Abstract
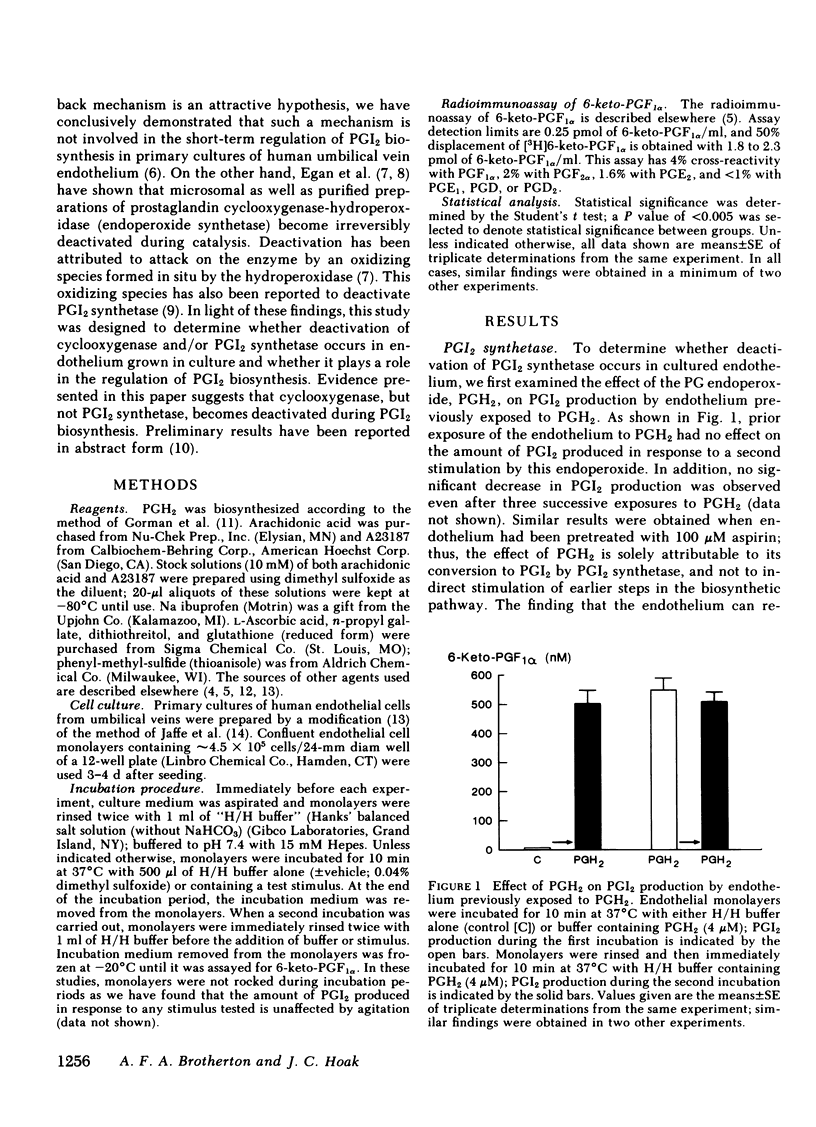
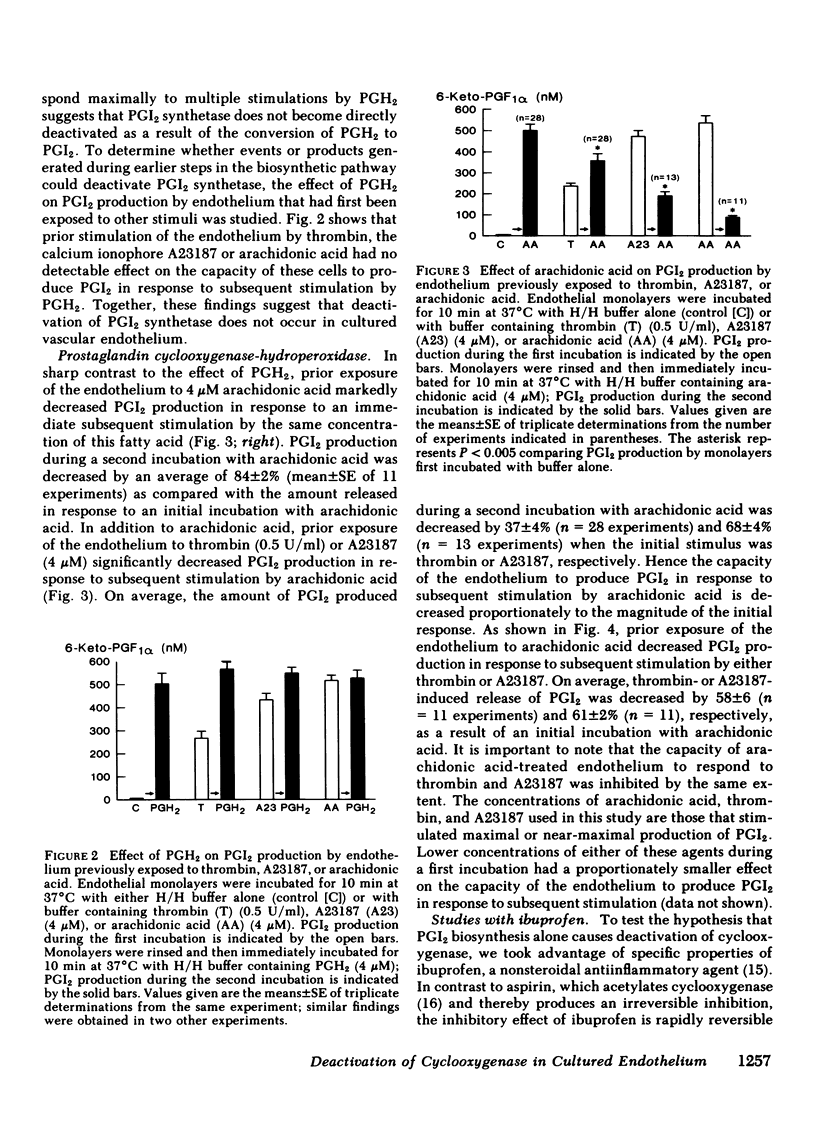
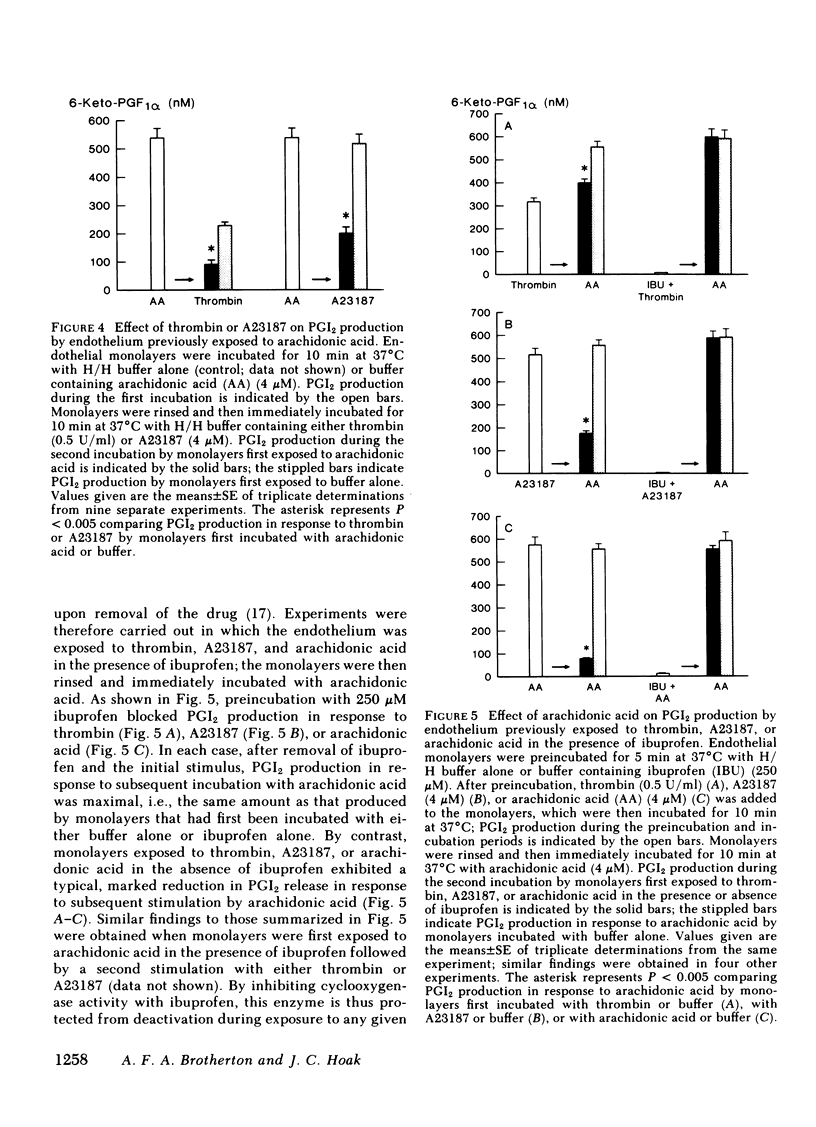
Primary monolayer cultures of human umbilical vein endothelium produce prostacyclin (PGI2) in response to stimulation by thrombin, ionophore A23187, arachidonic acid, and the prostaglandin endoperoxide, PGH2. None of these treatments had a significant effect on the capacity of the endothelium to produce PGI2 in response to subsequent stimulation by PGH2. By contrast, endothelium initially exposed to thrombin, A23187, or arachidonic acid produced approximately 37, 68, and 84% less PGI2, respectively, upon subsequent stimulation by arachidonic acid. These findings suggest that PGI2 biosynthesis in cultured endothelium results in deactivation of cyclooxygenase-hydroperoxidase but not PGI2 synthetase. To test the hypothesis that PGI2 biosynthesis alone causes deactivation of cyclooxygenase, thrombin, A23187, and arachidonic acid were added to monolayers that had been preincubated with ibuprofen (250 microM), a rapidly reversible, competitive inhibitor of this enzyme. After removal of the ibuprofen and the initial stimulus, PGI2 production in response to subsequent stimulation by arachidonic acid was maximal. These findings suggest that the metabolism of arachidonic acid itself causes a direct deactivation of cyclooxygenase. After an initial exposure to arachidonic acid, PGI2 production in response to a second stimulation by arachidonic acid was restored to approximately 34, 69, and 74% of maximal, after recovery periods of 1, 24, and 48 h, respectively. We conclude that the regulation of PGI2 biosynthesis in normal vascular endothelium may be in part a function of the activity and biosynthesis of cyclooxygenase-hydroperoxidase and the deactivation of this enzyme may be a primary factor limiting the capacity of the endothelium to produce PGI2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beetens J. R., Claeys M., Herman A. G. Antioxidants increase the formation of 6-oxo-PGF1 alpha by ram seminal vesicle microsomes. Biochem Pharmacol. 1981 Oct;30(20):2811–2815. doi: 10.1016/0006-2952(81)90419-6. [DOI] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Role of Ca2+ and cyclic AMP in the regulation of the production of prostacyclin by the vascular endothelium. Proc Natl Acad Sci U S A. 1982 Jan;79(2):495–499. doi: 10.1073/pnas.79.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton A. F., Macfarlane D. E., Hoak J. C. Prostacyclin biosynthesis in vascular endothelium is not inhibited by cyclic AMP. Studies with 3-isobutyl-1-methylxanthine and forskolin. Thromb Res. 1982 Dec 1;28(5):637–647. doi: 10.1016/0049-3848(82)90155-4. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Baptista E. M., Kennicott K. L., VandenHeuvel W. J., Walker R. W., Fagerness P. E., Kuehl F. A., Jr Oxidation reactions by prostaglandin cyclooxygenase-hydroperoxidase. J Biol Chem. 1981 Jul 25;256(14):7352–7361. [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Beveridge G. C., Marnett L. J., Kuehl F. A., Jr Direct and indirect involvement of radical scavengers during prostaglandin biosynthesis. Adv Prostaglandin Thromboxane Res. 1980;6:153–155. [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Kuehl F. A., Jr Reduction of hydroperoxides in the prostaglandin biosynthetic pathway by a microsomal peroxidase. J Biol Chem. 1979 May 10;254(9):3295–3302. [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gorman R. R., Sun F. F., Miller O. V., Johnson R. A. Prostaglandins H1 and H2. Convenient biochemical synthesis and isolation. Further biological and spectroscopic characterization. Prostaglandins. 1977 Jun;13(6):1043–1053. doi: 10.1016/0090-6980(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Ham E. A., Egan R. W., Soderman D. D., Gale P. H., Kuehl F. A., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979 Apr 10;254(7):2191–2194. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre B. A., Philp R. B. Effect of three nonsteroidal anti-inflammatory agents on platelet function and prostaglandin synthesis in vitro. Thromb Res. 1978 Jan;12(1):67–77. doi: 10.1016/0049-3848(78)90086-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A., Vane J. R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977 Jan 1;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- Parks W. M., Hoak J. C., Czervionke R. L. Comparative effect of ibuprofen on endothelial and platelet prostaglandin synthesis. J Pharmacol Exp Ther. 1981 Nov;219(2):415–419. [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Ogletree M. L., Lefer A. M., Nicolaou J. C. Antibodies which antagonise the effects of prostacyclin. Nature. 1978 Jul 6;274(5666):64–65. doi: 10.1038/274064a0. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971 Nov;246(21):6700–6702. [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]


