Abstract
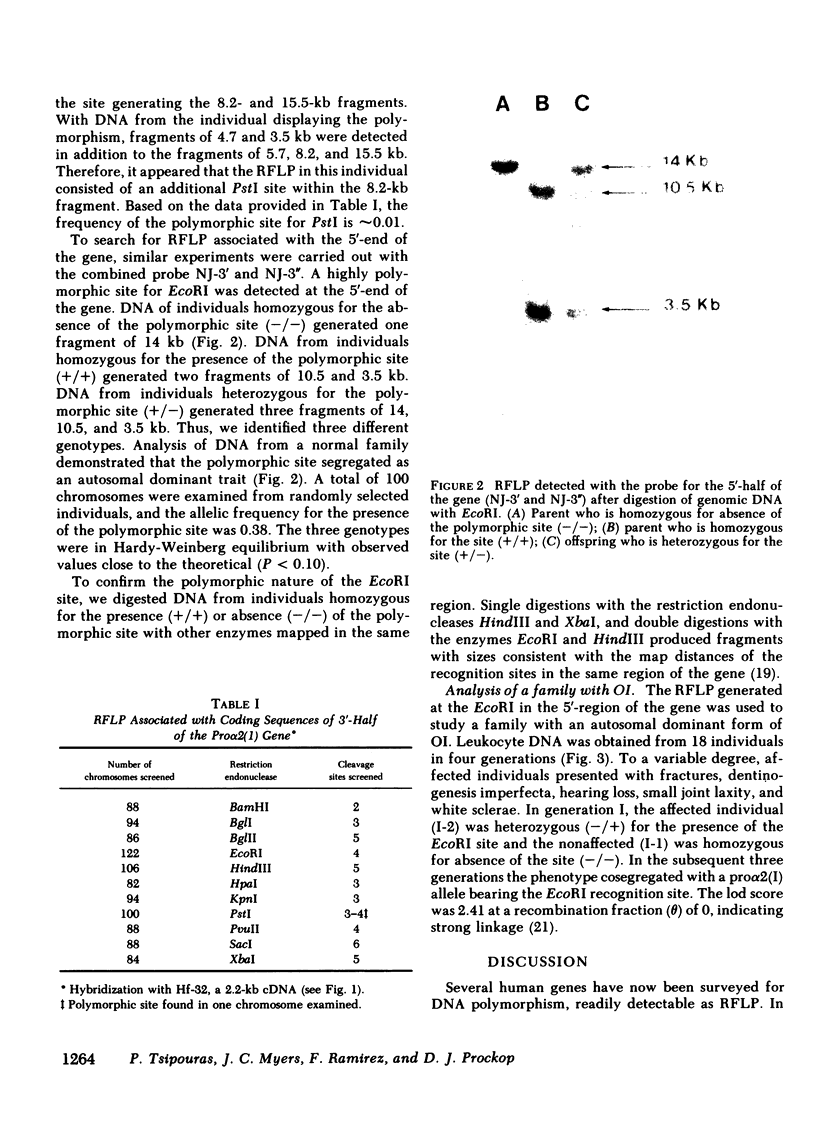
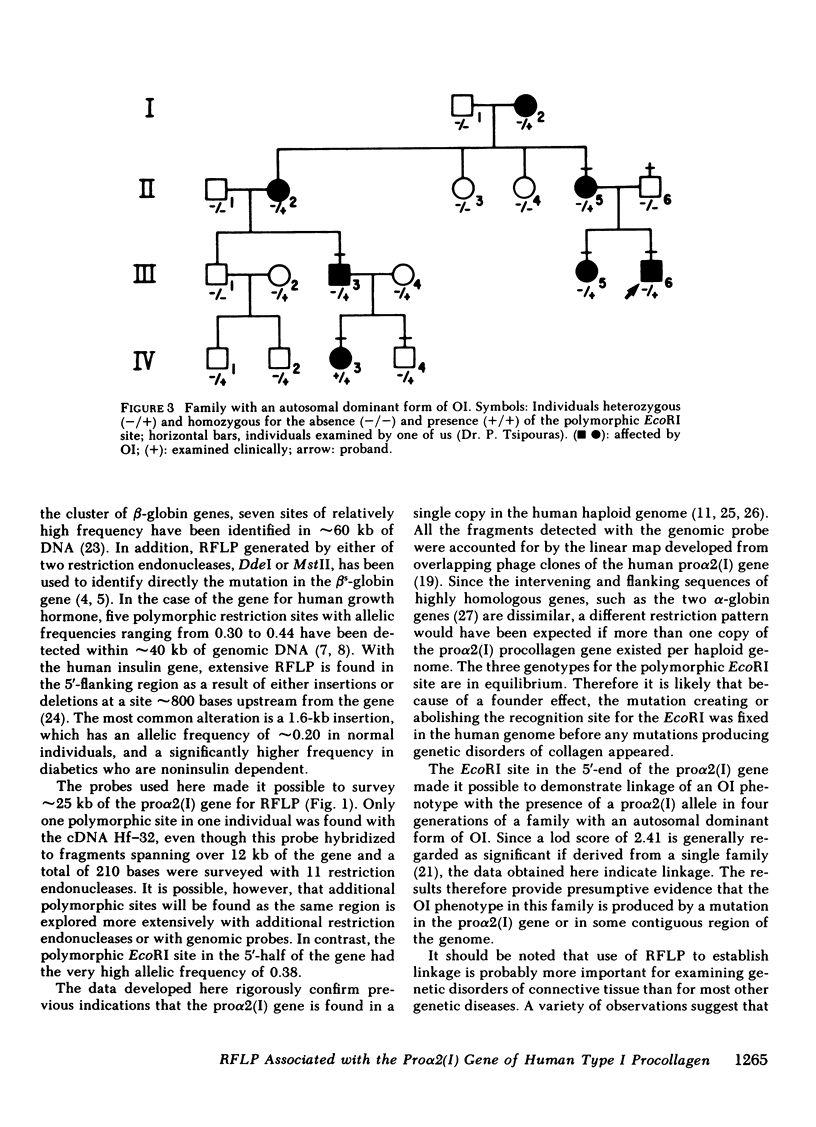
One cloned complementary DNA and one genomic subclone were used to detect restriction fragment length polymorphism associated with the pro alpha 2(I) gene for human type I procollagen. The restriction fragments obtained from examination of 30-122 chromosomes confirmed previous indications that the pro alpha 2(I) gene is found in a single copy in the human haploid genome. One highly polymorphic site was detected with EcoRI in the 5'-half of the gene. The restriction site polymorphism at the site had an allelic frequency of 0.38, and it generated two fragments of 10.5 and 3.5 kilobase in homozygous individuals. The restriction fragment length polymorphism generated at the EcoRI site was used to study affected and non-affected individuals in four generations of a family with an autosomal dominant form of osteogenesis imperfecta. The data demonstrated a linkage of the phenotype to a pro alpha 2(I) allele with a lod score of 2.41 at a recombination fraction (theta) of 0. The data therefore provided presumptive evidence that osteogenesis imperfecta in this family is caused by a mutation in the pro alpha 2(I) gene or some contiguous region of the genome. The relatively high frequency of polymorphism at the EcoRI site makes it useful for studying a broad range of genetic disorders in which mutations in type I procollagen are suspected. In addition, the polymorphic site should provide useful markers for linkage studies with other loci located on human chromosome 7.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Siegel R. C., Peterson K. E., Rowe D. W., Holbrook K. A., Smith L. T., Chang Y. H., Fu J. C. Marfan syndrome: abnormal alpha 2 chain in type I collagen. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7745–7749. doi: 10.1073/pnas.78.12.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. A sensitive new prenatal test for sickle-cell anemia. N Engl J Med. 1982 Jul 1;307(1):30–32. doi: 10.1056/NEJM198207013070105. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Driesel A. J., Schumacher A. M., Flavell R. A. A Hind III restriction site polymorphism in the human collagen alpha 1 (I)-like gene on chromosome No. 7. Hum Genet. 1982;62(2):175–176. doi: 10.1007/BF00282310. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Williams J., Sharp P., Sambrook J. Physical mapping of temperature-sensitive mutations of adenoviruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):439–446. doi: 10.1101/sqb.1974.039.01.056. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Byers P. H., Holbrook K. A. Genetic disorders of collagen metabolism. Adv Hum Genet. 1982;12:1–87. doi: 10.1007/978-1-4615-8315-8_1. [DOI] [PubMed] [Google Scholar]
- Huerre C., Junien C., Weil D., Chu M. L., Morabito M., Van Cong N., Myers J. C., Foubert C., Gross M. S., Prockop D. J. Human type I procollagen genes are located on different chromosomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6627–6630. doi: 10.1073/pnas.79.21.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Junien C., Weil D., Myers J. C., Van Cong N., Chu M. L., Foubert C., Gross M. S., Prockop D. J., Kaplan J. C., Ramirez F. Assignment of the human pro alpha 2(I) collagen structural gene (COLIA2) to chromosome 7 by molecular hybridization. Am J Hum Genet. 1982 May;34(3):381–387. [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Phillips J. A., 3rd, Boehm C. D., Vik T. A., Mahoney M. J., Ritchey A. K. Prenatal diagnosis of beta-thalassemias by amniocentesis: linkage analysis using multiple polymorphic restriction endonuclease sites. Blood. 1980 Nov;56(5):926–930. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Bank A. Organization of human delta--and beta-globin genes in cellular DNA and the presence of intragenic inserts. Cell. 1978 Sep;15(1):15–23. doi: 10.1016/0092-8674(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Friedman J., McCarthy B. J. DNA sequence analysis of a mouse pro alpha 1 (I) procollagen gene: evidence for a mouse B1 element within the gene. Mol Cell Biol. 1982 Nov;2(11):1362–1371. doi: 10.1128/mcb.2.11.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Little P. F., Kazazian H. H., Jr, Boehm C. D. Improved detection of the sickle mutation by DNA analysis: application to prenatal diagnosis. N Engl J Med. 1982 Jul 1;307(1):32–36. doi: 10.1056/NEJM198207013070106. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Hjelle B. L., Seeburg P. H., Zachmann M. Molecular basis for familial isolated growth hormone deficiency. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6372–6375. doi: 10.1073/pnas.78.10.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Parks J. S., Hjelle B. L., Herd J. E., Plotnick L. P., Migeon C. J., Seeburg P. H. Genetic analysis of familial isolated growth hormone deficiency type I. J Clin Invest. 1982 Sep;70(3):489–495. doi: 10.1172/JCI110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Rotwein P. S., Chirgwin J., Province M., Knowler W. C., Pettitt D. J., Cordell B., Goodman H. M., Permutt M. A. Polymorphism in the 5' flanking region of the human insulin gene: a genetic marker for non-insulin-dependent diabetes. N Engl J Med. 1983 Jan 13;308(2):65–71. doi: 10.1056/NEJM198301133080202. [DOI] [PubMed] [Google Scholar]
- Steinmann B., Tuderman L., Peltonen L., Martin G. R., McKusick V. A., Prockop D. J. Evidence for a structural mutation of procollagen type I in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem. 1980 Sep 25;255(18):8887–8893. [PubMed] [Google Scholar]
- Vogeli G., Avvedimento E. V., Sullivan M., Maizel J. V., Jr, Lozano G., Adams S. L., Pastan I., de Crombrugghe B. Isolation and characterization of genomic DNA coding for alpha 2 type I collagen. Nucleic Acids Res. 1980 Apr 25;8(8):1823–1837. doi: 10.1093/nar/8.8.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. H., Cheah K. S., Grosveld F. G., Dahl H. H., Solomon E., Flavell R. A. Isolation and characterization of a human collagen alpha 1(I)-like gene from a cosmid library. Nucleic Acids Res. 1982 Mar 25;10(6):1981–1994. doi: 10.1093/nar/10.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]