Abstract
Background
Autoimmune thyroid disease (AITD) pathogenesis may result from a loss of immune tolerance to thyroid antigens. Regulatory T cells (Tregs) control immune responses, prevent excessive inflammation, and may be dysfunctional in AITD. We investigated the role of Tregs in Hashimoto's thyroiditis (HT) and Graves' disease (GD), complicated by Down syndrome (DS). Our goal was to identify differences in CD4+CD25high Treg function or number in patients with GD and HT, compared to healthy controls (HC).
Methods
Treg number was assessed by flow cytometric analysis in samples from 20 AITD patients (seven GD, 13 HT), nine HC, and seven individuals with DS, a genetic disorder associated with multiple autoimmune disorders including AITD. Treg function was assessed by the inhibition of proliferation (radioactive thymidine incorporation into DNA) of blood-derived T effector (Teff) cells by Tregs in a coculture. Various methods of stimulation were contrasted. Cytokine levels were determined in conditioned media from the co-cultures.
Results
No differences were found in the frequency of Tregs as a percentage of CD4+ cells between AITD and HC. AITD Tregs were less capable of inhibiting the proliferation of Teff cells when compared to HC; however, the impairment was dependent on the type of stimulation used. DS patients without AITD exhibited normal Treg function. We observed few differences in cytokine production between HC and AITD patients.
Conclusions
Tregs from AITD patients are partly dysfunctional, possibly explaining their autoimmunity. Future work will elucidate the diagnostic potential and pathophysiology of Tregs in AITD.
Introduction
Autoimmune thyroid disease (AITD) is a common disorder affecting 1%–4% of the overall population (1,2). AITD is subdivided into two main groups, Graves' disease (GD) and Hashimoto's thyroiditis (HT). Although GD is associated with hyperthyroidism and patients with HT more often exhibit hypothyroidism, these two conditions have significant overlap (3–6). There is frequently a co-occurrence of both thyroid disorders within a family and transition over time from one AITD to the other within an individual, suggesting a shared immunoregulatory defect. Individuals with GD and HT have a significantly increased risk of developing other autoimmune diseases, with a frequency of approximately 10% in GD and 14% in HT (7). Autoimmune diseases that occur with increased prevalence in AITD include type 1 diabetes (T1DM), celiac disease, Addison's disease, vitiligo, and rheumatoid arthritis (7,8).
Cell-mediated and humoral immunity both play roles in AITD. In HT and GD there is a loss of tolerance to thyroid antigens and lymphocyte infiltration into the thyroid gland (3). In HT, a diffuse lymphocyte infiltration leads to the formation of germinal centers and the destruction of thyrocytes (9). In GD, lymphocyte infiltration into the gland results in hypervascularity with a lack of significant thyrocyte destruction (3). In both conditions, B cells produce autoantibodies against thyroid antigens. Antibodies to thyroid peroxidase are present in the majority of patients with HT and GD (10). In GD autoantibodies cause a nonphysiological activation of the thyrotropin receptor resulting in hyperthyroidism (11).
Regulatory T cells (Tregs) are a subset of CD4+ T cells that have drawn tremendous interest due to their role in maintaining tolerance by suppressing the immune response and preventing autoimmune diseases (12). Tregs comprise ∼5%–10% of CD4+ T cells and can be identified by the expression of the transcription factor Foxp3 and high surface expression of CD25. These cells function through several mechanisms, including cell to cell contact (13) and the production of immunosuppressive cytokines, such as transforming growth factor (TGF)-β and interleukin (IL)-10, which inhibit antigen-specific T-cell responses (14,15).
Treg defects are thought to play a role in the development of numerous autoimmune diseases, including AITD, T1DM, and multiple sclerosis. Notably, mutations in Foxp3, a transcriptional repressor that is a key modulator of Treg function, result in IPEX syndrome, a syndrome involving severe multisystem autoimmune disease (16). A few groups have investigated Tregs in AITD patients; however, these studies have yielded conflicting results. In looking at the number of Tregs in AITD patients, two studies showed no deficit in Treg number, while another study found that only untreated GD patients had a significant decrease in the percentage of circulating Tregs (17,18).
Beyond assessing Treg number, limited studies have also evaluated Treg function in AITD, and these have yielded inconsistent results. One study showed impaired Treg function in a small number of individuals with AITD, although the authors did not differentiate between HT and GD (19). In contrast, another study found that untreated GD, euthyroid GD, and euthyroid HT patients had Tregs with similar suppressive function to HC (20).
Due to the limited number of studies reported, lack of differentiation between GD and HT patients, and conflicting results, we assessed Treg number and function in GD and HT. Another limitation of previous studies is that they did not investigate patients with AITD who have additional inflammatory diseases to determine if Treg function is more severely impaired. The current study focuses on patients with multiple autoimmune disorders and Down syndrome, a population with a high propensity for multiple autoimmune diseases including AITD. It is hoped that through a more complete understanding of Tregs in AITD we can develop better screening and prevention strategies for at-risk patients and improve therapeutic options for AITD and other autoimmune diseases.
Materials and Methods
Study population
Peripheral blood samples were obtained from 20 patients with AITD, nine HC, as well as seven individuals with DS (four of which had AITD). Diagnosis of GD and HT was based on the presence of anti–thyroid peroxidase antibodies, antithyroglobulin antibodies, and thyroid stimulating immunoglobulins. HC had no known history of autoimmune disease, negative anti–thyroid peroxidase and antithyroglobulin antibodies, and negative celiac antibodies. Exclusion criteria were as follows: steroids or immunosuppressants in the past 6 months, pregnancy, or a T-cell deficiency. Patients were recruited from Rainbow Babies and Children's Hospital, University Hospitals Case Medical Center, and Your Diabetes Endocrine Nutrition Group, Inc., Cleveland, Ohio. Blood samples were obtained with informed consent under Institutional Review Board approved protocols.
Cell isolation and quantification by flow cytometry
Peripheral blood mononuclear cells (PBMCs) were prepared from heparinized venous blood by density-gradient centrifugation (Ficoll-hypaque, Sigma-Aldrich, St. Louis, MO, 250 g, 25 min) within 3 hours of blood being drawn. Cells recovered from the gradient interface were washed twice in phosphate-buffered saline (PBS). A portion of the PMBCs were removed prior to staining to be used as irradiated, autologous (auto) or allogenic (allo) antigen-presenting cells (APCs). PMBCs used as APCs were irradiated at 3000 rad using a 137Cs irradiator (JL Shepherd, Mark 1 Model 68, San Fernando, CA).
For cell marker staining and cell sorting, CD4 and CD25 staining was performed on the remaining PBMCs using APC-conjugated anti-CD4 (Invitrogen, Carlsbad, CA) and phycoerythrin (PE)-conjugated anti-CD25 (BD Biosciences, San Jose, CA). Freshly isolated PBMCs were incubated with appropriate concentrations of antibodies for 30 minutes at 4°C in the dark. To confirm that CD4+CD25high T cells were FoxP3 positive, PE-conjugated antihuman FoxP3, fluorescein isothiocyanate (FITC)–conjugated anti-CD4, and APC-conjugated anti-CD25 antibodies were obtained from eBioscience (San Diego, CA). Cells were permeabilized and stained according to the manufacturer's protocol.
Cells were sorted and quantified on a BD Biosciences FACSAria based on their CD4 and CD25 expression. CD4+CD25high T cells (Tregs) were gated as the population of CD25high T cells with slightly lowered expression of CD4 as previously described (21,22). The CD4 cells that did not have CD25 bright expression were collected and used as T effector (Teff) cells. Upon reanalysis by flow cytometry, the purity of the sorted Teff and Treg cells was >95%.
Proliferation assay
Freshly sorted Teff cells (CD4+CD25−) and Tregs (CD4+CD25high) were cultured at varying ratios (20,000:0, 20,000:10,000, 20,000:5000, and 20,000:2500) in triplicate in round-bottomed 96-well plates (BD Biosciences) in complete RPMI 1640 medium ThermoScientific (Logan, UT), containing 10% fetal bovine serum (Sigma-Aldrich). Plate-bound anti-CD3 (0.2 μg/well OKT3, Ortho Diagnostic Systems, Rochester, NY), soluble anti-CD28 (0.05 μg/well, Ancell, Bayport, MN), and irradiated, auto-PBMCs (50,000 cells/well) were used to stimulate proliferation. In a subset of patients, irradiated allo-PBMCs from healthy donors were used alone or in combination with anti-CD3 and anti-CD28 to stimulate T cells.
Cells were cultured at 37°C in humidified 5% carbon dioxide. On day 4 of culture, media samples were obtained for cytokine measurements, and 0.5 μCi/well [3H]-thymidine (New England Nuclear, Boston, MA) was added for an additional 15–18 hours. Cell lysates were harvested with a Unifilter-96 Harvester (Perkin Elmer, Waltham, MA), and radioactive DNA incorporated into proliferating cells was measured as counts per minute (cpm) with a 1450 MicroBeta TriLux scintillation counter (Perkin Elmer, Waltham MA). Due to donor variability, the percentage of maximal proliferation was compared between groups rather than cpm. The percentage of proliferation was determined by (cpm incorporated in co-culture/cpm of the Teff population alone)×100.
Cytokine determination
Cytokine production from stimulated cells was measured on a subset of individuals (four HC and 14 with AITD) using a commercially available multiplex kit (Mesoscale Discovery, Gaithersburg, MD). The following cytokines were analyzed: TGF-β, IL-10, IL-4, IL-17, IL-5, IL-2, and interferon (IFN)-γ.
Statistical analysis
To determine statistical significance, the Mann–Whitney test was performed for two-group comparisons and a one-way ANOVA Bonferonni or Tukey test for multiple group comparisons using GraphPad Prism software (San Diego, CA); p values less than 0.05 were considered significant.
Results
Patient demographics
Samples were obtained from nine HC, 13 patients with HT, and seven patients with GD. There was a similar age range among all groups. More females participated than males in both the HC and AITD groups, and a similar sex ratio was maintained. A subset of patients with AITD had multiple autoimmune or inflammatory diseases. One GD patient had ulcerative colitis, four HT patients had one additional autoimmune disease (T1DM, CREST syndrome, systemic lupus erythematosis), and two HT patients had two additional autoimmune diseases (T1DM and celiac or pernicious anemia). Data from these patients are summarized in Table 1. We studied a separate population of patients with DS with and without AITD. These data are summarized in Table 2. All patients with HT were being treated with levothyroxine. Of the GD patients, three who had received radioactive iodine ablation and four patients who underwent thyroidectomy were taking levothyroxine. One GD patient went into remission while receiving antithyroid medications and was not taking any medication at the time of the study.
Table 1.
Characteristics of Healthy Controls and Individuals with Autoimmune Thyroid Disease
| Healthy controls | AITD | |
|---|---|---|
| Number of patients | 9 | 20 |
| Age, mean (range) | 34 (13–46) | 32 (10–56) |
| Female, n (%) | 7 (78) | 16 (80) |
| AITD: HT/GD | N/A | 13/7 |
| Mean duration of AITD in years (range) | N/A | 7 (0–23) |
| No. with multiple autoimmune disease (% with ≥3 autoimmune diseases, % with type 1 diabetes) | N/A | 7 (29, 57) |
AITD, autoimmune thyroid disease; HT, Hashimoto's thyroiditis; GD, Graves' disease; N/A, not applicable.
Table 2.
Characteristics of Individuals with Down Syndrome With and Without Autoimmune Thyroid Disease
| DS with AITD | DS without AITD | |
|---|---|---|
| Number of patients | 4 | 3 |
| Age, mean (range) | 18 (12–23) | 11 (10–12) |
| Female, n (%) | 2 (50) | 3 (100) |
| AITD: HT/GD | 3/1 | N/A |
| Mean duration of AITD in years (range) | 6 (2–10) | N/A |
| No. with multiple autoimmune disease | 0 | N/A |
DS, Down syndrome.
CD4+CD25high cells are Foxp3+
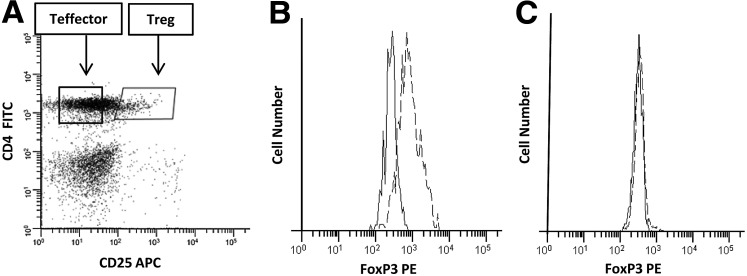
Tregs were defined during flow cytometric analysis as CD4 positive, CD25 bright cells. Consistent with other studies (23), the Treg cells exhibited a slightly lower expression of CD4 than the bulk of CD4+ T cells. An example of the gating strategy used to identify and isolate Tregs is shown in Figure 1A. To confirm that the gated CD4+CD25high cells exhibited phenotypic characteristics of Tregs, we stained these cells for the Treg-associated transcription factor FoxP3. The CD4+CD25high cells uniformly expressed FoxP3 (Fig. 1B). In contrast, the Teff cells (CD4+CD25−) showed no FoxP3 staining (Fig. 1C). Besides phenotypic properties of Tregs, our sorted Treg cells also demonstrated functional properties of Tregs as demonstrated by their potent ability to suppress Teff cell proliferation in HC (Fig. 2).
FIG. 1.
Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs). (A) PBMCs were stained with antigen-presenting cell (APC)-conjugated anti-CD4 (y-axis) and phycoerythrin (PE)-conjugated anti-CD25 (x-axis) and analyzed by flow cytometry. The CD4+CD25high cells exhibiting a slightly reduced expression of CD4 were considered regulatory T cells (Tregs). (B, C) PMBCs were stained either with PE-conjugated antihuman FoxP3 or an isotype control as well as fluorescein isothiocyanate (FITC)-conjugated anti-CD4 and APC-conjugated anti-CD25 and analyzed by flow cytometry. (B) Histogram demonstrating FoxP3 expression in gated CD4+CD25high cells (Tregs). (C) Histogram demonstrating absence of FoxP3 expression in gated CD4+CD25− cells (T effector [Teff] cells). ---, FoxP3 stain; ---, isotype control.
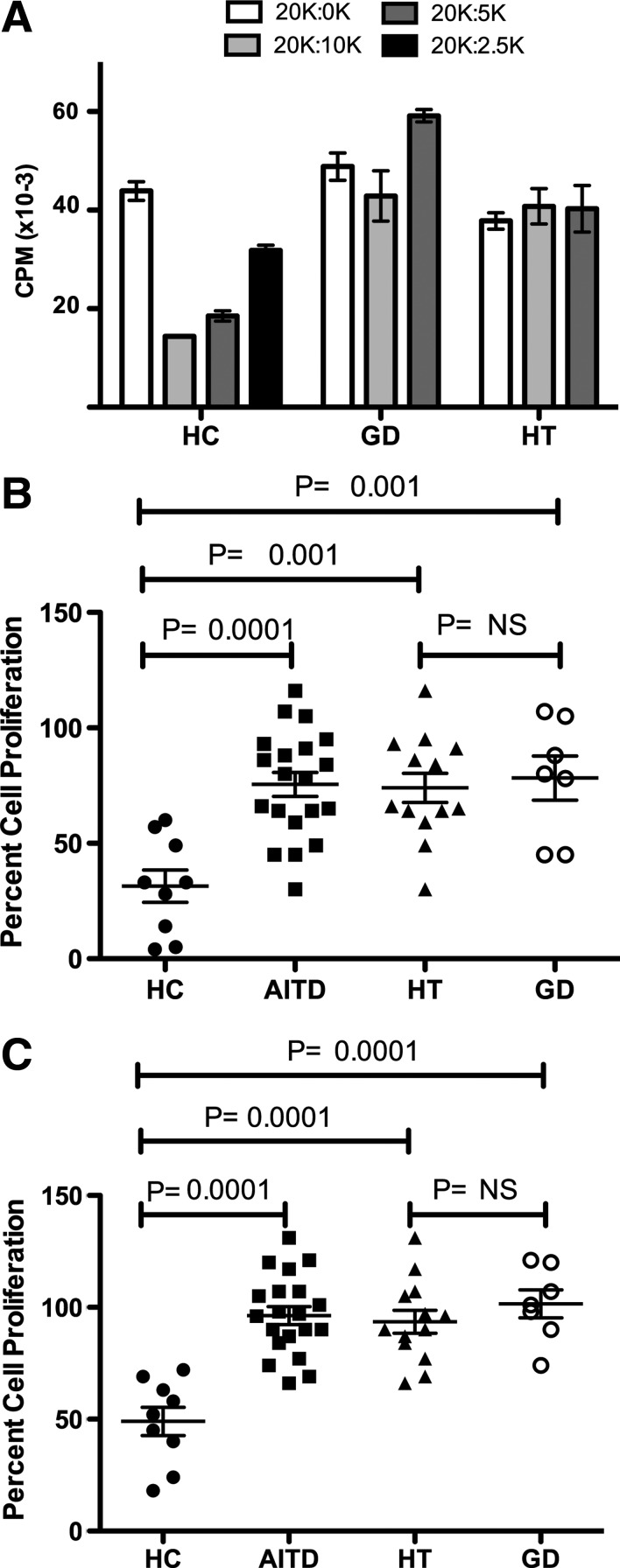
FIG. 2.
Tregs are immunosuppressive in healthy controls (HC), but not AITD. (A) Representative T-cell proliferation. CD4+ T cells from one HC subject, one GD patient, and one HT patient were activated at various ratios of Teff to Treg. Effector cell density was maintained at 20,000 cells/well, and Treg populations were titered from 10,000 to 2500. [3H]-thymidine was added for the final 15–18 hours of culture, and proliferation was measured by scintillation counting. (B, C) Percentage proliferation of activated Teff cells when cocultured with various concentrations of Tregs. (B) A 20,000:10,000 ratio of Teff to Treg. (C) A 20,000:5000 ratio of Teff to Treg. Horizontal bars show the mean with SEM. A one-way ANOVA Bonferonni test was used for multiple group comparisons in (B) and (C).
Frequency of CD4+ T cells and CD4+CD25high Tregs is unchanged in AITD
Some studies have suggested that the frequency of Tregs is altered in certain autoimmune conditions though it is not clear whether this occurs in AITD (24,25). Therefore, we sought to re-examine this question. No significant differences were found in the frequency of CD4+CD25high T cells as a percentage of CD4+ T cells between AITD patients (6.1%±0.4%) and HC (6.5%±0.4%; p=0.18). There was also no apparent difference in the frequency of CD4+ T cells as a percentage of mononuclear cells between AITD patients (42.1%±2.1%) and HC (44.2%±2.4%; p=0.1).
The suppressive function of Tregs from AITD patients is reduced compared to HC
Prior to assessing the function of Tregs in suppressing T-cell proliferation, we tested the proliferative capacity of CD4+CD25− Teff cells isolated from AITD and HC patients. Teff cells proliferated well in response to plate-bound anti-CD3 antibody, soluble anti-CD28 antibody, and auto-APC stimulation. There was no difference in the level of proliferation between groups. When Teff cells were cultured without Tregs (20,000:0) proliferation ranged from 11,604 to 74,419 cpm. The mean cpm for HC, HT, and GD were 33,081±5633, 32,093±6169, and 38,685±8160, respectively (p=0.7837). In agreement with previous reports, Tregs were anergic to stimulation (cpm<10,000).
In cocultures of Treg and Teff cells at varying ratios, a significant reduction in the level of suppression of proliferation was observed in patients with AITD as compared to HC. An example of a single donor from each group is shown in Figure 2A. In aggregate at a 20,000:10,000 ratio, the percent cell proliferation in AITD patients and HC were 75.5%±5.2% and 31.4%±7%, respectively (Fig. 2B; p=0.0001). Treg suppression was similarly impaired in HT and GD. At a 20,000:10,000 ratio the percent cell proliferation was 74.0%±6.3% in HT versus 78.3%±9.6 in GD (Fig. 2B; p=NS). When each AITD group was compared to the HC separately, both HT and GD Tregs remained significantly less suppressive (Fig. 2B; p=0.001 and p=0.001, respectively). Similar significant differences in suppressive capacity in AITD, HT, and GD were seen at the 20,000:5000 ratio (Fig. 2C). There was no apparent relationship between duration of thyroid disease or sex and Treg suppression. The percentage proliferation at a 20,000:10,000 ratio was 79.3%±8.8% (AITD ≤2 years) and 73.9%±6.5% (AITD>2 years) and 76.4%±5.6% (female) and 71.8%±14.4% (male).
Cytokine profiles were minimally altered in AITD
Cytokine secretion was analyzed in the conditioned medium obtained from cultures with CD4+CD25− T cells alone and cocultures of CD4+CD25− T cells and CD4+CD25high T cells. We observed few differences in cytokine production between HC and patients with AITD. The production of IL-10 from coculture was significantly less in AITD compared to HC (p=0.03). In patients with AITD, there was significantly more IL-17 production and significantly less IL-2 in coculture compared with CD4+CD25− T cells alone (Table 3). Though few differences were observed in cytokine levels, measurements were highly variable between donors as is commonly found in these assays making it difficult to definitively demonstrate the involvement of particular cytokines in AITD.
Table 3.
Cytokine Production in the Presence and Absence of Autoimmune Thyroid Disease
| Presence of AITD | 20,000:0 | 20,000:10,000 | p | |
|---|---|---|---|---|
| TGF-β | − | 904±374.0 | 839.5±355.4 | 0.34 |
| + | 947.4±105.5 | 908.6±99.41 | 0.36 | |
| IL-10 | − | 149.4±50.65 | 181±63.07a | 0.44 |
| + | 94.92±27.27 | 131.4±29.66a | 0.1253 | |
| IL-4 | − | 38.68±24.37 | 43.73±25.47 | 0.3429 |
| + | 21.02±5.65 | 24.14±5.46 | 0.3962 | |
| IL-17 | − | 331.8±142.7 | 598±276.6 | 0.1833 |
| + | 290.6±80.83 | 912.8±254.5 | 0.0228* | |
| IL-5 | − | 21.08±14.38 | 38.22±13.32 | 0.2 |
| + | 185.2±76.54 | 299.7±105.3 | 0.2863 | |
| IL-2 | − | 1002±683.4 | 298.4±217.7 | 0.3429 |
| + | 1510±418.9 | 524.5±222.2 | 0.0228* | |
| IFN-γ | − | 34,720±18,436 | 57,694±31,612 | 0.3429 |
| + | 34,046±15,544 | 23,754±10,662 | 0.4361 |
Conditioned media from Teff cells cultured alone and cocultured with Tregs at 20,000:10,000 were evaluated at 96 hours. Samples were obtained from four HC and 14 patients with AITD. Data represent mean and SEM (pg/mL); p values represent a comparison of 20,000:0 and 20,000:10,000.
Significant decrease in IL-10 production in coculture of AITD versus HC.
p value<0.05.
TGF, transforming growth factor; IL, interleukin; IFN, interferon.
Additional autoimmune disease does not compound Treg dysfunction
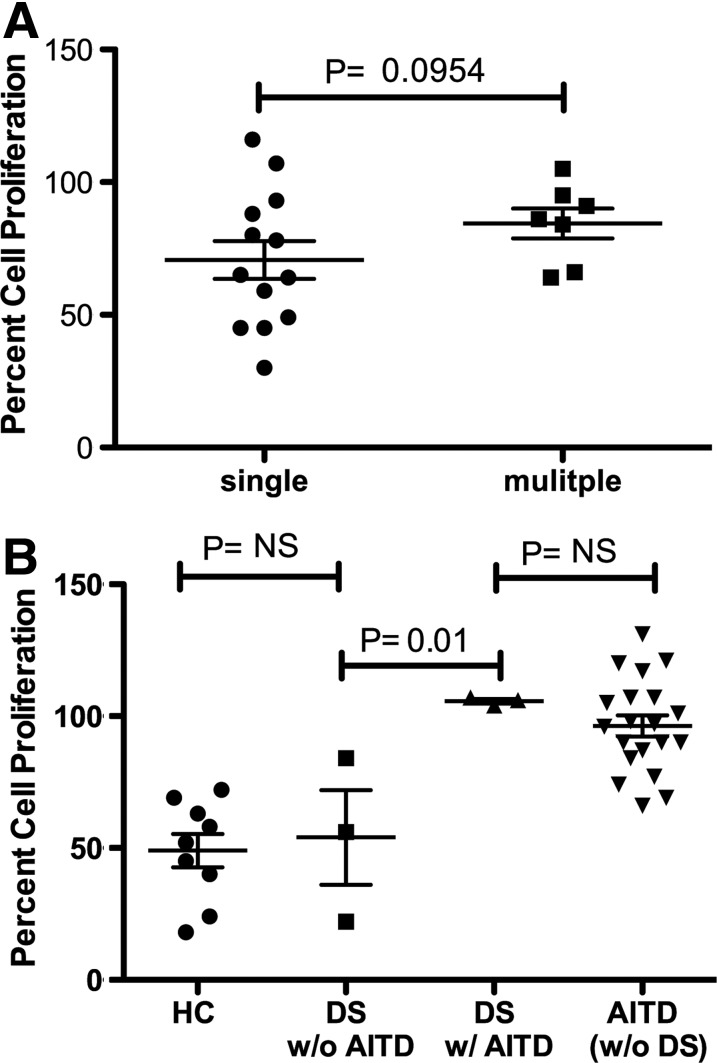
Due to the fact that a significant portion of AITD patients develop multiple autoimmune disorders, it was of interest to assess if these patients exhibit more profound Treg dysfunction. Treg function was not found to be more impaired in individuals with multiple (AITD and at least one additional autoimmune disease) versus single disease (AITD alone) (Fig. 3A).
FIG. 3.
No difference in impairment of Treg suppression in patients with increased susceptibility to autoimmune disease. (A) AITD alone versus those with AITD and at least one additional autoimmune disease. Percentage proliferation of 20,000 activated Teff cells when cocultured with 10,000 Tregs. A one-tailed Mann–Whitney t test was used to compare the groups. (B) Assessment of Treg function in patients with DS. Percentage proliferation of activated Teff cells when cocultured with various concentrations of Tregs. A 20,000:5000 ratio of Teff to Treg. A one-way ANOVA Bonferonni test was used for multiple group comparisons.
Treg activity is not impaired in DS patients without autoimmune disease
Individuals with DS have an extremely high predisposition to autoimmune disease, with AITD being the most common. The reason why DS patients are highly susceptible to autoimmune disease is not known. We sought to determine if autoimmune susceptibility in DS is due to an alteration in Treg function or number. In individuals with DS who did not have autoimmune disease, Treg number (data not shown) and function were not deficient. Tregs had similar suppressive activity in patients with DS without AITD as HC (Fig. 3B). Treg function was also found to be similarly impaired in DS individuals with AITD compared with other individuals with AITD.
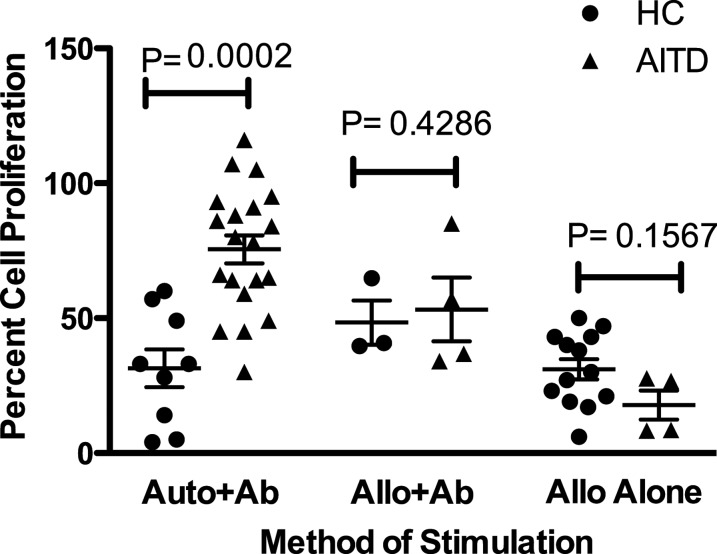
Method of stimulation of Teff cells obscures differences in Treg function between HC and AITD
Impairment in Treg function was found to be dependent on the type of stimulation. In contrast to a clear Treg impairment in AITD when auto-PBMCs, anti-CD3, and anti-CD28 antibodies were used as a stimulus, there was no significant difference in Treg suppression of proliferation between HC and those with AITD when allo-PBMCs were used in combination with anti-CD3 and anti-CD28 antibodies or when allo-PBMCs were used alone (Fig. 4). Due to this unexpected finding, we also assessed Treg function in a group of patients with T1DM, an autoimmune disease in which Treg impairment is widely recognized. When allo-PBMCs were used alone as a stimulus, there was no significant difference in Treg function between T1DM and HC (data not shown).
FIG. 4.
The method of T-cell stimulation affects the ability to detect a difference between Treg suppression in HC (●) versus AITD (▲) patients. CD4+CD25− T cells and Tregs were cultured at a 20,000:10,000 ratio. Cells from AITD patients and HC were stimulated with auto- or allo-PBMCs plus anti-CD3 and anti-CD28 antibodies or allo-PBMCs alone. Proliferation was measured by [3H]-thymidine incorporation at 4 days. Impairment in suppression of AITD patients was only observed when auto-PBMCs plus anti-CD3 and anti-CD28 antibodies were used. A two-tailed Mann–Whitney t test was used to compare the groups.
Discussion
Regulatory T cells play a prominent role in the maintenance of self-tolerance and suppression of excessive immune responses in health and disease (26,27). Defective Treg activity is associated with many autoimmune conditions such as T1DM, multiple sclerosis, and rheumatoid arthritis, but there are limited and conflicting data on the role of Tregs in AITD (12,19,20). We demonstrated a defect in Treg function in both HT and GD, despite the distinct pathophysiology of these diseases. This suggests there may be a shared immunoregulatory defect. Treg function was not affected by multiple autoimmune disease, which may be attributable to an inability to detect more impairment with the methodology used in these experiments. It is of particular interest that there was no difference in Treg function based on duration of AITD since patients ranged from newly diagnosed to 23 years duration. This suggests a lifelong alteration in Treg function versus a transient phenomenon. Interestingly, one patient with GD went into remission, and this single AITD patient exhibited Treg function similar to HC, suggesting that recovery of the Treg function may correlate with, and possibly correct, disease status. Longitudinal studies on a larger cohort will be necessary to investigate this phenomenon and may possibly impact innovative treatment strategies. The effects of the type of treatment and treatment duration on Treg function need to be examined in future studies. In addition, a predictive nature of Treg function in autoimmune diseases has been found in other diseases such as systemic lupus erythematosus (SLE). For instance, in vitro suppression of the proliferation of Teff cells by Tregs isolated from inactive SLE patients was comparable to that of normal individuals, whereas Tregs isolated from patients with active SLE poorly suppressed the proliferation (28).
Since DS patients are highly susceptible to autoimmune disease, we were surprised to find that Treg function was not impaired in DS individuals without AITD. This suggests that DS itself is not associated with Treg dysfunction. However, given the small number of DS patients in this study, more patients need to be assessed in order to verify this finding.
We also assessed the number of circulating Tregs in AITD. No differences in the number of Tregs or total number of CD4+ T cells were observed between AITD, HT, GD, and HC. Confirmation of this finding, which has been discrepant between previous studies (17,18,20), is important because it may affect future therapeutic approaches. If there is a deficiency in Treg number, then enhancing that number through cell therapy or drug treatment may be a reasonable strategy. If the number of Tregs is normal and these cells are dysfunctional, it would likely be necessary for AITD patients to receive either cellular or molecular stimuli that can reactivate normal Treg function.
Since Treg function is known to be mediated by cytokine production and cell-to-cell contact (13–15), we investigated the potential role of cytokines in Treg dysfunction found in AITD. Our data suggest that cytokine production is not likely playing a major role in the Treg dysfunction. Few differences were observed in cytokines produced during cocultures of Tregs and Teff cells. The only significant findings included the production of less IL-2 and more IL-17 in AITD from coculture experiments. It was recently reported that Tregs can produce IL-17, and higher IL-17 production by Tregs is associated with reduced suppressive activity (29,30). Therefore, higher IL-17 levels in our autoimmune patients were associated with their Treg dysfunction. Since few differences in cytokines were found in our study, it is likely that defects in cell to cell contact may be more important in explaining the functional impairment of Tregs from AITD patients.
Our study revealed that the method of stimulation of T-cell proliferation is critical to appreciate Treg dysfunction in AITD. In vitro, Teff cells can be induced to proliferate by various agents, including anti-CD3 and anti-CD28 antibodies and accessory cells. When allo-PBMCs (instead of auto-PBMCs) were used as accessory cells to stimulate T-cell proliferation, no impairment in Treg function was apparent in AITD. This is consistent with our unpublished observations in T1DM and raises the critical question of how accurately in vitro proliferation assays truly represent the function of these cells in vivo. This issue was previously noted by Brusko et al. (31), who discussed the value and limitations of the various in vitro assays. These observations suggest an important role for APCs in AITD as well. While it seems that a defect in Tregs is contributing to the pathophysiology of AITD, the pathogenesis is likely to be much more complex than an issue limited to Tregs alone. In other autoimmune conditions, APC dysfunction has been linked to impaired suppression of T cells. Jin et al. (32) studied this question in T1DM and found that the source of APCs affected Treg-mediated suppression of Teff cell proliferation. Future studies are warranted to determine why the source of the APC affects Treg function in vitro and to better understand how these cells interact in vivo.
Certain major histocompatibility (MHC) antigens have been associated with autoimmune diseases, including AITD. It has been proposed that the MHC phenotype can influence proliferative responses to mitogens and cytokine generation (33–35). Thus, differences in MHC may influence Teff cell proliferation in patients with autoimmune disease. Future studies are needed to characterize differences in the MHC profile of APCs from HC and AITD because some defects in Treg function may only be highlighted by identifying a corresponding defect in APCs (32).
Our study provides a partial explanation as to why there may be many seemingly conflicting results on the function of Tregs in autoimmune disease, including AITD, because different groups utilize a variety of methods to induce T-cell proliferation (31). For example, one study on primary Sjögren's syndrome revealed effective suppression of Teff by Tregs, with the authors concluding that CD4CD25 high regulatory T cells are not impaired in these patients (24). The proliferation assay in that study involved anti-CD3 and anti-CD28 antibodies as stimulators without the addition of PBMCs or APCs. It is unclear if the same results would have been achieved had the authors used auto-APCs. In addition to the means of stimulation, other variables, such as the duration of cell culture, the method of measuring proliferation (tritium uptake by proliferating cells or cell tracking dye), and the isolation method of the Treg population may affect results. One advantage of our study was the use of flow cytometric sorting, which provides a higher purity than using column-based methods. We therefore recommend exercising caution when interpreting studies utilizing in vitro cultures, an artificial environment that can be altered to produce differing results.
A better understanding of Treg function in AITD is important for a variety of reasons. For example, it may enable earlier identification of patients who will develop AITD and other autoimmune diseases. At this time, it is recommended that certain high-risk individuals, such as those with T1DM, Turner syndrome, or DS, have thyroid function studies performed annually or every other year (36–38). This leads to significant cost, time, and undue anxiety. If we better understand which individuals will develop AITD, such frequent screening may not be necessary.
An enhanced awareness of the role of Tregs in AITD may also aid in the development of new therapeutics that work by improving their function. Treg-based therapies for HT would be particularly useful in young children who require frequent lab monitoring and levothyroxine dose adjustments to ensure proper growth and brain development (39). In GD, current therapeutic options are limited to antithyroid medications, radioactive iodine ablation, and surgery, all of which are associated with significant risks (40).
Ultimately, a predictive gene signature and Treg functional diagnostic test for AITD along with therapeutics that can prevent or reverse Treg dysfunction in AITD would have a tremendous public health and economic impact.
Acknowledgments
We thank Nancy Roizen, MD, Karen Horowitz, MD, and Daniel Weiss, MD, for help recruiting patients and Jonida Toska and Wendy Goodman, PhD, for technical assistance. We thank Paul Hartman, MS, and the William T. Dahms, MD, CRU, for assistance with cytokine analysis. This work was supported by the K12 Career Research Development Program (HD057581-05) (AG), Fellows Research Award Program at Rainbow Babies and Children's Hospital (AG), Clinical and Translational Science Collaborative of Cleveland grant (UL1TR000439 and UL1RR024989) (AG), DK-054213 (ADL), AI-083609 (ADL), and AT-006I536 (DW).
Disclosure Statement
No competing financial interests exist.
References
- 1.Juan CJ. Endocrine autoimmunity. In: Greenspan FS, editor. Basic and Clinical Endocrinology. Lange; New York: 2011. pp. 27–45. [Google Scholar]
- 2.Doi F. Kakizaki S. Takagi H. Murakami M. Sohara N. Otsuka T. Abe T. Mori M. Long-term outcome of interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. Liver Int. 2005;25:242–246. doi: 10.1111/j.1478-3231.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- 3.Cappa M. Bizzarri C. Crea F. Autoimmune thyroid diseases in children. J Thyroid Res. 2010;2011:675703. doi: 10.4061/2011/675703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling MA. Thyroid disorders in childhood and adolescence. In: Fletcher J, editor; McGonigal C, editor. Pediatric Endocrinology. Third. Saunders; Philadelphia, PA: 2008. pp. 227–253. [Google Scholar]
- 5.Tomer Y. Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: from gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 6.Ban Y. Greenberg DA. Davies TF. Jacobson E. Concepcion E. Tomer Y. Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93:3589–3596. doi: 10.1210/jc.2008-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boelaert K. Newby PR. Simmonds MJ. Holder RL. Carr-Smith JD. Heward JM. Manji N. Allahabadia A. Armitage M. Chatterjee KV. Lazarus JH. Pearce SH. Vaidya B. Gough SC. Franklyn JA. Prevalence, relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1–9. doi: 10.1016/j.amjmed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins RC. Weetman AP. Disease associations with autoimmune thyroid disease. Thyroid. 2002;12:977–988. doi: 10.1089/105072502320908312. [DOI] [PubMed] [Google Scholar]
- 9.Totterman TH. Maenpaa J. Gordin A. Makinen T. Taskinens E. Andersson LC. Hayry P. Blood and thyroid-infiltrating lymphocyte subclasses in juvenile autoimmune thyroiditis. Clin Exp Immunol. 1977;30:193–199. [PMC free article] [PubMed] [Google Scholar]
- 10.Trbojević B. Djurica S. (Diagnosis of autoimmune thyroid disease) Srp Arh Celok Lek. 2005;133(Suppl 1):25–33. doi: 10.2298/sarh05s1025t. (In Serbian.) [DOI] [PubMed] [Google Scholar]
- 11.Gossage AA. Munro DS. The pathogenesis of Graves‘ disease. Clin Endocrinol Metab. 1985;14:299–330. doi: 10.1016/s0300-595x(85)80036-0. [DOI] [PubMed] [Google Scholar]
- 12.Fehervari Z. Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 15.Tang Q. Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett CL. Christie J. Ramsdell F. Brunkow ME. Ferguson PJ. Whitesell L. Kelly TE. Saulsbury FT. Chance PF. Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 17.Pan D. Shin YH. Gopalakrishnan G. Hennessey J. De Groot LJ. Regulatory T cells in Graves' disease. Clin Endocrinol (Oxf) 2009;71:587–593. doi: 10.1111/j.1365-2265.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang H. Zhao S. Tang X. Li J. Zou P. Changes of regulatory T cells in Graves' disease. J Huazhong Univ Sci Technolog Med Sci. 2006;26:545–547. doi: 10.1007/s11596-006-0515-6. [DOI] [PubMed] [Google Scholar]
- 19.Marazuela M. Garcia-Lopez MA. Figueroa-Vega N. de la Fuente H. Alvarado-Sanchez B. Monsivais-Urenda A. Sanchez-Madrid F. Gonzalez-Amaro R. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 20.Mao C. Wang S. Xiao Y. Xu J. Jiang Q. Jin M. Jiang X. Guo H. Ning G. Zhang Y. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves' disease. J Immunol. 186:4734–4743. doi: 10.4049/jimmunol.0904135. [DOI] [PubMed] [Google Scholar]
- 21.Baecher-Allan C. Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C. Brown JA. Freeman GJ. Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 23.Goodman WA. Young AB. McCormick TS. Cooper KD. Levine AD. Stat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppression. J Immunol. 186:3336–3345. doi: 10.4049/jimmunol.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottenberg JE. Lavie F. Abbed K. Gasnault J. Le Nevot E. Delfraissy JF. Taoufik Y. Mariette X. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun. 2005;24:235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Luczynski W. Stasiak-Barmuta A. Urban R. Urban M. Florys B. Hryszko M. Lower percentages of T regulatory cells in children with type 1 diabetes—preliminary report. Pediatr Endocrinol Diabetes Metab. 2009;15:34–38. [PubMed] [Google Scholar]
- 26.Brusko TM. Putnam AL. Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 27.Chatenoud L. Salomon B. Bluestone JA. Suppressor T cells—they're back and critical for regulation of autoimmunity! Immunol Rev. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 28.Valencia X. Yarboro C. Illei G. Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 29.Voo KS. Wang YH. Santori FR. Boggiano C. Wang YH. Arima K. Bover L. Hanabuchi S. Khalili J. Marinova E. Zheng B. Littman DR. Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beriou G. Costantino CM. Ashley CW. Yang L. Kuchroo VK. Baecher-Allan C. Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brusko TM. Hulme MA. Myhr CB. Haller MJ. Atkinson MA. Assessing the in vitro suppressive capacity of regulatory T cells. Immunol Invest. 2007;36:607–628. doi: 10.1080/08820130701790368. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y. Chen X. Podolsky R. Hopkins D. Makala LH. Muir A. She JX. APC dysfunction is correlated with defective suppression of T cell proliferation in human type 1 diabetes. Clin Immunol. 2009;130:272–279. doi: 10.1016/j.clim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto S. Michalski JP. Berman MA. McCombs C. Mechanism of a lymphocyte abnormality associated with HLA-B8/DR3: role of interleukin-1. Clin Exp Immunol. 1990;79:227–232. doi: 10.1111/j.1365-2249.1990.tb05183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallden J. Ilonen J. Roivainen M. Ludvigsson J. Vaarala O. Effect of HLA genotype or CTLA-4 polymorphism on cytokine response in healthy children. Scan J Immunol. 2008;68:345–350. doi: 10.1111/j.1365-3083.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 35.Lio D. Candore G. Romano GC. D'Anna C. Gervasi F. Di Lorenzo G. Modica MA. Potestio M. Caruso C. Modification of cytokine patterns in subjects bearing the HLA-B8,DR3 phenotype: implications for autoimmunity. Cytokines Cell Mol Ther. 1997;3:217–224. [PubMed] [Google Scholar]
- 36.2012 Executive summary: Standards of medical care in diabetes—2012. Diabetes Care. 35(Suppl 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bondy CA Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 38.Bull MJ Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 39.LaFranchi S. Diagnosis and treatment of hypothyroidism in children. Compr Ther. 1987;13:20–30. [PubMed] [Google Scholar]
- 40.Rivkees SA. Pediatric Graves' disease: controversies in management. Horm Res Paediatr. 2010;74:305–311. doi: 10.1159/000320028. [DOI] [PubMed] [Google Scholar]