011a). In both of these studies, the P scenario showed no effects at PP2. This point is particularly important because the respiratory measurements are not invasive, and the same animals are then assessed for the other parameters. For this reason, we expect to see consistency with exposure and response as well as among outcome parameters within the same exposure in order to conclude that an outcome was truly biologically significant. At PP3, where EF50 in the P scenario showed the greatest change in magnitude (although not statistically significant), bronchoalveolar lavage studies showed increases in neutrophils, which were associated with the zinc concentration in univariate analyses. However, in the respiratory studies, zinc had no influence on flow parameters in either univariate or random forest analyses.
Penh was also significantly decreased at PP2 in the P scenario and in the combined data. Since increased Penh is considered an adverse effect, the biological significance of decreases in this parameter is unclear. Studies comparing two different approaches to whole body plethysmography (flow and pressure) in animals (Lomask, 2006; Lundblad et al., 2002; Mitzner & Tankersley, 2003) have shown that Penh derived from single chamber whole body plethysmography cannot be used as an accurate index of bronchoconstriction. Indeed, Lomask (2006) suggests that Penh is only meaningful when applied to flow-based whole body plethysmography, which measures the difference between the thoracic and nasal flows. Since our system used pressure-based whole body plethysmography without measurement of nasal flows, it may be argued that the finding of changes in Penh in our study has little value. Therefore, although we report Penh findings, these results are never emphasized.
PO scenario
The PO scenario contains the secondary reaction products of power plant emissions alone, i.e., unneutralized sulfuric acid. Although this scenario may be the purest test of the toxicity of secondary particles from power plant emissions, the scenario, with its high concentrations of strong acidity, is never encountered in ambient air due to neutralization by atmospheric ammonia (Kang et al., 2011; Spengler et al., 1990). The changes in breathing pattern evoked by the PO scenario consisted of an increase in respiratory frequency with a corresponding decrease in Ti and Te. These changes were strongest at PP2 and to a lesser extent at PP3; PP1 showed minor changes with this scenario. PEF was non-significantly decreased at PP1 and PP3 and only marginally significantly at PP2 along with a marginally significant decrease in TV. When all plants were combined, increased f and decreased Ti and Te were all marginally significant at p < 0.05. The PO scenario was characterized by the formation of acid sulfate at all three plants (PP1-36.1 ± 7.7 μg/m3; PP2- 71.6 ± 17.0 μg/m3; PP3- 68.9 ± 16.8μg/m3) (Kang et al., 2011), and the random forest ranking listed acid sulfate as the component having the strongest association with changes in F, Ti and Te, but these were not significant in univariate analyses.
Data from our control scenarios at PP3 are illustrative in evaluating the impact of specific components on respiratory changes. Very few strongly significant findings were observed with the control scenarios, and all were observed with the O scenario: reduced Ti and Te (with a marginally significant increase in f) and reduced EIP. These findings would suggest that the strong acidity in the O scenario increases frequency, a finding that has been widely reported (Amdur, 1989; Amdur et al., 1952a; Amdur et al., 1952b). However, although f increased, tidal volume did not change and MV, in fact, showed a nonsignificant increase, such that we did not observe a rapid, shallow breathing pattern but simply an increase in frequency. However, this pattern is not entirely reflected in the power plant emissions exposures, with the PO scenarios causing increased f at PP2 and PP3 (Figure 2), with corresponding decreases in Ti and Te, but only PP2 had decreases in TV, PEF and EF50 (all p < 0.05). At the same time, neither the PO scenario nor acid sulfate had any effect on lung inflammation (Godleski et al., 2011) or in vivo chemiluminescence of the lung (Lemos et al., 2011). Therefore, lack of consistency across outcomes lessens the impact of this breathing pattern finding.
POS scenario
The POS scenario, which actually had higher acidity (in the form of acid sulfate) than the PO scenario (Table 1), did not induce changes in F, Ti and Te, a somewhat puzzling result. Indeed, at individual plants, the POS scenario induced minimal effects. We also observed a marginally significant increase in f with the S control scenario, but a nonsignificant result with the OS scenario.
In comparing POS results from the in vivo chemiluminescence studies on the lungs of the same animals (Lemos et al., 2011), it can be seen that an increase in lung chemiluminescence was only observed at PP2 (where the only strongly significant breathing pattern finding for POS was a decrease in TV). In comparing the POS scenario respiratory results with bronchoalveolar lavage results, also on the lungs of the same animals (Godleski et al., 2011a), increases in total cells and macrophages were observed with the POS scenario at PP1 and PP3, and these reached marginal significance (p < 0.05) when all plants were combined. No significant changes in PMNs, generally a more accurate marker of inflammatory processes, were observed. Taken together, despite the fact that the POS scenario induced minimal changes in respiratory parameters, this scenario did result in some other pulmonary changes in the same animals.
The importance of sulfuric acid as a respiratory irritant has been extensively studied in the past (Amdur, 1989; Amdur et al., 1952a; Amdur et al., 1952b). Some studies have reported enhanced effects with sulfuric acid-coated particles (Chen et al., 1992; Chen et al., 1991). EF50 (the flow at the midpoint of the expiratory time) has been suggested as a robust confirmatory marker of bronchoconstriction (Glaab et al., 2006; Glaab et al., 2002). As discussed above, we observed some changes in f, Ti, and Te in the PO scenario, but not in the POS scenario, despite higher acidity (at least at PP1 and PP2). We also observed no robustly significantly changes in PIF, PEF, or EF50 at any of the plants in response to scenarios containing high sulfuric acid. Coupled with our failure to find strongly significant and consistent associations with acid sulfate itself, our findings suggest that acidity does not play a major role in our respiratory results.
PONS scenario
In the PONS scenario, the addition of NH3 to partially neutralize strong acidity increased mass, dramatically increased neutralized sulfate, and resulted in a decrease in respiratory airflow parameters (PEF and EF50). These decreases were robust at PP1 and PP2, and in the combined data, but were not significant at PP3. In univariate analyses, the decrease in these outcomes was associated with NH4 neutralized sulfate, and EC. However, the random forest analysis for both PEF and EF50 failed to rank neutral sulfate in the top 5 variables. However, there was some consistency with other TERESA studies. We observed significant lung in vivo chemiluminescence for the PONS scenario at PP2, although not at PP1, where significant changes in flow parameters were observed (Lemos et al., 2011). We also observed some degree of consistency with the lung inflammatory studies in these animals (Godleski et al., 2011a) in that the PONS scenario produced significant increases in total cells and macrophages at PP1 and PP3, although not at PP2. It should be noted that we did observe consistent results with NH4 and PEF reductions in both univariate and random forest analyses. Since NH4 and neutralized sulfate are highly correlated, these results suggest that the PONS scenario may be more responsible for these findings than the individual components, a conclusion confirmed in Table 4.
Control scenarios (O, OS and S)
These scenarios lacked primary particles and were used to assess contributions of primary particles to the reaction mixtures and the formation of secondary particles. The findings of the O and OS scenarios were discussed in part in relationship to the PO and POS scenarios.
To further understand the differences in f, Ti, and Te responses between the O/OS and PO/POS scenarios, we can examine the associations between the SOA and its precursors. In univariate analyses and illustrated in the scatter plots, pinene was associated with a nonsignificant decrease in frequency and inconsistent changes in Ti and Te, as well as ranking relatively high in random forest analyses. However, pinene reacted in the aging chamber and therefore exposure concentrations were very low (maximum of 8 ppb (Kang et al., 2011)). Useful measures of the reaction products include OC, formaldehyde, acetaldehyde, acetone, and total aldehydes, but none of these parameters was significantly associated with changes in f, Ti, or Te. Previous studies (Rohr et al., 2002) have reported reductions in frequency indicative of sensory irritation in response to pinene oxidation products; however, no such changes were observed with pinene alone. Thus, although it is unclear why the POS scenario appears to produce a lesser response than the PO scenario, it is possible that some of the organic materials are having and irritant effect to reduce breathing rate, rather than increasing it as the simpler PO scenario did. It should be noted that we only investigated a-pinene as an SOA precursor, and of course responses could differ significantly with (an) other VOC(s).
Differences by power plant
Although differences in plant pollution control devices, and differences in the coal used by each plant were part of the design of these studies, analyses of these differences may only be inferred from the differences in outcomes in individual plants controlled for scenario. However, differences in measured component concentrations from plant to plant and scenario complicate the interpretation of such analyses. Nevertheless, certain trends, which are analyzed in Figure 2, are worthy of discussion. In Figure 2, all scenarios at PP3 have non-significant increases in inspiratory flow, whereas PP1 and PP2 all have decreases in inspiratory flow with all scenarios. Thus, comparing these outcomes while controlling for scenario resulted in a significant difference among plants (p = 0.019). Similarly, PP1 and PP2 have decreases in tidal volume with all scenarios, but PP3 has nonsignificant increases in these measurements in almost all scenarios, and this led to a highly significant difference when controlling for scenario (p < 0.0001). Differences in outcome trends can also be appreciated for measures of expiratory and inspiratory pause. There were no differences in animals, personnel, mobile laboratory use, or outcome analyses to account for these differences at the three power plants. The stack extraction system at PP3 was different from PP1 and PP2 because of the differences in pollution controls at PP3. Although the outcome trends seen at PP3 are in general not adverse outcomes, more research is needed before it can be determined as to whether the pollution control system influenced these outcomes.
Role of sulfates
Because these differences in aerosol component composition determined by scenarios with additional differences contributed by power plant configuration, it was possible to assess dose response of individual components. As expected sulfur compound concentrations dominated most scenarios (Kang et al., 2011). In a recent review of sulfate health effects (Schlesinger, 2007), Schlesinger emphasizes the lack of toxicological effects with partially to totally neutralized sulfate in laboratory toxicological studies. Our data appear to support this conclusion, since we do not observe consistency in findings with varied statistical methods, and nor do the findings of varying pulmonary endpoints consistently compare. In the associations observed in this study with neutral sulfate and flow parameters (PEF and EF50), neutral sulfate concentrations ranged from 5.6 to 139 μg/m3 over all scenarios at all plants. Previous studies evaluating sulfate effects on airflow or bronchoconstrictive effects in humans have reported no effects at 133 and 116 μg/m3 (Stacy et al., 1983) or 100 μg/m3 (Utell et al., 1983). However, some epidemiological panel studies as well as toxicological studies utilizing CAPs have reported associations between sulfate and adverse respiratory effects (Grahame & Schlesinger, 2005; Neas et al., 1996; Raizenne et al., 1996). These studies have found it difficult to determine the extent to which the sulfate associations are due to sulfate itself, to other components that are highly correlated, or to interactions within the complex mixture. In our study, since plant/scenario explained more of the variance than individual components including sulfates, we suggest that the changes observed here are more likely related to the complex mixture than to neutral sulfate. Hypotheses forwarded to explain differing results observed in single-component laboratory sulfate studies vs. studies utilizing complex mixtures include the possibility of the formation of organosulfate materials in the atmosphere, or complexation with sulfate leading to an increase in the bioavailability of metals. Recent work investigating cardiopulmonary effects of organosulfate compounds has reported little evidence for increased toxicity of these materials (McDonald et al., 2010).
Role of organic components
EC figured prominently in many of the analyses. In particular, EC was consistently and strongly associated with reductions in MV, PEF, and EF50 in both univariate and random forest analyses. As described in some detail by Kang et al. (2011), EC concentrations in this study were abnormally high in many cases. These elevations were considered artifactual and were attributed at least in part to pyrolyzed OC erroneously reported as EC, since power plants emit very low concentrations of EC. Furthermore, the associations with EC that we observed are likely not due to EC itself as this material is generally inert. It is more likely that any effects observed were due to adsorbed organic materials that were able to more effectively reach pulmonary regions.
We also observed interesting findings with respect to the measured pinene oxidation products: formaldehyde, acetaldehyde, acetone, and total aldehydes. In particular, these four parameters were strongly or marginally associated with increases in Penh, which may be considered physiologically meaningful (see the caveat above, however). This is consistent with findings of reductions in airflow in response to pinene oxidation products(Rohr et al., 2002), as well as other reports of specific aldehyde-related bronchoconstrictive effects (Matsuse et al., 2007; Thompson & Grafström, 2008). Additional research on the possible role played by these compounds is warranted.
Role of metals
Metals did not appear to play a large role in the TERESA respiratory responses, although there were some consistent results. Aluminum, a component of coal fly ash, was negatively associated with f in both univariate and random forest analyses. This element has been linked with pulmonary inflammatory effects in CAP studies(Clarke et al., 2000). Silicon, lead, and magnesium were all strongly associated with a reduction in TV in univariate analyses, but these findings were not confirmed by random forest. Nickel was consistently associated with an increase in TV; however, this change is generally not considered to be of pathophysiological significance. Similarly, sodium was related to increased MV, again not considered an adverse response.
Role of gases
We observed several strong associations in univariate analyses with gases, including NO with increased frequency and decreased Te, and NO2 with increased Pause (a parameter not generally reported and of limited physiological meaning) and Penh. Marginally significant associations were observed between ozone and Pause; NO and decreased Ti and increased PIF; and NO2 and decreased MV and PIF. By design, gaseous co-pollutant concentrations in the exposure scenarios were low through the use of denuders (Kang et al., 2011). For example, maxima for ozone, NO2, and SO2 over all scenarios at all plants were 29 ppb, 18 ppb, and 73 ppb, respectively. Therefore, any associations observed with gases are likely not reflective of a true biological association, but rather, because gases were common to all scenarios, they may be serving as tracers for scenarios.
Possible physiological mechanisms
Examination of the breathing pattern data can provide insight into the possible physiological mechanism at play. For example, a reduction in frequency coupled with an increase in EIP is indicative of a sensory irritant effect, mediated by the trigeminal nerve (Alarie, 1966). A reduction in frequency coupled with an increase in EEP is suggestive of pulmonary irritation, as is a rapid, shallow breathing pattern (increased f concomitant with reduced Ti and Te, and decreased TV) (Vijayaraghavan et al., 1993). Finally, as discussed earlier, changes in flow parameters such as PEF and EF50 can be indicative of a bronchoconstrictive effect, particularly when occurring simultaneously with elongated Te and reduced TV (Vijayaraghavan et al., 1993). Our data indicate the occurrence of pathophysiological effects, with the PO scenario at PP2, where we observed a significant increase in f (with reductions in Ti and Te) and decrease in TV. As mentioned earlier, this rapid, shallow breathing pattern has been well documented with exposures to strong acids. In this scenario, acidic sulfate was 108 μg/m3, the highest of all scenario/plant combinations, and roughly two orders of magnitude higher than typical environmental concentrations (Dockery et al., 1992). While there was significantly increased EIP in the P scenario at PP3, this was not accompanied by a reduction in f, so sensory irritation was not evident. Reductions in flow parameters (PEF and EF50) did not co-occur with increased Te; therefore, we have less confidence in the occurrence of a frank bronchoconstrictive effect. However, with the PONS scenario at PP1 and PP2 in which PEF, EF50, TV and MV were all decreased at levels of significance ranging from p < 0.05 in one instance (TV in PP2) to p < 0.005 in three instances (PEF and EF50 on PP1, and MV on PP2) to p < 0.0005 in the remaining 4 measurements, these decreases cannot be ignored even though prolonged Te was not observed.
Study strengths and weaknesses
Due to differences in the number of animals used per scenario and per plant, statistical power to detect an effect in ANOVA analyses differs. For example, as shown in Table 1, the number of days that each scenario was run ranged from 3 to 12 at PP1, 4 to 8 at PP2, and 4 to 8 at PP3. This imbalance does not affect the univariate or random forest analyses, but the scenario-specific analyses, especially for PONS at PP1 (with 12 days of exposure, or triple the statistical power of the PO scenario at that plant), should be interpreted accordingly.
A primary strength of this study was the collection of a rich exposure data set, which allowed us to examine relationships between individual components. However, comparison of the strength of associations between scenario and the components defining the scenario showed no differences. This indicates that individual components defining a scenario could not be identified as causative for an effect related to a scenario. This may be due to either (i) the complexity of the scenarios, and our related inability to fully capture the dynamics of the system; and/or (ii) the high correlations between many of the components, particularly within a given scenario.
Consistency between univariate findings and random forest results should provide a greater degree of confidence in the effects observed. When we examined the more robustly significant associations in the univariate analyses and compared them to the top random forest variables for a given exposure variable, relatively few exposure parameters were consistent. These include the following: (i) Frequency was significantly associated with Al (negative) and NO (positive), and in random forest analyses these two components were ranked 2 and 4, respectively; (ii) Ti was marginally associated with pinene (positive), and this parameter was ranked 3 by random forest; (iii) Te was associated with NO (negative), which was ranked 3 by random forest; (iv) TV was associated with nickel and magnesium (increase), which were ranked 5 and 6, respectively, in random forest analyses; (v) MV was significantly associated with EC and NO2 (negative) and these parameters were ranked 1 and 4, respectively, by random forest, as well as Na (positive), which was ranked 5 by random forest; (vi) PIF was associated with EC (negative), which was ranked 1, as well as Na and Mg (positive) which were ranked 3 and 4, respectively; (vii) PEF was associated with EC and NH4 (negative), which were ranked 1 and 3 in random forest analyses; and (viii) EF50 was associated with EC and NH4 (negative), which were ranked 1 and 6 by random forest. Again, these findings confirm the assessment shown in Table 4, that scenario was a much stronger determinant of outcome than individual components.
Conclusions
Overall, we observed a number of significant breathing pattern changes in response to exposure to power plant-derived particles and added constituents. Significant clinical changes in breathing pattern, such as specific irritant effects were difficult to dissect from the results because, we tended to observe a number of isolated changes in certain respiratory parameters. Some individual exposure scenario components appeared to be more strongly and consistently related to respiratory parameter changes; however, the specific scenario investigated remained a better predictor of response than individual components, suggesting that it is the chemical reactions with other compounds (from different sources) in the atmosphere that may increase the toxicity of emitted gasses and primary particles from coal-fired power plants.
Ongoing studies from our laboratory are using the TERESA approach to evaluate the toxicity of traffic related pollution; comparison of these data with the findings reported here plus the existing database of toxicological and epidemiological studies will give us a better understanding of the contribution of different sources to the morbidity and mortality associated with exposure to air pollution.
Acknowledgments
The authors thank the local universities, veterinary clinics, and suppliers who made an extraordinary effort to make a logistically very complex project possible.
Footnotes
Declaration of interest
This project was supported by the Electric Power Research Institute (Contract EP-P10983/C5530/56546), the U.S. Environmental Protection Agency Center for Particle Health Effects at the Harvard School of Public Health (grant R827353), and the Harvard NIEHS Center for Environmental Health (grant ES00002). This work was also prepared with the support of the U.S. Department of Energy (DOE) under award DE-FC26-03NT41902, and a grant from the State of Wisconsin. However, any opinions, findings, conclusions, or recommendations expressed herein are those of the authors, and do not necessarily reflect the views of the U.S. EPA or DOE. The Electric Power Research Institute (EPRI) employs Annette C. Rohr.
References
- Alarie Y. Irritating properties of airborne materials to the upper respiratory tract. Arch Environ Health. 1966;13:433–449. doi: 10.1080/00039896.1966.10664593. [DOI] [PubMed] [Google Scholar]
- Amdur MO. Health effects of air pollutants: Sulfuric acid, the old and the new. Environ Health Perspect. 1989;81:109–13. doi: 10.1289/ehp.8981109. discussion 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdur MO, Schulz RZ, Drinker P. Toxicity of sulfuric acid mist to guinea pigs. A M A Arch Ind Hyg Occup Med. 1952a;5:318–329. [PubMed] [Google Scholar]
- Amdur MO, Silverman L, Drinker P. Inhalation of sulfuric acid mist by human subjects. A M A Arch Ind Hyg Occup Med. 1952b;6:305–313. [PubMed] [Google Scholar]
- Chen LC, Miller PD, Amdur MO, Gordon T. Airway hyperresponsiveness in guinea pigs exposed to acid-coated ultrafine particles. J Toxicol Environ Health. 1992;35:165–174. doi: 10.1080/15287399209531606. [DOI] [PubMed] [Google Scholar]
- Chen LC, Peoples SM, Amdur MO. Pulmonary effects of sulfur oxides on the surface of copper oxide aerosol. Am Ind Hyg Assoc J. 1991;52:187–191. doi: 10.1080/15298669191364578. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Catalano PJ, Koutrakis P, Murthy GG, Sioutas C, Paulauskis J, Coull B, Ferguson S, Godleski JJ. Urban air particulate inhalation alters pulmonary function and induces pulmonary inflammation in a rodent model of chronic bronchitis. Inhal Toxicol. 1999;11:637–656. doi: 10.1080/089583799196781. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Coull B, Reinisch U, Catalano P, Killingsworth CR, Koutrakis P, Kavouras I, Murthy GG, Lawrence J, Lovett E, Wolfson JM, Verrier RL, Godleski JJ. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Environ Health Perspect. 2000;108:1179–1187. doi: 10.1289/ehp.001081179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Rossi CA, Lawrence J, Long MS, Diaz EA, Lim RH, McEwen B, Koutrakis P, Godleski JJ. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ Health Perspect. 2010;118:769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull BA, Schwartz J, Wand MP. Respiratory health and air pollution: Additive mixed model analyses. Biostatistics. 2001;2:337–349. doi: 10.1093/biostatistics/2.3.337. [DOI] [PubMed] [Google Scholar]
- Coull BA, Wellenius GA, Gonzalez-Flecha B, Diaz EA, Godleski JJ. Methods for statistical analysis of TERESA health data. Inhal Toxicol. 2011;23(S2):31–41. doi: 10.3109/08958378.2010.566291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwent RG. Atmospheric Chemestry. In: Holgate ST, editor. Air pollution and health. San Diego, Calif.; London: Academic Press; 1999. pp. 51–62. [Google Scholar]
- Dockery DW, Schwartz J, Spengler JD. Air pollution and daily mortality: Associations with particulates and acid aerosols. Environ Res. 1992;59:362–373. doi: 10.1016/s0013-9351(05)80042-8. [DOI] [PubMed] [Google Scholar]
- Glaab T, Hecker H, Stephan M, Baelder R, Braun A, Korolewitz R, Krug N, Hoymann HG. Comparison of non-invasive measures of cholinergic and allergic airway responsiveness in rats. Acta Physiol (Oxf) 2006;186:301–308. doi: 10.1111/j.1748-1716.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- Glaab T, Hoymann HG, Hohlfeld JM, Korolewitz R, Hecht M, Alarie Y, Tschernig T, Braun A, Krug N, Fabel H. Noninvasive measurement of midexpiratory flow indicates bronchoconstriction in allergic rats. J Appl Physiol. 2002;93:1208–1214. doi: 10.1152/japplphysiol.01121.2001. [DOI] [PubMed] [Google Scholar]
- Godleski JJ, Diaz EA, Lemos M, Long M, Ruiz P, Gupta T, Kang CM, Coull B. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA)-power plant studies: Assessment of cellular responses. Inhal Toxicol. 2011a;23(S2):60–74. doi: 10.3109/08958378.2010.563804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski JJ, Rohr A, Kang C-M, Diaz EA, Ruiz P, Koutrakis P. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Introduction and overview. Inhal Toxicol. 2011b;23(S2):95–103. doi: 10.3109/08958378.2010.568019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health E3 Inst. 2000;(91):5–88. discussion 89–103. [PubMed] [Google Scholar]
- Grahame T, Schlesinger R. Evaluating the health risk from secondary sulfates in eastern North American regional ambient air particulate matter. Inhal Toxicol. 2005;17:15–27. doi: 10.1080/08958370590885672. [DOI] [PubMed] [Google Scholar]
- Heinrich U, Muhle H, Hoymann HG, Mermelstein R. Pulmonary function changes in rats after chronic and subchronic inhalation exposure to various particulate matter. Exp Pathol. 1989;37:248–252. doi: 10.1016/s0232-1513(89)80062-3. [DOI] [PubMed] [Google Scholar]
- Holman C. Sources of Air Pollution. In: Holgate ST, editor. Air pollution and health. San Diego, Calif.; London: Academic Press; 1999. pp. 115–148. [Google Scholar]
- Hoymann HG. New developments in lung function measurements in rodents. Exp Toxicol Pathol. 2006;57(Suppl 2):5–11. doi: 10.1016/j.etp.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hoymann HG. Invasive and noninvasive lung function measurements in rodents. J Pharmacol Toxicol Methods. 2007;55:16–26. doi: 10.1016/j.vascn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Kang CM, Gupta T, Ruiz PA, Wolfson JM, Ferguson ST, Lawrence JE, Rohr AC, Godleski J, Koutrakis P. Aged particles derived from emissions of coal-fired power plants: The TERESA field results. Inhal Toxicol. 2011 doi: 10.3109/08958371003728040. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos M, Diaz EA, Gupta T, Kang C-M, Ruiz P, Coull B, Gonzalez-Flecha B. Cardiac and pulmonary oxidative stress in rats exposed to realistic emissions of source aerosols. Inhal Toxicol. 2011 doi: 10.3109/08958378.2011.601433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomask M. Further exploration of the Penh parameter. Exp Toxicol Pathol. 2006;57(Suppl 2):13–20. doi: 10.1016/j.etp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol. 2002;93:1198–1207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- Matsuse H, Fukushima C, Shimoda T, Sadahiro A, Kohno S. Effects of acetaldehyde on human airway constriction and inflammation. Novartis Found Symp. 2007;285:97–106. doi: 10.1002/9780470511848.ch7. discussion 106. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Doyle-Eisele M, Campen MJ, Seagrave J, Holmes T, Lund A, Surratt JD, Seinfeld JH, Rohr AC, Knipping EM. Cardiopulmonary response to inhalation of biogenic secondary organic aerosol. Inhal Toxicol. 2010;22:253–265. doi: 10.3109/08958370903148114. [DOI] [PubMed] [Google Scholar]
- Mitzner W, Tankersley C. Interpreting Penh in mice. J Appl Physiol. 2003;94:828–31. doi: 10.1152/japplphysiol.00815.2002. author reply 831. [DOI] [PubMed] [Google Scholar]
- Neas LM, Dockery DW, Burge H, Koutrakis P, Speizer FE. Fungus spores, air pollutants, and other determinants of peak expiratory flow rate in children. Am J Epidemiol. 1996;143:797–807. doi: 10.1093/oxfordjournals.aje.a008818. [DOI] [PubMed] [Google Scholar]
- Nikolov MC, Coull BA, Catalano PJ, Diaz E, Godleski JJ. Statistical methods to evaluate health effects associated with major sources of air pollution: A case study of breathing patterns during exposure to concentrated Boston air particles. Journal of the Royal Statistical Society Series C. 2008:357–378. [Google Scholar]
- Raizenne M, Neas LM, Damokosh AI, Dockery DW, Spengler JD, Koutrakis P, Ware JH, Speizer FE. Health effects of acid aerosols on North American children: Pulmonary function. Environ Health Perspect. 1996;104:506–514. doi: 10.1289/ehp.96104506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr AC, Wilkins CK, Clausen PA, Hammer M, Nielsen GD, Wolkoff P, Spengler JD. Upper airway and pulmonary effects of oxidation products of (+)-alpha-pinene, d-limonene, and isoprene in BALB/c mice. Inhal Toxicol. 2002;14:663–684. doi: 10.1080/08958370290084575. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Gupta T, Kang CM, Lawrence JE, Ferguson ST, Wolfson JM, Rohr AC, Koutrakis P. Development of an exposure system for the toxicological evaluation of particles derived from coal-fired power plants. Inhal Toxicol. 2007a;19:607–619. doi: 10.1080/08958370701353148. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Lawrence JE, Ferguson ST, Wolfson JM, Koutrakis P. A counter-current parallel-plate membrane denuder for the non-specific removal of trace gases. Environ Sci Technol. 2006;40:5058–5063. doi: 10.1021/es060563w. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Lawrence JE, Wolfson JM, Ferguson ST, Gupta T, Kang CM, Koutrakis P. Development and evaluation of a photochemical chamber to examine the toxicity of coal-fired power plant emissions. Inhal Toxicol. 2007b;19:597–606. doi: 10.1080/08958370701353361. [DOI] [PubMed] [Google Scholar]
- Ruppert D, Wand MP, Caroll RJ. Semiparametric Regression. Cambridge University Press; 2003. [Google Scholar]
- Saldiva PH, Massad E, Caldeira MP, Calheiros DF, Saldiva CD, Böhm GM. The study of mechanical properties of rats lungs by whole body plethysmography. Acta Physiol Pharmacol Latinoam. 1985;35:109–117. [PubMed] [Google Scholar]
- Schlesinger RB. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: A critical review. Inhal Toxicol. 2007;19:811–832. doi: 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- Spengler J, Brauer M, Koutrakis P. Acid Air and Health. Environ Sci Technol. 1990;24:946–956. [Google Scholar]
- Stacy RW, Seal E, Jr, House DE, Green J, Roger LJ, Raggio L. A survey of effects of gaseous and aerosol pollutants on pulmonary function of normal males. Arch Environ Health. 1983;38:104–115. doi: 10.1080/00039896.1983.10543989. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Grafström RC. Mechanistic considerations for formaldehyde-induced bronchoconstriction involving S-nitrosoglutathione reductase. J Toxicol Environ Health Part A. 2008;71:244–248. doi: 10.1080/15287390701598259. [DOI] [PubMed] [Google Scholar]
- Utell MJ, Morrow PE, Speers DM, Darling J, Hyde RW. Airway responses to sulfate and sulfuric acid aerosols in asthmatics. An exposure-response relationship. Am Rev Respir Dis. 1983;128:444–450. doi: 10.1164/arrd.1983.128.3.444. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R, Schaper M, Thompson R, Stock MF, Alarie Y. Characteristic modifications of the breathing pattern of mice to evaluate the effects of airborne chemicals on the respiratory tract. Arch Toxicol. 1993;67:478–490. doi: 10.1007/BF01969919. [DOI] [PubMed] [Google Scholar]