Abstract
Background
Autoimmune and non-autoimmune thyroiditis frequently occur in persons with hepatitis C virus (HCV) infection. Treatment with interferon alpha (IFNα) is also associated with significant risk for the development of thyroiditis. To explore HCV–thyroid interactions at a cellular level, we evaluated whether a human thyroid cell line (ML1) could be infected productively with HCV in vitro.
Methods and Results
ML1 cells showed robust surface expression of the major HCV receptor CD81. Using a highly sensitive, strand-specific reverse transcription polymerase chain reaction assay, positive-sense and negative-sense HCV RNA were detected in ML1 cell lysates at days 3, 7, and 14 postinfection with HCV. HCV core protein was expressed at high levels in ML1 supernatants at days 1, 3, 5, 7, and 14 postinfection. The nonstructural protein NS5A was also detected in ML1 cell lysates by Western blotting. HCV entry into ML1 cells was shown to be dependent on the HCV entry factors CD81 and SR-B1/CLA1, while IFNα inhibited HCV replication in ML1 cells in a dose-dependent manner. Supernatants from HCV-infected ML1 cells were able to infect fresh ML1 cells productively, suggesting that infectious virions could be transferred from infected to naïve thyroid cells in vivo. Additionally, HCV infection of ML1 cells led to increased expression of the pro-inflammatory cytokine IL-8.
Conclusions
For the first time, we have demonstrated that HCV can infect human thyroid cells in vitro. These findings strongly suggest that HCV infection of thyrocytes may play a role in the association between chronic HCV infection and thyroid autoimmunity. Furthermore, the thyroid may serve as an extrahepatic reservoir for HCV viral replication, thus contributing to the persistence of viral infection and to the development of thyroid autoimmunity.
Introduction
Globally, 130–170 million people are infected with the hepatitis C virus (HCV) (1). While hepatocytes represent the major site of viral replication, multiple studies provide evidence for replication of HCV in several extrahepatic tissues and peripheral blood mononuclear cells, including granulocytes, monocytes/macrophages, dendritic cells, B lymphocytes, and T lymphocytes (2). In addition to the hepatic disease associated with HCV infection, a number of systemic complications and autoimmune disorders are associated with chronic HCV infection, including mixed cryoglobulinemia, arthralgia, paresthesia, myalgia, pruritus, sicca syndrome, and endocrine-metabolic complications (3). Notably, autoimmune and nonautoimmune thyroiditis are frequent in persons with HCV infection (4). HCV RNA is also present in thyroid tissue from patients with chronic HCV, suggesting that infection of the thyroid occurs in vivo (5,6). Moreover, HCV treatment with interferon alpha (IFNα) is associated with significant risk for the development of thyroiditis (7,8).
While HCV infection is associated with autoimmune thyroiditis, the mechanisms by which HCV triggers thyroiditis are largely unknown. We hypothesized that infection of thyroid cells by hepatitis C virions could contribute to the development of autoimmune thyroiditis by triggering local inflammation. Indeed, we previously reported high levels of the HCV receptor CD81 in human thyroid cells in primary cultures (9). Furthermore, incubation of thyroid cells with the HCV envelope 2 (E2) glycoprotein resulted in E2 binding to thyroid cells and increased expression of interleukin 8 (IL-8), an important pro-inflammatory cytokine. Thyroid cells incubated with E2 continued to proliferate normally and did not undergo apoptosis in contrast to previous findings in hepatocytes. To explore possible HCV–thyroid interactions at the cellular level further, we evaluated whether a human thyroid cell line could be infected productively with HCV in vitro.
Materials and Methods
Cell culture
Huh7.5 cells were provided by Apath LLC (St. Louis, MO) and maintained in Dulbecco's modified Eagle's medium—high glucose, supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 mg/mL), and non-essential amino acids. The human thyroid cell line ML1 was kindly provided by Dr. Johann Schoenberger (University of Regensburg, Regensburg, Germany). This cell line was derived from a differentiated follicular thyroid carcinoma (10). More than 90% of ML1 cells express thyroglobulin, chondroitin sulfate, and vimentin antigens. ML1 cells also uptake iodine and/or glucose and constitutively release triiodothyronine into culture supernatants, indicating that they represent a suitable model for biological studies of HCV–thyrocyte interactions. ML1 cells were maintained in Dulbecco's modified Eagle's medium containing 100 μM sodium pyruvate, 2 mM L-glutamine, 1 mg/mL glucose, and 3.7 g/L NaHCO3, and supplemented with 10% fetal calf serum, 100 U penicillin/mL, and 100 μg/mL streptomycin. ML1 and Huh7.5 cells were cultured at 37°C in 5% CO2, and the medium was replaced every two to four days.
Production of infectious HCV particles and infection of ML1 cells
The Huh7.5JFH1 cell line—which releases infectious HCV genotype 2a virions into the cell culture supernatant—was provided by Dr. Guangxiang Luo (University of Kentucky, Lexington, Kentucky) (11) and maintained in Dulbecco's modified Eagle's medium with 10% FBS and 5 μg/mL of blasticidin. Infectious virions (JFH1) were harvested from the supernatants of Huh7.5JFH1 cells, spun at high speed to pellet cellular debris, and stored at −80°C prior to use. For all experiments, 2×105 ML1 or Huh7.5 cells were infected for 4 h with 50 μL of virus (∼11.6 ng/mL of HCV core protein) in a 24-well plate. The virus was then removed, and cells were washed with phosphate-buffered saline (PBS) to remove the unbound virus.
Surface CD81 expression
A total of 5×105 Huh7.5 or ML1 cells in 1 mL of PBS were incubated with either 0.5 μL of either fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD81 monoclonal antibody (clone JS- 81; catalog no. 551108; BD Biosciences Pharmingen, San Jose, CA) or FITC-conjugated isotype control (IgG1 kappa, clone X40; catalog no. 340755; BD Biosciences Pharmingen). Cells were incubated for 30 min at 4°C, spun at ∼200 g at 4°C, and washed twice with PBS. Cells were resuspended in 1 mL of PBS, and surface expression was evaluated using a Coulter EPICS XL-MCL Flow Cytometer (Beckman Coulter, Inc., Fullerton, CA). At least 10,000 events were recorded per incubation.
Qualitative strand-specific rTth reverse transcription polymerase chain reaction
RNA from cell lysates was extracted using TRIzol (Invitrogen, Carlsbad, CA), washed, and resuspended in 50 μL of DEPC-treated dH2O. RNA from 140 μL of culture supernatant was extracted using the QIAamp Viral RNA Kit (Qiagen, Valencia, CA), and eluted in 60 μL of DEPC-treated dH2O. HCV RNA was detected utilizing a previously described qualitative strand-specific rTth reverse transcription polymerase chain reaction (RT-PCR) assay (12). PCR primers included the HCV-II sense primer (5′-CAC TCC CCT GTG AGG AAC T-3′, nucleotides [nt] 38–56 of the 5′UTR) and the HCV-I antisense primer (5′-TGG ATG CAC GGT CTA CGA GAC CTC-3′, nt 342–320). Thirty cycles of PCR (94°C for 30 sec, 58°C for 1 min, and 72°C for 2 min) were performed, and PCR products (295 base pairs in length) were visualized by gel electrophoresis.
ELISA for HCV core or NS3 protein
HCV core protein was quantified in cell culture supernatants and/or cell lysates by the HCV Core Antigen ELISA Kit (Cell Biolabs, Inc., San Diego, CA) with a lower limit of detection of 1 ng/mL. HCV NS3 was quantified using the HCV NS3 ELISA Kit (BioFront Technologies, Tallahassee, FL) with a lower limit of detection of 9 ng/mL.
Inhibition of HCV replication with anti-CD81, anti-CLA1, or IFNα
To evaluate cellular factors involved in viral entry, the mouse anti-human CD81 monoclonal antibody (CBL579; Millipore Corp., Billerica, MA), the mouse anti-human CLA1 monoclonal antibody (BDB610882; Thermo Fisher Scientific. Asheville, NC), or mouse IgG1k isotype control antibody (MH1013; Invitrogen Corp., Camarillo, CA) were incubated with ML1 or Huh7.5 cells on the day prior to and during HCV infection. Incubation with 0.1 ng, 10 ng, or 1000 ng consensus interferon (Infergen®; Three Rivers Pharmaceuticals, LLC, Cranberry Township, PA) was performed one day before and during viral infection.
Infection with patient-derived serum
The virus was prepared from three individuals infected with HCV genotype 1a—1795 (HCV RNA 6.62 log IU/mL), 1800 (5.01 log IU/mL), and 1870 (6.83 log IU/mL)—by incubating 100 μL of patient serum, 900 μL of PBS, and 300 μL of polyethylene glycol (PEG) overnight at 4°C. This mixture was then centrifuged for 20 min at ∼1600 g. Infections were performed with 200 μL of virus-containing supernatant per well of a 24-well plate for 4 h prior to washing cells three times with PBS to remove the input virus. HCV RNA and HCV core protein were detected at day 3 postinfection, as described above.
IL-8 expression
Cells were infected as described above. At day 3 postinfection, cell culture supernatants were removed and diluted 1:10 with PBS. IL-8 protein expression was quantified using the Human IL-8 ELISA Kit (Invitrogen Corp.) with a lower limit of detection of 5 pg/mL.
Results
CD81 is expressed on the human thyroid cell line ML1
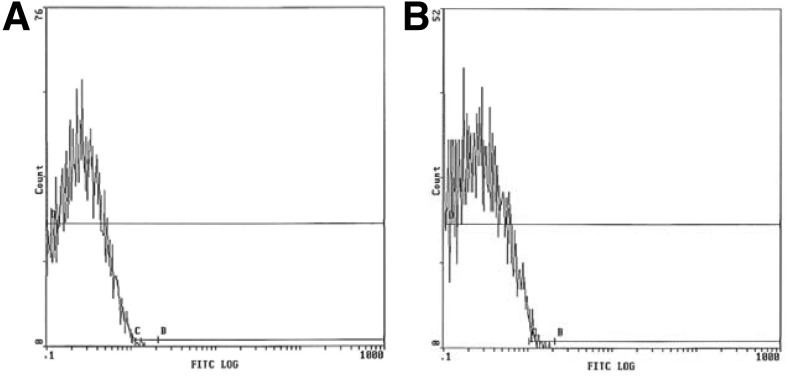
When examining human thyroid cells in primary culture, we previously reported expression of CD81, as well as binding of the HCV E2 protein (9). In order to examine the potential for ML1 cells to be infected by HCV, we evaluated surface expression of CD81 on the human thyroid cell line ML1. As shown in Figure 1, ML1 cells showed robust surface expression of CD81. As expected, the human hepatocyte-derived cell line Huh7.5 also expressed surface CD81. CD81 mRNA was also detected in DNase-treated ML1 and Huh7.5 cells (data not shown). These data suggest that ML1 cells express at least one receptor necessary for HCV infection; therefore, viral entry and replication might be possible.
FIG. 1.
Surface expression of CD81 receptor on Huh7.5 (A) or ML1 (B) cells; staining for isotype-matched control antibody is shown in gray.
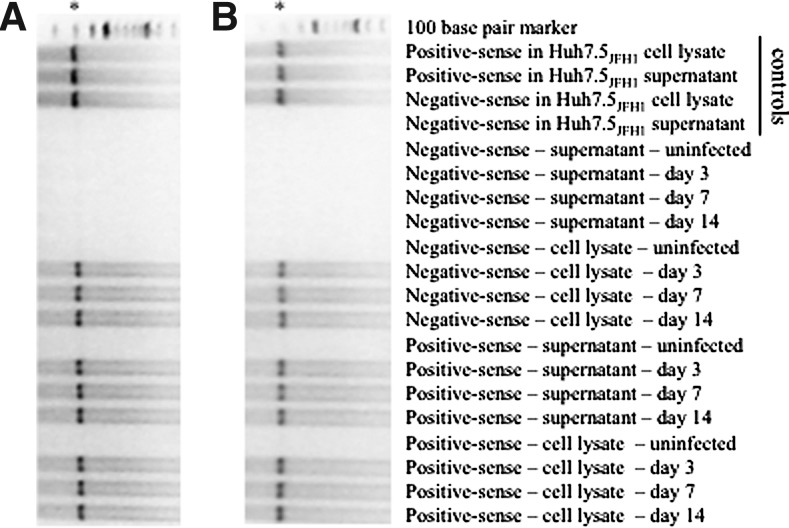
Detection of positive-sense and negative-sense HCV RNA in ML1 cells
Because virions contain positive-sense RNA genomes, the detection of positive-sense HCV RNA is not sufficient to demonstrate replication within a cell. Instead, a more accurate measure of actively replicating virus is the detection of negative-sense HCV RNA (i.e., replicative intermediates). Using a highly sensitive, qualitative RT-PCR, positive-sense and negative-sense HCV RNA were evaluated in the supernatants and cell lysates from Huh7.5 and ML1 cells after incubation with JFH1. As expected, HCV RNA was detected in the hepatocyte-derived cell line Huh7.5 after incubation with JFH1; both positive-sense and negative-sense HCV RNA were detected in cell lysates at days 3, 7, and 14 postinfection (Fig. 2A). The Huh7.5JFH1 cell line was evaluated as a positive control. Productive infection of the Huh7.5JFH1 cell line was confirmed by detection of positive-sense HCV RNA in cell lysates and supernatants, while negative-sense HCV RNA was detected only in Huh7.5JFH1 cell lysates as expected.
FIG. 2.
Qualitative reverse transcriptase polymerase chain reaction (PCR) for the detection of positive-sense and negative-sense hepatitis C virus (HCV) RNA at days 3, 7, and 14 postinfection of Huh7.5 (A) or ML1 (B) cells; asterisks denote the 295 bp PCR product.
Productive infection with HCV was observed in JFH1-treated ML1 cells. Positive-sense and negative-sense HCV RNA were detected in cell lysates at days 3, 7, and 14 postinfection (Fig. 2B), while positive-sense HCV RNA—but not negative-sense HCV RNA—was detected in supernatants from ML1 cells infected with JFH1 at days 3, 7, and 14 postinfection. As expected, positive-sense and negative-sense HCV RNA were not detected in cell lysates or supernatants from uninfected ML1 or uninfected Huh7.5 cells.
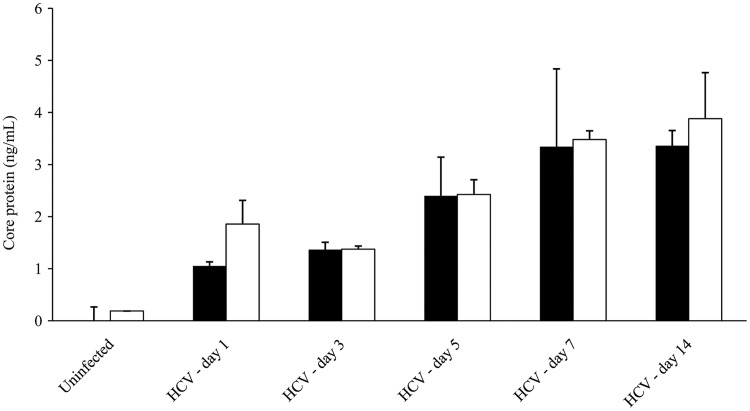
Detection of HCV core protein in ML1 cells
HCV-infected ML1 cells were further evaluated for viral protein production. As shown in Figure 3B, HCV core protein was expressed at high levels in ML1 supernatants at days 1, 3, 5, 7, and 14 postinfection with JFH1. As a positive control, HCV core protein expression was also evaluated in supernatants from Huh7.5 cells infected with JFH1 (Fig. 3A). HCV-infected ML1 and HCV-infected Huh7.5 cells released similar levels of core protein. Using Western blot assays, we also detected HCV NS5A protein—a nonstructural protein that is absent from infectious virions but is an important regulator of cellular pathways and viral replication in infected cells—in the cell lysates from ML1 (or Huh7.5) cells at day 3 postinfection with JFH1 (data not shown).
FIG. 3.
HCV core protein levels at days 1, 3, 5, 7, and 14 postinfection of Huh7.5 (■) or ML1 (□) cells.
HCV infection of ML1 cells is dependent on CD81 and CLA1
CD81 is a major entry factor for HCV infection of hepatocytes (13). Therefore, we evaluated whether ML1 infection with JFH1 was CD81-dependent. As shown in Figure 4A, incubation with an anti-CD81 antibody was highly efficient at inhibiting JFH1 infection (80.8% to 93.5% inhibition compared to HCV infection in the absence of antibody) of ML1 cells. As expected, anti-CD81 antibody also efficiently inhibited JFH1 infection (93.1% to 97.2%) of Huh7.5 cells.
FIG. 4.
HCV NS3 protein levels in cell lysates at day 3 postinfection of Huh7.5 (■) or ML1 (□) cells in the presence/absence of anti-CD81 (A) or anti-CLA1 (B) at dilutions of 1:100, 1:500, and 1:2500.
Scavenger receptor class B type 1 (SR-B1/CLA1) plays an important role in HCV entry into hepatocytes (14). As shown in Figure 4B, anti-CLA1 antibody inhibited JFH1 infection, although inhibition was less pronounced in ML1 cells (66.3% to 94.4%) compared to Huh7.5 cells (92.2% to 95.8%).
IFNα limits HCV replication in ML1 cells
IFNα is a potent inhibitor of HCV replication in hepatocytes. Therefore, we evaluated the ability of IFNα to inhibit JFH1 replication within ML1 cells. As shown in Figure 5, IFNα inhibited JFH1 replication in both ML1 (31.7%, 85.3%, and 90.1% in the presence of 0.1 ng, 10 ng, and 1000 ng IFNα respectively) and Huh7.5 (37.5%, 80.5%, and 88.1%) cells in a dose-dependent manner.
FIG. 5.
HCV core protein levels at day 3 postinfection of Huh7.5 (■) or ML1 (□) cells in the presence/absence of interferon alpha at concentrations of 0.1 ng, 10 ng, and 1000 ng.
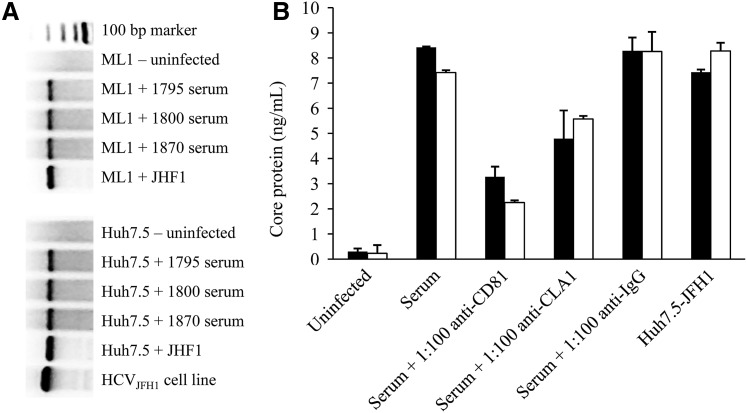
Patient-derived HCV infects ML1 cells. JFH1 is a genotype 2a strain of HCV. To explore the ability of genotype 1—the most common genotype in the United States—to replicate within ML1 cells further, we precipitated virus from three HCV RNA–positive individuals (1795, 1800, and 1870) and infected ML1 or Huh7.5 cells with these patient-derived HCVs. At day 3 postinfection, negative-sense HCV RNA—a replication intermediate—was detected by qualitative RT-PCR in both cell types indicating infection (Fig. 6A), while uninfected cells were RT-PCR negative. Similarly, positive-sense HCV RNA was detected in both cell types but not uninfected cells (data not shown). Using HCV derived from one individual (1800), we further explored the ability of receptor antibodies to block viral infection. As shown in Figure 6B, patient-derived HCV achieved similar levels of infection compared to JFH1. Incubation with anti-CD81 partially blocked infection in both cell types (69.7% for ML1 and 61.1% for Huh7.5), although anti-CLA1 was less efficient at blocking infection in both cell types (24.9% for ML1 and 43.1% for Huh7.5). An IgG1k isotype control antibody had no effect on viral infection.
FIG. 6.
(A) Qualitative reverse transcriptase PCR for the detection of negative-sense HCV RNA in cell lysates at day 3 postinfection of Huh7.5 or ML1 cells with serum from individuals 1795, 1800, and 1870. The HCVJFH1 cell line is included as a positive control. (B) HCV core protein levels at day 3 postinfection of Huh7.5 (■) or ML1 (□) cells with serum from 1800 in the presence/absence of anti-CD81, anti-CLA1, or isotype control antibodies at dilutions of 1:100. Infection of both cell lines with JFH1 is included as a positive control.
HCV infection of ML1 cells produces infectious virions
We evaluated whether ML1 cells infected with JFH1 could produce infectious virions capable of subsequent rounds of infection. ML1 cells (or Huh7.5 cells as positive controls) were first infected with JFH1 for four hours, as in other experiments. Supernatants were then harvested at day 3 postinfection and used to infect HCV naïve ML1 or Huh7.5 cells. As shown in Figure 7, robust levels of HCV infection were achieved in both ML1 and Huh7.5 cells, suggesting the production of infectious virions that could be transferred to naïve cells.
FIG. 7.
ML1 cells were infected with HCV for 4 h, supernatants harvested at day 3 postinfection, and used to infect fresh Huh7.5 (■) or ML1 (□) cells. HCV core protein levels were measured at day 3 postinfection to demonstrate production of infectious virions.
HCV infection upregulates IL-8 expression
We have previously reported that the HCV E2 protein induces IL-8 expression in thyrocytes (9). Therefore, we examined further whether HCV infection of ML1 cells similarly induced IL-8 production. As shown in Figure 8, supernatant IL-8 protein levels were 10.8 pg/mL and 41.4 pg/mL for supernatants from Huh7.5 and ML1 cells respectively. At day 3 postinfection with HCV, IL-8 protein levels increased to 401.9 pg/mL for Huh7.5 cells and 407.2 pg/mL for ML1 cells, suggesting that HCV infection induced a pro-inflammatory cytokine response in both cell types.
FIG. 8.
Interleukin 8 protein levels in Huh7.5 (■) or ML1 (□) cell culture supernatants—diluted 1:10 with PBS—at day 3 postinfection.
Discussion
Among the extrahepatic complications associated with chronic HCV infection, autoimmune thyroid diseases are very common (4,15–17). Indeed, there is considerable epidemiological evidence for an association between chronic HCV infection and thyroid disorders (4,16,18–21). While the frequency of thyroid antibodies (TAb) in chronic HCV patients varies, prevalence rates of up to 42% have been reported (22). Pooling of data from all studies on HCV infection (as measured by either HCV antibodies or RNA) and thyroid autoimmunity has also demonstrated a significant increased risk of thyroiditis in HCV patients (23). Moreover, IFNα—the mainstay of therapy for chronic HCV infection—adds additional risk for thyroiditis (24–28). These and other complications of IFNα therapy frequently necessitate stopping therapy or reducing the dose, thereby interfering with effective management of chronic HCV infection. However, the mechanisms by which chronic HCV infection and IFNα therapy trigger thyroiditis are unclear. Here, we show for the first time that direct viral infection of thyroid cells may play a key role in the autoimmune thyroiditis triggered by HCV infection.
Does infection of thyrocytes play a role in triggering thyroid autoimmunity in HCV-infected patients? Mechanistically, HCV infection of thyroid cells can trigger autoimmune thyroiditis by induction of: (1) changes in self antigen expression, or exposure of cryptic epitopes; (2) local inflammation and cytokine release, resulting in activation of autoreactive T cells by bystander mechanisms; (3) molecular mimicry between viral antigens and thyroid antigens; and (4) aberrant expression of MHC class II molecules on thyroid cells (29). Several host cell factors play a role in mediating HCV attachment and entry, including CD81, SR-B1, the low-density lipoprotein (LDL) receptor, and the tight junction proteins claudin-1 and occludin (30). While the expression of all of these factors has not been shown in the thyroid to date, we have shown that human thyrocytes express CD81, bind HCV virions, and activate the tissue inflammatory response (9).
Cytokines play an important role in the immunopathogenesis of thyroiditis (31), and a number of cytokines are regulated by HCV (32). For instance, IL-8 is elevated in the serum of persons with chronic HCV compared to uninfected controls and is associated with hepatic inflammation, severe hepatitis activity, and decreased susceptibility to interferon therapy (33–36). IL-8 expression in thyrocytes is also upregulated by HCV E2 protein (9) and by HCV infection itself (Fig. 8). Because IL-8 is known to antagonize the antiviral effects of IFN (37,38), increased expression of IL-8 may facilitate HCV persistence in thyrocytes. Other cytokines may also be involved in the pathogenesis of HCV-mediated thyroiditis and warrant careful investigation in future studies.
To date, only two studies have evaluated the presence of HCV replication within the thyroid in vivo. While examining extrahepatic sites of HCV replication in patients with AIDS, Laskus et al. detected negative-strand HCV RNA in the thyroid from two of eight individuals (6). A recent study by Bartolome et al. reported both positive-sense and negative-sense HCV RNA in the thyroid of three HCV seropositive patients, while HCV RNA was not detected in the thyroid tissues from eight HCV seronegative patients (5). These studies are consistent with our current findings and strongly suggest that direct HCV infection of human thyroid cells occurs both in vitro and in vivo.
Extrahepatic reservoirs of HCV replication may play a role in viral persistence. However, accurately demonstrating extrahepatic HCV replication has been challenging due to the limited cellular tropism of certain HCV isolates in vitro. Thus, most studies have been performed using cells and tissues collected from HCV-infected persons and not by directly infecting human nonhepatic cells, as performed in the current study. Because hepatitis C virions themselves contain positive-sense RNA genomes, the detection of positive-strand HCV RNA is not sufficient to demonstrate HCV replication; rather, detection of actively replicating viral genomes—as indicated by negative-strand HCV RNA (i.e., replicative intermediates)—is necessary. Indeed, using highly sensitive, strand-specific RT-PCR, we demonstrated the presence of negative-strand HCV RNA in human thyroid cell lysates infected with HCV. Moreover, the production of new viral proteins and infectious virions was confirmed in the ML1 human thyroid cell line.
The various systems utilized to examine HCV infection in vitro have been the subject of several comprehensive reviews (39,40). Despite its use in studying the complete HCV life cycle, it is unclear whether the high level of replication achieved by JFH1 in hepatocytes is due to the source from which it was isolated or whether it represents a unique HCV sequence that is infrequent in vivo. The JHF1 strain of HCV is highly hepatotropic and is not capable of infecting lymphocytes (31,32); thus, it is intriguing that JFH1 was able to infect another extrahepatic cell type—ML1/thyrocytes. As an alternative to the JHF1-based cell culture system, several studies have previously shown that clinical isolates can infect primary hepatocytes (41–43). Others have reported infection of hepatoma cell lines with HCV clinical isolates (44), although this was not shown in all studies (45). Such disparate findings may be the result of differences in HCV genotypes, the infection methodology, and/or the titer of virus used to establish infection. Given these differences, we utilized both experimental approaches with respect to HCV infection of thyrocytes. Importantly, the preliminary data presented here suggest that both the JFH1-based replication system, as well as serum-derived HCV, could serve as complementary models for evaluating HCV–thyrocyte interactions. However, it should be noted that a more thorough investigation into the host cell molecules (13) that regulate HCV entry and subsequent genome replication within thyrocytes is now warranted to determine how to best limit HCV replication in the thyroid. Furthermore, additional thyroid cell lines, as well as primary thyroid cultures, should be examined for their susceptibility to HCV infection in vitro. Finally, assessing the relative proportion of thyroid-derived versus liver-derived virus would be helpful in determining the overall contribution of the thyroid to HCV disease pathogenesis.
Here, we provide strong evidence that the thyroid may serve as an extrahepatic reservoir of HCV replication. There are few studies examining the role of extrahepatic replication in HCV persistence. For example, HCV RNA—including negative-strand replicative intermediates—may persist in PBMCs after spontaneous or treatment-induced resolution of HCV infection (46–52). This has led some authors to speculate that low-level replication of HCV in PBMCs may lead to reactivation of HCV after treatment cessation and/or predict response to therapy. In a similar manner, replication of HCV in the thyroid may not only induce thyroid-specific disease but could also contribute to the reemergence of HCV after treatment cessation if available HCV therapies do not effectively eradicate viral replication. Moreover, persistence of HCV infection in the thyroid could induce sustained immune activation that triggers and maintains thyroid autoimmunity.
In summary, we have demonstrated for the first time that HCV can infect human thyroid cells and replicate within them in vitro. These findings strongly suggest that HCV infection of thyrocytes plays a role in the association between chronic HCV infection and thyroid autoimmunity. Our findings present a novel mechanism for the induction of thyroid autoimmunity by infectious agents.
Acknowledgments
This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and by the VA Biomedical Laboratory Research and Development Merit Award and by grants DK61659, DK67555, and DK073681 from NIDDK (to Y.T.). The authors would like to thank Ms. Eleanor Powell for her critical evaluation of this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alter M. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackard JT. Kemmer N. Sherman K. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 3.Cacoub P. Poynard T. Ghillani P. Charlotte F. Olivi M. Piette JC. Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42:2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Antonelli A. Ferri C. Pampana A. Fallahi P. Nesti C. Pasquini M. Marchi S. Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Bartolomé J. Rodríguez-Iñigo E. Quadros P. Vidal S. Pascual-Miguelañez I. Rodríguez-Montes JA. García-Sancho L. Carreño V. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. J Med Virol. 2008;80:1588–1594. doi: 10.1002/jmv.21269. [DOI] [PubMed] [Google Scholar]
- 6.Laskus T. Radkowski M. Wang L. Vargas H. Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398–1401. doi: 10.1002/hep.510280531. [DOI] [PubMed] [Google Scholar]
- 7.Andrade LJ. D'Oliveira A., Jr Silva CA. Nunes P. França LS. Malta AM. Paraná R. A meta-analysis of patients with chronic hepatitis C treated with interferon-alpha to determine the risk of autoimmune thyroiditis. Acta Gastroenterol Latinoam. 2011;41:104–110. [PubMed] [Google Scholar]
- 8.Tomer Y. Blackard JT. Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akeno N. Blackard JT. Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31:339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schönberger J. Bauer J. Spruss T. Weber G. Chahoud I. Eilles C. Grimm D. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J Mol Med. 2000;78:102–110. doi: 10.1007/s001090000085. [DOI] [PubMed] [Google Scholar]
- 11.Cai Z. Zhang C. Chang KS. Jiang J. Ahn BC. Wakita T. Liang TJ. Luo G. Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. J Virol. 2005;79:13963–13973. doi: 10.1128/JVI.79.22.13963-13973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiasa Y. Blackard JT. Lin W. Kamegaya Y. Horiike N. Onji M. Schmidt EV. Chung RT. Cell-based models of sustained, interferon-sensitive hepatitis C virus genotype 1 replication. J Virol Methods. 2006;132:195–203. doi: 10.1016/j.jviromet.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meredith LW. Wilson GK. Fletcher NF. McKeating J. Hepatitis C virus entry: beyond receptors. Rev Med Virol. 2012;22:182–193. doi: 10.1002/rmv.723. [DOI] [PubMed] [Google Scholar]
- 14.Scarselli E. Ansuini H. Cerino R. Roccasecca RM. Acali S. Filocome E. Traboni C. Nicosia A. Cortese R. Vitelli A. The human scavenger receptor class B type 1 is a novel candidate receptor for the hepatis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pateron D. Hartmann DJ. Duclos-Vallee JC. Jouanolle H. Beaugrand M. Latent autoimmune thyroid disease in patients with chronic HCV hepatitis. J Hepatol. 1992;16:244–245. doi: 10.1016/s0168-8278(05)80124-2. [DOI] [PubMed] [Google Scholar]
- 16.Quaranta JF. Tran A. Régnier D. Letestu R. Beusnel C. Fuzibet JG. Thiers V. Rampal P. High prevalence of antibodies to hepatitis C virus (HCV) in patients with anti-thyroid autoantibodies. J Hepatol. 1993;18:136–138. doi: 10.1016/s0168-8278(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 17.Mohran ZY. Abdel Kader NA. Abdel Moez AT. Abbas A. Subclinical autoimmune thyroid disorders in Egyptian patients with untreated chronic hepatitis C virus infection. J Egypt Soc Parasitol. 2010;40:45–56. [PubMed] [Google Scholar]
- 18.Boadas J. Rodríguez-Espinosa J. Enríquez J MF. Martínez-Cerezo FJ. González P. Madoz P. Vilardell F. Prevalence of thyroid autoantibodies is not increased in blood donors with hepatitis C virus infection. J Hepatol. 1995;22:611–615. doi: 10.1016/0168-8278(95)80216-9. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Soto L. Gonzalez A. Escobar-Jimenez F. Vazquez R. Ocete E. Olea N. Salmeron J. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158:1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 20.Ganne-Carrie N. Medini A. Coderc E. Seror O. Christidis C. Grimbert S. Trinchet JC. Beaugrand M. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14:189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 21.Preziati D. La Rosa L. Covini G. Marcelli R. Rescalli S. Persani L. Del Ninno E. Meroni PL. Colombo M. Beck-Peccoz P. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132:587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 22.Tomer Y. Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? Am J Med. 2004;117:60–61. doi: 10.1016/j.amjmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli A. Ferri C. Fallahi P. Ferrari SM. Ghinoi A. Rotondi M. Ferrannini E. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16:563–572. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 24.Carella C. Amato G. Biondi B. Rotondi M. Morisco F. Tuccillo C. Chiuchiolo N. Signoriello G. Caporaso N. Lombardi G. Longitudinal study of antibodies against thyroid in patients undergoing interferon-alpha therapy for HCV chronic hepatitis. Horm Res. 1995;44:110–114. doi: 10.1159/000184606. [DOI] [PubMed] [Google Scholar]
- 25.Carella C. Mazziotti G. Morisco F. Rotondi M. Cioffi M. Tuccillo C. Sorvillo F. Caporaso N. Amato G. The addition of ribavirin to interferon-alpha therapy in patients with hepatitis C virus-related chronic hepatitis does not modify the thyroid autoantibody pattern but increases the risk of developing hypothyroidism. Eur J Endocrinol. 2002;146:743–749. doi: 10.1530/eje.0.1460743. [DOI] [PubMed] [Google Scholar]
- 26.Marazuela M. García-Buey L. González-Fernández B. García-Monzón C. Arranz A. Borque MJ. Moreno-Otero R. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44:635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 27.Roti E. Minelli R. Giuberti T. Marchelli S. Schianchi C. Gardini E. Salvi M. Fiaccadori F. Ugolotti G. Neri TM. Braverman LE. Multiple changes in thyroid function in patients with chronic active HCV hepatitis treated with recombinant interferon-alpha. Am J Med. 1996;101:482–487. doi: 10.1016/s0002-9343(96)00259-8. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe U. Hashimoto E. Hisamitsu T. Obata H. Hayashi N. The risk factor for development of thyroid disease during interferon-alpha therapy for chronic hepatitis C. Am J Gastroenterol. 1994;89:399–403. [PubMed] [Google Scholar]
- 29.Tomer Y. Davies T. Infection, thyroid disease and autoimmunity. Endocr Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 30.Zeisel MB. Fofana I. Fafi-Kremer S. Baumert T. Hepatitis C virus entry into hepatocytes: molecular mechanisms and targets for antiviral therapies. J Hepatol. 2011;54:566–576. doi: 10.1016/j.jhep.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Ganesh BB. Bhattacharya P. Gopisetty A. Prabhakar B. Role of cytokines in the pathogenesis and suppression of thyroid autoimmunity. J Interferon Cytokine Res. 2011;31:721–731. doi: 10.1089/jir.2011.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallahi P. Ferri C. Ferrari SM. Corrado A. Sansonno D. Antonelli A. Cytokines, HCV-related disorders. Clin Develop Immunol. 20122012:468107. doi: 10.1155/2012/468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda R. Ishimura N. Ishihara S. Chowdhury A. Moriyama N. Nogami C. Miyake T. Niigaki M. Tokuda A. Satoh S. Sakai S. Akagi S. Watanabe M. Fukumoto S. Intrahepatic expression of pro-inflammatory cytokine mRNAs and interferon efficacy in chronic hepatitis C. Liver. 1996;16:390–399. doi: 10.1111/j.1600-0676.1996.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 34.Shimoda K. Begum N. Shibuta K. Mora M. Bonkovsky H. Banner B. Barnard G. Interleukin-8 and hIRH (SDF1-a/PBSF) mRNA expression and histological activity index in patients with chronic hepatitis C. Hepatology. 1998;28:108–115. doi: 10.1002/hep.510280116. [DOI] [PubMed] [Google Scholar]
- 35.Polyak SJ. Khabar KS. Rezeiq M. Gretch D. Elevated levels of interleukin-8 in serum are associated with hepatitis C infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polyak SJ. Khabar KS. Paschal DM. Ezelle HJ. Duverlie G. Barber GN. Levy DE. Mukaida N. Gretch DR. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol. 2001;75:6095–6106. doi: 10.1128/JVI.75.13.6095-6106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khabar KS. Al-Zoghaibi F. Al-Ahdal MN. Murayama T. Dhalla M. Mukaida N. Taha M. Al-Sedairy ST. Siddiqui Y. Kessie G. Matsushima K. The alpha chemokine, interleukin 8, inhibits the antiviral action of interferon a. J Exp Med. 1997;186:1077–1085. doi: 10.1084/jem.186.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y. Wei L. Jiang D. Wang J. Cong X. Fei R. Antiviral action of interferon-alpha against hepatitis C virus replicon and its modulation by interferon-gamma and interleukin-8. J Gastroenterol Hepatol. 2007;22:1278–1285. doi: 10.1111/j.1440-1746.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 39.Brass V. Moradpour D. Blum H. Hepatitis C virus infection: in vivo and in vitro models. J Viral Hepat. 2007;14:64–67. doi: 10.1111/j.1365-2893.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 40.Burlone ME. Budkowska A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J Gen Virol. 2009;90:1055–1070. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- 41.Fournier C. Sureau C. Coste J. Ducos J. Pageaux G. Larrey D. Domergue J. Maurel P. In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J Gen Virol. 1998;79:2367–2374. doi: 10.1099/0022-1317-79-10-2367. [DOI] [PubMed] [Google Scholar]
- 42.Molina S. Castet V. Pichard-Garcia L. Wychowski C. Meurs E. Pascussi JM. Sureau C. Fabre JM. Sacunha A. Larrey D. Dubuisson J. Coste J. McKeating J. Maurel P. Fournier-Wirth C. Serum-derived hepatitis C virus infection of primary human hepatocytes is tetraspanin CD81 dependent. J Virol. 2008;82:569–574. doi: 10.1128/JVI.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rumin S. Berthillon P. Tanaka E. Kiyosawa K. Trabaud MA. Bizollon T. Gouillat C. Gripon P. Guguen-Guillouzo C. Inchauspé G. Trépo C. Dynamic analysis of hepatitis C virus replication and quasispecies selection in long-term cultures of adult human hepatocytes infected in vitro. J Gen Virol. 1999;80:3007–3018. doi: 10.1099/0022-1317-80-11-3007. [DOI] [PubMed] [Google Scholar]
- 44.Fukuhara T. Tani H. Shiokawa M. Goto Y. Abe T. Taketomi A. Shirabe K. Maehara Y. Matsuura Y. Intracellular delivery of serum-derived hepatitis C virus. Microbes Infect. 2011;13:405–412. doi: 10.1016/j.micinf.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarhan MA. Chen AY. Russell RS. Michalak T. Patient-derived hepatitis C virus and JFH-1 clones differ in their ability to infect human hepatoma cells and lymphocytes. J Gen Virol. 2012;93:2399–2407. doi: 10.1099/vir.0.045393-0. [DOI] [PubMed] [Google Scholar]
- 46.Pham T. MacParland S. Mulrooney P. Cooksley H. Naoumov N. Michalak T. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78:5867–5874. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong G. Lai L. Jiang Y. He Y. Su X. HCV replication in PBMC and its influence on interferon therapy. World J Gastroenterol. 2003;9:291–294. doi: 10.3748/wjg.v9.i2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radkowski M. Gallegos-Orozco J. Jablonska J. Colby T. Walewska-Zielecka B. Kubicka J. Wilkinson J. Adair D. Rakela J. Laskus T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology. 2005;41:106–114. doi: 10.1002/hep.20518. [DOI] [PubMed] [Google Scholar]
- 49.Taliani G. Badolato M. Lecce R. Poliandri G. Bozza A. Duca F. Pasquazzi C. Clementi C. Furlan C. De Bac C. Hepatitis C virus RNA in peripheral blood mononuclear cells: relation with response to interferon treatment. J Med Virol. 1995;47:16–22. doi: 10.1002/jmv.1890470105. [DOI] [PubMed] [Google Scholar]
- 50.Saleh M. Tibbs C. Koskinas J. Pereira L. Bomford A. Portmann B. McFarlane I. Williams R. Hepatic and extrahepatic hepatitis C virus replication in relation to response to interferon therapy. Hepatology. 1994;20:1399–1404. doi: 10.1002/hep.1840200604. [DOI] [PubMed] [Google Scholar]
- 51.Watkins-Riedel T. Ferenci P. Steindl-Munda P. Gschwantler M. Mueller C. Woegerbauer M. Early prediction of hepatitis C virus (HCV) infection relapse in nonresponders to primary interferon therapy by means of HCV RNA whole-blood analysis. Clin Infect Dis. 2004;39:1754–1760. doi: 10.1086/425614. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt W. Wu P. Brashear D. Klinzman D. Phillips M. LaBrecque D. Stapleton J. Effect of interferon therapy on hepatitis C virus RNA in whole blood, plasma, and peripheral blood mononuclear cells. Hepatology. 1998;28:1110–1116. doi: 10.1002/hep.510280428. [DOI] [PubMed] [Google Scholar]