Abstract
Background
Low birth weight has been linked with changes in thyroid function in adulthood, but it is unknown whether fetal programming or underlying genetic and environmental factors explains the association. We hypothesized that birth weight influences the pituitary-thyroid set point in adults.
Methods
A total of 152 birth weight–discordant monozygotic twin pairs with a median age of 57 years (interquartile range: 33–63) were ascertained from the Danish Twin Registry in 2010. Serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), and triiodothyronine (T3) levels were measured. Birth weights were retrieved from midwife records (individuals born before 1973) and the Danish Birth Record Registry (all other participants)
Results
Birth weight was inversely associated with serum levels of FT4 (β=−0.48 pmol/[L·kg], p=0.014) and serum T3 (β=−0.09 nmol/[L·kg], p=0.010), but not serum TSH after adjustment for age, sex, and current use of tobacco products, when the twins were investigated as singletons. Serum levels of TSH and T3 were similar in within twin-pair analyses, while serum FT4 was higher in twins with the lowest birth weight (median difference 0.3 mIU/L). When the analyses were repeated in twin pairs (n=46 pairs) characterized by extreme difference in birth weight (>0.5 kg), serum TSH, T3, and FT4 levels were similar in twins with high and low birth weight. The proportion of individuals with serum TSH level >4 mIU/L or <0.3 mIU/L was identical in both groups.
Conclusions
No overall evidence of an association between birth weight and adult pituitary-thyroid axis set point, after control for genetic and environmental factors, could be demonstrated.
Introduction
The fetal origin hypothesis states that adverse intrauterine conditions predispose to development of diseases in later life (1). Accordingly, low birth weight has been associated with several important chronic conditions, including hypertension, type 2 diabetes, and obesity in adults. Furthermore, low birth weight has been associated with the presence of thyroid autoantibodies suggesting that adult thyroid disease may be programmed in very early life (2). This observation has been further explored in a small number of studies with conflicting results. Phillips et al. (3) found higher levels of thyroid peroxidase antibodies (TPOAb) in the smaller monozygotic (MZ) twin, whereas Brix et al. (4) reported that birth weight and presence of thyroid autoantibodies, TPOAb and thyroglobulin antibodies, were unrelated in 512 Danish twin pairs. This finding was independent of thyroid autoantibody level or whether analyzed as dichotomized or continuous variables (4). Kajantie et al. (5) reported an association between small body size and risk of overt hypothyroidism in adulthood, which is at variance with our finding of no difference in birth weight between twin siblings discordant for clinically overt thyroid disease (6). Furthermore, it has been suggested that fetal growth and feeding influence thyroid function in adults, emphasizing that the interaction between early life factors and adult health is complex. Thus, in the Hertfordshire Cohort, breast-feeding beyond 1 year of age was associated with higher free thyroxine (FT4) levels, whereas an inverse association between birth weight and FT4 was detected in women who had been bottle-fed (7).
Thyroid function variables show substantial inter-individual variability, and each individual has a set point, at which secretion of thyroid-stimulating hormone (TSH) is changed (8,9). Due to the deviating and limited information retrieved from the above studies, the aim of the present investigation was to provide insight into the potential association between birth weight and the pituitary–thyroid axis set point in adults. Twins in general, and discordant MZ twins in particular, provide excellent opportunities for investigating the association between birth weight and the adult pituitary–thyroid axis set point because of the ability within twin-pair analyses to control for maternal factors, gestational age, early environment, and genetic background.
Therefore, to test the hypothesis that birth weight and the adult pituitary-thyroid axis set point is associated, birth weight–discordant MZ twins from the Danish Twin Registry were recruited for the present study. If birth weight has an independent impact on the set point of the pituitary-thyroid axis, twins from birth weight–discordant MZ pairs ought to show different levels of serum TSH and/or thyroid hormones.
Materials and Methods
Three hundred seventy-nine of the most birth weight–discordant (at least 200 gram) MZ twin pairs were selected among the 77,855 twin pairs in the Danish Twin Registry (10). Among these, 336 pairs were consecutively invited by mail to participate in the study, but a total of 178 pairs declined participation. Therefore, 158 were included in the study.
Only complete twin pairs reporting no present or recent (<6 months) pregnancy or type I diabetes were eligible. Also, individuals using thyroid hormones or antithyroid drugs were excluded from the study, which was approved by the Local Ethics Committee (S-20090033). All participants consented in writing to participate in the study.
Zygosity
DNA fingerprinting using 12 highly polymorphic microsatellite markers confirmed monozygosity. Identity in all markers indicated a probability of monozygosity >0.998. One twin pair was dizygotic, and subsequently excluded from all analyses.
Biochemistry
Blood samples were collected between 7 and 9 a.m. after an overnight fast of at least 8 hours and separated into plasma and serum, aliquoted, and stored at −80°C until analyzed. Serum TSH (normal range [NR] 0.3–4.0 mIU/L) was measured using a sandwich immunoassay, whereas both serum FT4 (NR 11.5–22.7 pmol/L) and serum triiodothyronine (T3; NR 1.0–2.6 nmol/L) were determined by a competitive immunoassay (Advia Centaur XP, Siemens, Germany). TSH, FT4, and T3 were not available in 2 twin pairs, and not measured in one pair because one of the twins was pregnant at the time of clinical assessment.
Birth weight
The Danish Birth Record Registry and midwife records provided information about birth weight on those born before and after 1973, respectively. Each study participant was asked to report if he or she had been the heaviest at birth. One twin pair disagreed on who was the heaviest at birth and this pair was excluded from all analyses. Twenty two questionnaires were only filled out by one twin. Thus, the difference in birth weight was verified twice by birth records as well as questionnaires in a total of 130 twin pairs.
Statistics
Data are presented as mean (SD) or median (interquartile range [IQR]) depending on distribution. In case of skewed data, logarithmic transformation was used before statistical testing. Chi-square was used to test the distribution of low and high serum TSH values, according to the reference interval (0.3 and 4.0 mIU/L).
Data were analyzed in two different ways. The study participants were initially evaluated as singletons to determine if birth weight was associated with serum TSH and thyroid hormone levels. Afterward, intrapair differences in thyroid hormone levels were investigated (heavy twin compared to light twin).
Associations between birth weight and serum TSH, FT4 as well as T3 concentrations were assessed using regression models. The first model (model A) included birth weight and either serum TSH, FT4, or T3 levels. The second model (model B) additionally included age and sex. Both models took into account the possible within-pair correlations by using the cluster option in STATA version 11, and it was used for all calculations (Stata Corp., College Station, TX).
Afterward, the twin with the higher birth weight was compared to the twin with the lower birth weight. Within twin-pair differences in serum TSH, FT4, and T3 were compared using the Student's t-test (paired).
The association between birth weight and the variables of the pituitary-thyroid axis within twin pairs was assessed by use of fixed-effect linear.
For exploratory analyses, the study population was stratified for subgroup analyses according to (i) >0.5 kg difference in birth weight (ii) within twin-pair agreement on birth weight difference (iii) age <40 or >40 years, and (iv) sex. Interaction between age groups and sex were tested using regression analyses.
Results
Basic anthropometrics
Analyses were performed in 152 twin pairs, since a total of 6 twin pairs were excluded from analyses due to zygosity, disagreement with regard to birth weight, treatment with thyroid hormone, pregnancy, and lack of blood samples (n=2).
Median age of the 144 women and 160 men were 57 (IQR 33–63) years and 47 (IQR 33–62) years, respectively. Mean birth weight in women was 2.5±0.5 kg and in men 2.7±0.6 kg). Adult height and weight were 165±6.0 cm and 65.1±10.6 kg in women, and 178±7.0 cm and 85.7±13.6 kg in men. Adult body mass index was 23.9±4.1 kg/m2 and 26.8±3.9 kg/m2 in women and men, respectively.
Median serum TSH levels were higher in women: 1.7 (IQR 1.2–2.7) vs. 1.5 (IQR 1.1–2.0) mIU/L, p=0.01; whereas serum FT4 and T3 levels were similar in women and men: 13.2 (IQR 12.2–14.5) vs. 13.6 (IQR 12.7–14.9) pmol/L, p>0.1, and 1.8 (IQR 1.6–2.1) vs. 1.8 (IQR 1.6–1.9) nmol/L, p=0.07, respectively.
Twins analyzed as single individuals
Using model A, which included birth weight and TSH, FT4, or T3, birth weight was inversely associated with serum T3 (regression coefficient [95% confidence interval, CI]: −0.09 [CI −0.15, −0.03], p<0.01), but not serum FT4 (−0.26 [CI −0.64, 0.12], p>0.1) or serum TSH (−0.25 [CI −0.55, 0.04], p=0.10) level. Serum T3 (−0.08 [CI −0.15, 0.02], p=0.014), but not FT4 (−0.36 [CI −0.75, 0.03], p=0.068) or TSH levels (−0.17 [CI −0.46, 0.12], p>0.1) was inversely associated with birth weight after adjustment for age and sex (model B).
Twins analyzed as twin pairs
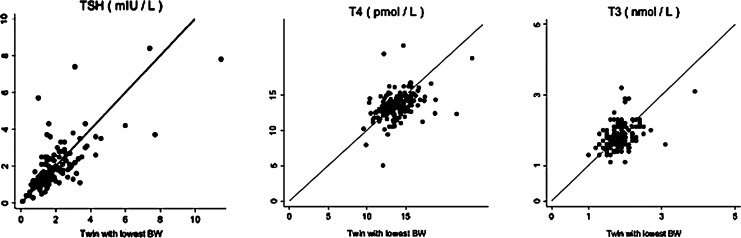
The distributions of TSH, FT4, and T3 are presented in Figure 1. No differences in the levels of serum TSH (mean difference : 0.03 [CI −0.10, 0.03] mIU/L, p>0.1) or T3 (0.01 [CI −0.04, 0.02] nmol/L, p>0.1) were observed (heaviest twin compared to cotwin), but serum FT4 was higher in those with the lowest birth weight (0.03 [CI −0.06, −0.01] pmol/L, p=0.02; Table 1).
FIG. 1.
Levels of thyroid-stimulating hormone (TSH), free thyroxine (FT4), and triiodothyronine (T3) in birth weight–discordant monozygotic twins.
Table 1.
Intrapair Differences in Thyroid Hormones in Birth Weight–Discordant Monozygotic Twins
| |
Highest birth weight compared to lowest |
|||||
|---|---|---|---|---|---|---|
| Twin pairs | TSH (mean [CI]) | p | T3 (mean [CI]) | p | FT4 (mean [CI]) | p |
| All (n=152) | −0.03 [−0.10, 0.03] | >0.1 | −0.01 [−0.04, 0.02] | >0.1 | −0.03 [−0.06, −0.01] | 0.02 |
| >0.5 kg BW difference (n=46) | −0.04 [−0.12, 0.05] | >0.1 | 0.02 [−0.04, 0.09] | >0.1 | −0.01 [−0.06, 0.03] | >0.1 |
| Age >40 years (n=78) | −0.02 [−0.13, 0.08] | >0.1 | −0.02 [−0.06, 0.02] | >0.1 | −0.04 [−0.08, 0.00] | 0.06 |
| Age ≤ 40 years (n=74) | −0.04 [−0.12, 0.04] | >0.1 | 0.00 [−0.05, 0.05] | >0.1 | −0.03 [−0.06, 0.01] | >0.1 |
| Women (n=72) | −0.01 [−0.12, 0.11] | >0.1 | 0.01 [−0.04, 0.06] | >0.1 | −0.01 [−0.05, 0.03] | >0.1 |
| Men (n=80) | −0.05 [−0.19, 0.02] | >0.1 | −0.03 [−0.07, 0.01] | >0.1 | −0.05 [−0.08, −0.02] | 0.01 |
| Double verification of BW (n=130) | −0.03 [−0.09, 0.04] | >0.1 | −0.01 [−0.04, 0.02] | >0.1 | −0.03 [−0.06, 0.01] | 0.07 |
CI, 95% confidence interval; TSH, thyroid-stimulating hormone; FT4, free thyroxine; T3, triiodothyronine; BW, birth weight.
In the subgroup analysis of twin pairs with >0.5 kg difference in birth weight, no differences in serum TSH, FT4, or T3 levels were identified (Table 1). Among males, the twin with the lowest birth weight had a higher serum FT4 concentration [mean difference: −0.05 (IQR −0.08 to −0.02) pmol/L, p=0.01], which was not the case among females (Table 1). In the subgroup analysis of individuals aged more than 40 years, serum FT4 concentration tended to be higher among twins with the lowest birth weight [mean difference: −0.04 (IQR −0.08–0.00) pmol/L, p=0.06] (Table 1). Neither of the subgroup-analyses showed differences in levels of serum TSH or T3 (Table 1).
Furthermore, regression analyses showed that there was no significant interaction between age groups and sex.
Fixed-effect models of intrapair differences in birth weight on intrapair differences in TSH and thyroid hormones showed no significant association between birth weight and serum TSH or T3, whereas birth weight was associated with levels of FT4 (coefficient: −0.68 [CI −1.28, −0.08], p=0.03; Table 2).
Table 2.
Fixed Effects Regression of Intrapair Difference in Birth Weight on Intrapair Differences in Thyroid Hormones
| |
Highest birth weight compared to lowest |
|||||
|---|---|---|---|---|---|---|
| |
TSH regression |
T3 regression |
FT4 regression |
|||
| Twin pairs | coefficient [CI] | p | coefficient [CI] | p | coefficient [CI] | p |
| All (n=152) | −0.08 [−0.36, 0.19] | >0.1 | −0.01 [−0.12, 0.09] | >0.1 | −0.68 [−1.29, −0.08] | 0.03 |
| >0.5 kg BW difference (n=46) | −0.12 [−0.34, 0.09] | >0.1 | 0.07 [−0.07, 0.21] | >0.1 | −0.22 [−1.08, 0.63] | >0.1 |
| Age >40 years (n=78) | 0.06 [−0.44, 0.55] | >0.1 | −0.09 [−0.24, 0.06] | >0.1 | −0.96 [−1.88, −0.03] | 0.04 |
| Age ≤ 40 years (n=74) | −0.18 [−0.49, 1.23] | >0.1 | 0.04 [−0.11, 0.19] | >0.1 | −0.49 [−1.31, 0.33] | >0.1 |
| Men (n=80) | −0.13 [−0.46, 0.20] | >0.1 | −0.07 [−0.19, 0.05] | >0.1 | −1.04 [−1.76, −0.31] | <0.01 |
| Women (n=72) | −0.03 [−0.50, 0.43] | >0.1 | 0.06 [−0.12, 0.23] | >0.1 | −0.26 [−1.27, 0.75] | >0.1 |
| Double verification of BW (n=130) | −0.08 [−0.39, 0.23] | >0.1 | −0.02 [−0.13, 0.09] | >0.1 | −0.60 [−1.30, 0.09] | 0.09 |
Subgroup analyses in women, twin pairs with double verification of birth weight, twin pairs with a difference in birth weight >0.5 kg, or age <40 years all demonstrated analogous results (Table 2). By contrast, subgroup analyses restricted to men or participants aged >40 years showed a significant association between intrapair differences in birth weight and serum FT4 level (coefficient: −1.04 [CI −1.76, −0.31], p<0.01 and −0.96 [CI −1.88, −0.03], p=0.04).
Seventeen (5.6%) individuals had a serum TSH >4.0 mIU/L, and eight of these individuals were heaviest at birth (p>0.1). Two individuals had a serum TSH concentration <0.3 mIU/L.
All the analyses were repeated after exclusion of those individuals who had a high or low level of TSH, but these alterations did not change the results.
Discussion
The main aim of our study was to investigate the association between early life factors, proxied by birth weight, and the set point of the pituitary-thyroid axis in adulthood. Because the discordant-twin design provides an opportunity to test this association while controlling for genetic and rearing environment (11), we recruited birth weight–discordant adult MZ twins from the Danish Twin Registry.
When twins were investigated as singletons, birth weight was inversely associated with serum FT4 and T3, which is in agreement with the observation made in bottle-fed women by Phillips et al. (7). However, analyses of the complete study population showed no within-pair differences in serum TSH or T3, but FT4 was higher in those with the lowest birth weight. Subgroup analyses showed that serum FT4 was higher in men but not in women with low birth weight. In contrast, when these analyses were repeated in twin pairs with a very substantial difference in birth weight (>0.5 kg), a difference in serum FT4 could not be confirmed. The absence of an a priori hypothesis concerning a sex- or age-specific difference in levels of TSH or thyroid hormones or any interaction between age and sex and TSH, T3, or FT4 suggest that the observed difference in FT4 in men may have been a spurious finding. In addition, the proportion of individuals with abnormal levels of serum TSH was equally distributed among twins with high and low birth weight. Intrapair differences in birth weight was inversely associated with intrapair differences in serum FT4, suggesting that adverse intrauterine conditions, that is, low birth weight, may cause increased levels of serum FT4 in adulthood. Bearing in mind that fetal programming is thought to prepare the fetus for a life with limited nutrition, we anticipated lower rather than higher serum FT4. There is no obvious explanation for this observation. However, it may be speculated that levels of serum FT4 were increased due to nutritional surplus.
We demonstrated an inverse association between birth weight and adult levels of thyroid hormones when twins were investigated as singletons; however, our study provides no compelling support for an association between birth weight and the pituitary-thyroid axis set point in adulthood. Rather, our results emphasize the importance of genetic and environmental factors for the association between birth weight and thyroid function. Accordingly, environmental factors, including most notably smoking behavior, may influence birth weight (12) as well as adult thyroid function (13), and studies of twins have shown that thyroid hormone levels are heritable (13,14). A number of genetic loci, as well as polymorphisms in different thyroid hormone pathway genes, such as phosphodiesterase 8B (15), the deiodinases (16–18), the TSH receptor (19,20), the thyroid hormone receptors (21), and thyroid hormone transporters (22,23), have been associated with serum levels of serum TSH, FT4, and T3. However, so far, their contribution to phenotypic variance is weak to modest, indicating that there is no single gene with a major regulatory influence on the pituitary-thyroid axis set point. Furthermore, individuals with the rare monogenic disease nonautoimmune hyperthyroidism, caused by an activating mutation in the TSH receptor, have lower mean birth weight compared to patients with inactivating TSHR mutations, further underlining that the association between birth weight and adult thyroid function may be under genetic regulation (24).
Our study has a number of limitations. First, twins are not necessarily representative of the general population with respect to the impact of birth weight on health status in adulthood. Nevertheless, since mortality rates in twins after the age of 6 years and singletons are alike (25), and as thyroid diseases are equally common in twins and singletons, despite a 1 kg lower birth weight in twins compared to singletons (11), it is reasonable to study the association between thyroid function and birth weight in twins. Second, we only performed single measurements of thyroid hormones accepting that there is some variation in the levels of thyroid hormones (8,26). Third, the limited number of statistically significant observations should be interpreted with caution due to the high number of tests performed. Fourth, we did not measure TPOAb. However, a large twin study previously showed that birth weight and the presence of TPOAb were unrelated (4). Therefore, autoimmunity is unlikely to have changed our results substantially. Also, the intraclass correlation of TPOAb is very high in euthyroid MZ twins (27). Furthermore, in MZ twins discordant for overt autoimmune thyroid disease, the apparently healthy cotwin most often carries antibodies against TPO or thyroglobulin (28). Therefore, the effect of discordant TSH levels within twin pairs is likely to be limited. Fifth, the age distribution of the study participants differed between men and women; nevertheless, there was no significant interaction between age and sex suggesting that variation in age distribution has no impact on the results. Finally, it may be suggested that fetal thyroid function in utero influences birth weight either directly or indirectly, for example, via growth or development of the pancreas. Since we had no data on fetal thyroid function, we were unable to assess the alternative explanation for the link between birth weight and adult thyroid function.
In aggregate, it appears that the association between birth weight and adult thyroid function is a complex interaction between environmental and genetic factors rather than the result of adverse intrauterine conditions, clinically manifesting as low birth weight. As a result, the present study provides no compelling support of an association between birth weight and adult thyroid status, indicating that early life factors and this essential endocrine regulator of human metabolism are unrelated. Moreover, even if adverse intrauterine conditions cause fetal programming of the pituitary-thyroid set point, the consequence appears not to convey any clinically relevant impact on these hormones in adulthood.
In conclusion, by studying birth weight–discordant MZ twins, we were able to investigate the association between birth weight and the function of the pituitary-thyroid axis in adulthood, while controlling for genetic and rearing environment. Based on highly informative twins, our study provides no support for an independent link between early life factors, measured as birth weight, and thyroid function in adulthood.
Acknowledgments
This study was supported by the European Union's Seventh Framework Programme (IDEAL. FP7/2007–2011) under grant agreement no. 259679, and by an unrestricted research grant by the Novo Nordisk Foundation. The Danish Twin Registry is supported by a grant from The National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology, and Innovation. We are indebted to Marianne Snorgaard and Frans Boedker (The Danish Twin Registry, University of Southern Denmark) for their assistance in the recruitment of study participants and study management.
Disclosure Statement
Authors declare that there is no conflict of interest
References
- 1.Barker DJ. Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Phillips DI. Cooper C. Fall C. Prentice L. Osmond C. Barker DJ. Rees Smith B. Fetal growth and autoimmune thyroid disease. Q J Med. 1993;86:247–253. [PubMed] [Google Scholar]
- 3.Phillips DI. Osmond C. Baird J. Huckle A. Rees-Smith B. Is birthweight associated with thyroid autoimmunity? A study in twins. Thyroid. 2002;12:377–380. doi: 10.1089/105072502760043440. [DOI] [PubMed] [Google Scholar]
- 4.Brix TH. Hansen PS. Rudbeck AB. Hansen JB. Skytthe A. Kyvik KO. Hegedus L. Low birth weight is not associated with thyroid autoimmunity: a population-based twin study. J Clin Endocrinol Metab. 2006;91:3499–3502. doi: 10.1210/jc.2006-1348. [DOI] [PubMed] [Google Scholar]
- 5.Kajantie E. Phillips DI. Osmond C. Barker DJ. Forsen T. Eriksson JG. Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J Clin Endocrinol Metab. 2006;91:4953–4956. doi: 10.1210/jc.2006-1093. [DOI] [PubMed] [Google Scholar]
- 6.Brix TH. Kyvik KO. Hegedus L. Low birth weight is not associated with clinically overt thyroid disease: a population based twin case-control study. Clin Endocrinol (Oxf) 2000;53:171–176. doi: 10.1046/j.1365-2265.2000.01025.x. [DOI] [PubMed] [Google Scholar]
- 7.Phillips DI. Barker DJ. Osmond C. Infant feeding, fetal growth and adult thyroid function. Acta Endocrinol (Copenh) 1993;129:134–138. doi: 10.1530/acta.0.1290134. [DOI] [PubMed] [Google Scholar]
- 8.Andersen S. Pedersen KM. Bruun NH. Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 9.Harrop JS. Ashwell K. Hopton MR. Circannual and within-individual variation of thyroid function tests in normal subjects. Ann Clin Biochem. 1985;22(Pt 4):371–375. doi: 10.1177/000456328502200407. [DOI] [PubMed] [Google Scholar]
- 10.Skytthe A. Kyvik KO. Holm NV. Christensen K. The Danish twin registry. Scand J Public Health. 2011;39:75–78. doi: 10.1177/1403494810387966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brix TH. Hegedus L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf ) 2012;76:457–464. doi: 10.1111/j.1365-2265.2011.04318.x. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein IM. Mongeon JA. Badger GJ. Solomon L. Heil SH. Higgins ST. Maternal smoking and its association with birth weight. Obstet Gynecol. 2005;106:986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 13.Hansen PS. Brix TH. Bennedbaek FN. Bonnema SJ. Kyvik KO. Hegedus L. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:2071–2077. doi: 10.1210/jc.2003-031999. [DOI] [PubMed] [Google Scholar]
- 14.Panicker V. Wilson SG. Spector TD. Brown SJ. Falchi M. Richards JB. Surdulescu GL. Lim EM. Fletcher SJ. Walsh JP. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clin Endocrinol (Oxf ) 2008;68:652–659. doi: 10.1111/j.1365-2265.2007.03079.x. [DOI] [PubMed] [Google Scholar]
- 15.Arnaud-Lopez L. Usala G. Ceresini G. Mitchell BD. Pilia MG. Piras MG. Sestu N. Maschio A. Busonero F. Albai G. Dei M. Lai S. Mulas A. Crisponi L. Tanaka T. Bandinelli S. Guralnik JM. Loi A. Balaci L. Sole G. Prinzis A. Mariotti S. Shuldiner AR. Cao A. Schlessinger D. Uda M. Abecasis GR. Nagaraja R. Sanna S. Naitza S. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. Am J Hum Genet. 2008;82:1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters RP. van den Beld AW. Attalki H. Toor H. de Rijke YB. Kuiper GG. Lamberts SW. Janssen JA. Uitterlinden AG. Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289:E75–E81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- 17.van der Deure WM. Hansen PS. Peeters RP. Uitterlinden AG. Fenger M. Kyvik KO. Hegedus L. Visser TJ. The effect of genetic variation in the type 1 deiodinase gene on the interindividual variation in serum thyroid hormone levels: an investigation in healthy Danish twins. Clin Endocrinol (Oxf ) 2009;70:954–960. doi: 10.1111/j.1365-2265.2008.03420.x. [DOI] [PubMed] [Google Scholar]
- 18.Panicker V. Cluett C. Shields B. Murray A. Parnell KS. Perry JR. Weedon MN. Singleton A. Hernandez D. Evans J. Durant C. Ferrucci L. Melzer D. Saravanan P. Visser TJ. Ceresini G. Hattersley AT. Vaidya B. Dayan CM. Frayling TM. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab. 2008;93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters RP. van Toor H. Klootwijk W. de Rijke YB. Kuiper GG. Uitterlinden AG. Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 20.Hansen PS. van der Deure WM. Peeters RP. Iachine I. Fenger M. Sorensen TI. Kyvik KO. Visser TJ. Hegedus L. The impact of a TSH receptor gene polymorphism on thyroid-related phenotypes in a healthy Danish twin population. Clin Endocrinol (Oxf ) 2007;66:827–832. doi: 10.1111/j.1365-2265.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen HG. van der Deure WM. Hansen PS. Peeters RP. Breteler MM. Kyvik KO. Sorensen TI. Hegedus L. Visser TJ. Identification and consequences of polymorphisms in the thyroid hormone receptor alpha and beta genes. Thyroid. 2008;18:1087–1094. doi: 10.1089/thy.2008.0236. [DOI] [PubMed] [Google Scholar]
- 22.van der Deure WM. Hansen PS. Peeters RP. Kyvik KO. Friesema EC. Hegedus L. Visser TJ. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology. 2008;149:5307–5314. doi: 10.1210/en.2008-0430. [DOI] [PubMed] [Google Scholar]
- 23.Friesema EC. Visser WE. Visser TJ. Genetics and phenomics of thyroid hormone transport by MCT8. Mol Cell Endocrinol. 2010;322:107–113. doi: 10.1016/j.mce.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya B. Campbell V. Tripp JH. Spyer G. Hattersley AT. Ellard S. Premature birth and low birth weight associated with nonautoimmune hyperthyroidism due to an activating thyrotropin receptor gene mutation. Clin Endocrinol (Oxf ) 2004;60:711–718. doi: 10.1111/j.1365-2265.2004.02040.x. [DOI] [PubMed] [Google Scholar]
- 25.Christensen K. Vaupel JW. Holm NV. Yashin AI. Mortality among twins after age 6: fetal origins hypothesis versus twin method. BMJ. 1995;310:432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen EA. Petersen PH. Blaabjerg O. Hansen PS. Brix TH. Hegedus L. Establishment of reference distributions and decision values for thyroid antibodies against thyroid peroxidase (TPOAb), thyroglobulin (TgAb) and the thyrotropin receptor (TRAb) Clin Chem Lab Med. 2006;44:991–998. doi: 10.1515/CCLM.2006.166. [DOI] [PubMed] [Google Scholar]
- 27.Hansen PS. Brix TH. Iachine I. Kyvik KO. Hegedus L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins. Eur J Endocrinol. 2006;154:29–38. doi: 10.1530/eje.1.02060. [DOI] [PubMed] [Google Scholar]
- 28.Brix TH. Hansen PS. Kyvik KO. Hegedus L. Aggregation of thyroid autoantibodies in first-degree relatives of patients with autoimmune thyroid disease is mainly due to genes: a twin study. Clin Endocrinol (Oxf ) 2004;60:329–334. doi: 10.1111/j.1365-2265.2004.01983.x. [DOI] [PubMed] [Google Scholar]