Abstract
Hyperbilirubinemia is common among patients exposed to atazanavir (ATV), but its long-term significance is not well documented. The objective was to analyze hyperbilirubinemia (incidence, regression, determinants, and outcome) among 1150 HIV-positive patients followed-up in a prospective cohort between 2003 and 2012. Cumulative incidence of hyperbilirubinemia grades 3–4 and its probability of regression were estimated using Kaplan-Meier method. Cox proportional hazards model was used to study the determinants. Generalized estimating equation (GEE) regression was used to evaluate the association between hyperbilirubinemia grades 3–4 and adverse health outcome. Eight years cumulative incidence of hyperbilirubinemia was 83.6% (95% CI:79.0–87.7) and 6.6% (95% CI:4.7–9.2) among ATV users and non-users, respectively. This clinical outcome fluctuated considerably, as most patients exposed to ATV (91%) regressed, transiently, to lower grade at some point during follow-up. Determinants were atazanavir (HR=147.90, 95% CI: 33.64–604.18), ritonavir (HR=5.18, 95% CI:2.33–11.48), zidovudine (HR=2.62, 95% CI:1.07–6.46), and age (HR=1.04 95% CI:1.01–1.08). Alcohol consumption and others non-antiretroviral medications including hepatotoxic and recreational drugs were not available for analyses. Incidence of hyperbilirubinemia was very high among ATV users and, although regression to lower grade was frequent in the clinical follow-up of these patients, this was usually transient as the mean level of bilirubin stayed at a relatively high level. Importantly, long-term hyperbilirubinemia was not associated with adverse health outcome.
Introduction
Atazanavir (ATV), approved by the United States Food and Drug Administration in 2003, is a protease inhibitor drug widely used in highly active antiretroviral therapies (HAART) of HIV-infected patients. According to United States Department of Health and Human Service (DHHS) guidelines, ATV is included in one of the fourth first-line combination regimens for treatment-naive HIV-infected patients.1 ATV is frequently associated with hyperbilirubinemia. It has been identified for its ability to inhibit bilirubin UDP-glucoronyltransferase (UGT) enzymatic activity, leading to increase of unconjugated serum bilirubin, like other previously used protease inhibitors, such as Indinavir.2,3 Clinical signs of jaundice and scleral icterus are most of the recognized consequences of hyperbilirubinemia and these esthetic consequences may lead ultimately to loss of adherence to treatment.4
Some authors have shown that the incidence of hyperbilirubinemia of grades 3 and 4 (bilirubin elevation greater than 2.5 and 5 times upper limit of normal (ULN), respectively) can reach 44.6–49% in ATV users.5,6 Use of ritonavir with ATV as a booster has also been identified as a major determinant of hyperbilirubinemia, since this antiretroviral increased plasma ATV concentrations.6 Other risk factors may exacerbate hyperbilirubinemia in the context of ATV use, such as low CD4 and abnormal bilirubin at the beginning of the treatment, as well as use of ribavirin (antiretroviral treatment used for hepatitis C). The UGT1A1 variant has also been associated with hyperbilirubinemia in ATV users.7 On the other hand, being a female and use of nonnucleoside reverse trancriptase inhibitors (NNRTIs) (possibly related to the pharmacological interaction of atazanavir with efavirenz) have been associated with a protective effect against hyperbilirubinemia.6,8–12
Although hyperbilirubinemia is common in ATV exposed patients, there is a paucity of data regarding the long-term significance and the clinical epidemiology of this problem in a large cohort of HIV-infected patients. A previous randomized study with 96 weeks of follow-up showed that hyperbilirubinemia associated with boosted ATV was not a barrier to achieve virologic response and that it did not affect quality of life and adherence to ATV.13 In an effort to document the long-term cumulative incidence of hyperbilirubinemia (grades 3 and 4) and its probability of regression to lower grade, as well as to characterize the determinants of hyperbilirubinemia and its impact on health outcome, we conducted a 9-year follow-up cohort study among HIV-infected patients exposed to antiretroviral therapies (ARVs).
Methods
Patients and data collection
We used data from an open prospective cohort of 2352 HIV-infected patients at the Clinique Médicale du Quartier Latin in Montreal, Canada. A detailed description of the design and methods of the study has been published previously.14 The cohort started in July 1997; recruitment and follow-up are still ongoing. All data were used initially to enhance follow-up and to better manage patients; participants did not receive money incentive. All participants signed an informed-consent form at enrollment, the first appointment related to their HIV diagnosis (100% of participation rate). As ATV was approved on June 20, 2003 by the U.S. Food and Drug Administration, follow-ups of patients (exposed and unexposed) were considered in this analysis only from June 20, 2003 to March 7, 2012. The study protocol was approved by the research ethics committee of Sainte-Justine Hospital in Montreal. Data collected include sociodemographic information, a complete history of ARVs, and results of laboratory tests, such as CD4 cell counts, HIV viral load, and total bilirubin (conjugated and unconjugated), which have generally been done every 3 months since July 1997. All clinical data were collected prospectively and retrieved in patient's medical chart, although certain socio-demographics data such as race, socioeconomic status, and sexual orientation were gathered retrospectively by physician's survey.
Measurement of ATV exposure status and other determinants
The prescription of every ARV was documented with the starting and ending date in medical charts. ATV exposure was evaluated from the medical chart for every patient. Patients who had taken ATV alone or in combination for at least 3 months were considered to be exposed to ATV. Patients who were exposed to ATV less than 3 months were excluded. Patients exposed to any ARV therapies except ATV were considered to be unexposed. All patients became at risk on June 20, 2003 if they were exposed to any ARVs. All patients not exposed to ARVs or not entered in the cohort at that date entered in the analysis (became at risk) in the course of follow-up at the time they became exposed to any ARVs. For ATV exposure and for any ARV exposure, no measurement of medication adherence was available except the prescription and information on the starting and ending date. All ARVs were considered in time-varying frame, based on prescriptions from the medical chart.
Viral load was categorized in four categories: 0–400 copies/ml, 401–10,000, 10,001–99,999, and 100,000 or more for modeling. CD4 cell count was also categorized in three categories: 0–200, 200–499, and 500 or more. Viral load, CD4, and bilirubin at baseline were defined at appointment at or after June 20, 2003. Liver enzymes, aspartate transaminase (AST) and alanine transaminase (ALT), categorized as grade 1 to 4 (defined with 40 UI/L for ULN at 1.25–2.5 X ULN, >2.5–5.0 X ULN, >5–10 X ULN, and >10 X ULN, respectively) were also considered. Age at baseline, sex (male or female), race (black, white, and else), sexual orientation (heterosexual or homosexual), monthly income (under or over $1500 by month), and type of employment (working full time or other kind or work), which are known to reflect global health and lifestyle, have been considered as potential determinants of hyperbilirubinemia.
Outcomes
Hyperbilirubinemia
Total bilirubin results were retrieved from medical charts, and patients must had at least two measurements of total bilirubin to be included. Hyperbilirubinemia grades were defined at every visit on basis of ULN set to 1.2 mg/dL. Grades 1, 2, 3, and 4 are equal to >1–1.5 X ULN, >1.5–2.5 X ULN, >2.5–5 X ULN, and >5 ULN, respectively. As we considered that hyperbilirubinemia of grades 3 and 4 is of clinical relevance, failure (outcome) was defined as any bilirubin elevation to or by more than 2.5 X ULN. Two different definitions for hyperbilirubinemia outcome were defined: one for any occurrence of grades 3 and 4 (failure considered to occur after one measure of grade 3 or 4) and another one for persistent occurrence (failure was considered to occur only after two consecutive measures of grade 3 or 4 separated by at least 3 months).
Adverse health outcome
Adverse health outcome was defined using a composite measure of variables including: viral load, CD4, ALT, and AST. Patient must have one of these conditions to be considered as having developed health adverse outcome: viral load >400 HIV-ARN copies/mL, CD4 <500 cell counts, ALT>= grade 3 or AST>= grade 3.
Statistical analysis
Incidence of hyperbilirubinemia of grades 3 and 4
The cumulative incidence of hyperbilirubinemia of grades 3 and 4 was estimated using Kaplan-Meier technique according to exposure status (ATV exposure versus other ARVs exposure). Two different curves were estimated: one for any occurrence of grades 3 and 4 and another one for persistent occurrence (two consecutive measures of grade 3 or 4 separated by at least 3 months). For both curves, patients were followed until a failure occurred or, for censored observations, the most recent recorded visit for which a total bilirubin measurement was available. We used the log-rank test to assess the significance of difference in incidences of hyperblirubinemia between exposure status (ATV versus other ARVs).
Determinants of hyperbilirubinemia of grades 3 and 4
Determinants of hyperbilirubinemia have been analyzed with Cox proportional Hazard regression modeling with time-dependent variables (ATV exposure, every ARV individually, ALT, AST) and non-time-dependent variables (age at baseline, sex, race, sexual orientation, monthly income, type of employment, viral load at baseline, CD4 at baseline). Hazard ratios (HR) and their respective 95% confidence intervals (CI) were estimated for the association between hyperbilirubinemia (occurrence defined with at least two consecutive measures of grade 3 or 4, separated by at least 3 months) and potential determinants. Variables with a p value higher than 0.25 in univariate analysis were excluded in the final multivariate model, except if they were considered of clinical significance or confounding (change the estimated relative risk by more than 10%). Interactions with efavirenz and ritonavir use were also analyzed.
Regression of hyperbilirubinemia of grades 3 and 4
Subanalysis for patients who developed persistent hyperbilirubinemia (grades 3 and 4 for more than 3 months) was undertaken using Kaplan-Meier analysis to estimate the probability of regression to lower grade. In order to describe the long-term distribution of bilirubin among these patients, we also estimated the mean level of bilirubin after every 3 months following the development of persistent hyperbilirubinemia.
Adverse health outcome
To analyze whether patients who developed hyperbilirubinemia have a higher risk of developing health adverse outcome, we also undertook generalized estimating equation (GEE) logistic regression models, which is essentially a logistic regression that correlates outcome and exposures cross-sectionally by taking into account the clustering within each individual caused by the repeated-measurements design. In this approach, correlation between outcome event is treated as a nuisance variable, allowing for inference based on the coefficients for the covariates in the model. Models incorporated an exchangeable correlation pattern for the repeated events. We estimated the adjusted odd ratios (OR) and their 95% CI for the association between this composite health adverse outcome and hyperbilirubinemia (categorized in five grades: from 0 to 4). In order to provide adjusted OR (control for potential confounding effect), possible confounders variables such as age, gender, race, sexual orientation, monthly income, type of employment, age, CD4, and viral load, and atazanavir, efavirenz, ritonavir, lopinavir, lamivudine, and zidovudine uses were included in the multivariate model.
All analyses were done using STATA SE version 11 (Stata, Corp.).
Results
Out of 2352 patients, 1946 were followed after June 20, 2003, 518 were excluded because they were not actively followed up at the clinic (had not been seen at least once in the last 2 years), 179 were excluded because they were not exposed to any ARVs, 55 were excluded because they were exposed to ATV less than 3 months, and 44 were excluded because they did not have at least two measurements of total bilirubin. The baseline characteristics of the 1150 patients included in the analysis are shown in Table 1. Stratification has been made according to ATV exposure measured at the end of follow-up (patients exposed to ATV in the course of follow-up versus patients exposed to all other ARVs except ATV). The median duration of exposure to ATV was 3.70 years (interquartile range (IQR): 1.88–5.02). Use of ritonavir among ATV users was common: 85.35% were exposed to ritonavir at the same time as ATV during the period of exposure.
Table 1.
Baseline Characteristics of 1150 HIV-Infected Patients, by Atazanavir Exposure Status at End of Follow-Upa
| Exposed to ATV N (%) N=389 (33.8) | Unexposed to ATV N (%) N=761 (66.2) | All N (%) N=1150 | |
|---|---|---|---|
| Sex | |||
| Male | 367 (94.3) | 736 (96.7) | 1103 (95.9) |
| Female | 22 (5.7) | 25 (3.3) | 47 (4.1) |
| Race | |||
| White | 339 (87.1) | 562 (73.9) | 901 (78.4) |
| Black | 15 (3.9) | 13 (1.7) | 28 (2.4) |
| Other | 12 (3.1) | 40 (5.3) | 52 (4.5) |
| Country of birth | |||
| Canada | 326 (83.8) | 536 (70.4) | 862 (75.0) |
| Other | 36 (9.3) | 81 (10.6) | 117 (10.2) |
| Sexual orientation | |||
| Homosexual | 319 (82.0) | 607 (79.8) | 926 (80.5) |
| Heterosexual | 34 (8.7) | 64 (8.4) | 98 (8.5) |
| Other | 22 (5.7) | 20 (2.6) | 42 (3.7) |
| Monthly income | |||
| ≤$1500/month | 63 (16.2) | 125 (16.4) | 188 (16.4) |
| >$1500/month | 275 (70.7) | 448 (58.9) | 723 (62.9) |
| Unknown | 0 (0) | 4 (0.5) | 4 (0.35) |
| Type of employment | |||
| Full time | 174 (44.7) | 298 (39.2) | 472 (41.0) |
| Else | 150 (38.6) | 242 (31.8) | 392 (34.1) |
| ALT | |||
| Grade 0 | 331 (85.1) | 651 (85.6) | 982 (85.4) |
| Grade 1 | 45 (11.6) | 86 (10.9) | 131 (11.4) |
| Grade 2 | 11 (2.3) | 17 (2.4) | 28 (2.4) |
| Grade 3 | 2 (0.5) | 6 (0.8) | 8 (0.7) |
| Grade 4 | 0 (0) | 1 (0.1) | 2 (0.2) |
| AST | |||
| Grade 0 | 357 (91.2) | 687 (90.3) | 1044 (90.8) |
| Grade 1 | 27 (6.9) | 58 (7.6) | 85 (7.4) |
| Grade 2 | 4 (1,0) | 11 (1.5) | 15 (1.3) |
| Grade 3 | 1 (0.3) | 5 (0.7) | 6 (0.5) |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) |
| Hyperbilirubinemia | |||
| Grade 0 | 247 (63.5) | 701 (92.1) | 948 (82.4) |
| Grade 1 | 70 (18.0) | 49 (6.4) | 119 (10.4) |
| Grade 2 | 47 (12.1) | 9 (1.2) | 56 (4.9) |
| Grade 3 | 24 (6.2) | 1 (0.1) | 25 (2.2) |
| Grade 4 | 1 (0.3) | 1 (0.1) | 2 (0.2) |
| Continuous variables | Median (IQR) | Median (IQR) | Median (IQR) | |
|---|---|---|---|---|
| Age | In years | 43.9 (38.6–49.1) | 43.6 (38.6–49.5) | 43.6 (38.6–49.4) |
| Follow-up duration | In years | 7.3 (4.2–8.2) | 6.5 (3.0–8.1) | 6.6 (3.5–8.2) |
| HIV-ARN viral load | (copies/mL) | 134 (49–1004) | 69 (49–873) | 82 (49–936) |
| CD4 cell count | T-cell/mm3 | 390 (250–570) | 410 (270–597) | 410 (270–590) |
| Total bilirubin | mg/dl | 1.0 (0.7–1.5)b | 0.8 (0.6–0.9) | 0.8 (0.6–1.0) |
ALT, alanine transaminase enzyme; AST, aspartate transaminase enzyme; ATV, atazanavir; IQR, interquartile range.
Baseline characteristics determined when patients become at risk (on or after June 20, 2003). Missing data are not listed. Total frequencies may differ slightly from total number of patients.
Baseline values of bilirubin were measured before the initiation of ATV.
The prevalences of hyperbilirubinemia of grade 3 or 4 at baseline were 6.4% in ATV users and at 0.3% in non-users. The median time of follow-up was 6.6 years ((IQR): 3.5–8.2). The median age at baseline was 43.6 years, and 4.1% of the cohort were women. The median number of bilirubin measurements per patient was 21 (IQR: 13–27) for ATV users and 18 (IQR: 9–25) for non-users. The median time between bilirubin measurements was 97 days (IQR: 84–126) for ATV users and 98 days (IQR: 84–126) for non-users.
Incidence of hyperbilirubinemia of grades 3 and 4
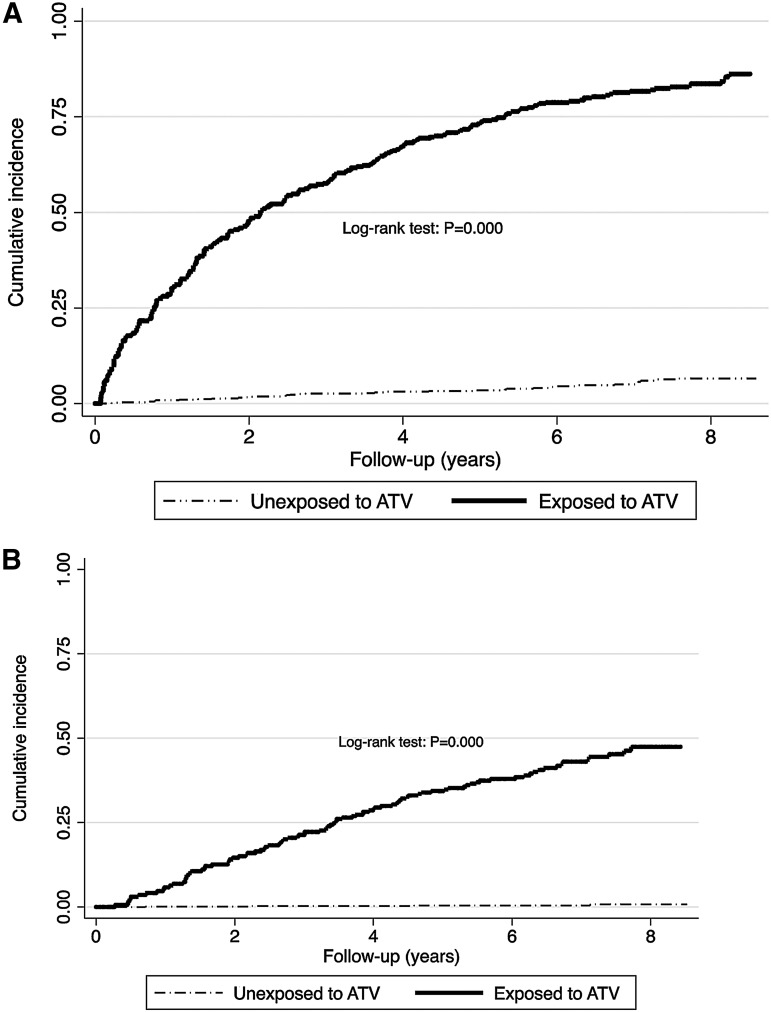
Cumulative incidence of hyperbilirubinemia of grades 3 and 4 (failure defined as one measure of grade 3 or 4) after 1, 5, and 8 years of exposure to ATV were 30.1% (95% CI: 23.5–38.0), 73.4% (95% CI: 67.9–78.7), and 83.6% (95% CI: 79.0–87.7), respectively (Fig. 1A). The mean and the median time to develop hyperbilirubinemia after ATV exposure were 3.3 years (%95 CI: 2.8–3.7) and 2.2 years (%95 CI: 1.6–2.8), respectively. Cumulative incidence in unexposed group (not exposed to ATV but exposed to any other ARVs) after 1, 5, and 8 years were 1.0% (95% CI: 0.4–2.0), 3.5% (95% CI: 2.4–5.1), and 6.6% (95% CI: 4.7–9.2), respectively. Log-rank test showed that the difference was statistically significant (p=0.000). Overall, there were 237 incident cases of hyperbilirubinemia of grades 3–4 for 5512.05 person-years [incidence rate (IR)=43.0 per 1000 person-years; 95% CI: 37.9–48.9]. In the ATV exposed group, 199 events occurred for 805.16 person-years (IR=247.2 per 1000 person-years; 95% CI: 215.1–284.0) and for the unexposed group, 38 events occurred for 4700.20 person-years (IR=8.1 per 1000 person-years; 95% CI: 5.9–11.1).
FIG. 1.
Kaplan-Meier graph of cumulative incidence of (A) hyperbilirubinemia grade 3 or 4 (outcome defined as the first measure of grade 3 or 4) and (B) persistent hyperbilirubinemia grade 3 or 4 (outcome defines as at least two consecutive measures of hyperbilirubinemia grade 3 or 4 (>2.5 X ULN). Out of 1150, 1122 were used for analysis : 28 were excluded because they had the outcome at baseline. ATV, atazanavir; P, p value; ULN, upper limit normal.
Figure 1B shows the cumulative incidence of persistent occurrence of hyperbilirubinemia grades 3 and 4 (defined as at least two consecutive measures of grade 3 or 4 separated by at least 3 months) over the 9 years period, by exposure status. Cumulative incidence of hyperbilirubinemia of grades 3 and 4 persisting for more than 3 months after 1, 5, and 8 years of exposure to ATV were 4.3% (95% CI: 2.1–8.9), 33.8% (95% CI: 27.9–40.7), and 46.8% (95% CI: 40.0–54.3), respectively. Cumulative incidences in unexposed group (unexposed to ATV but exposed to any other ARVs) after 1, 5, and 8 years were 0.1% (95% CI: 0.02–0.82), 0.3% (95% CI: 0.10–1.41), and 0.8% (95% CI: 0.27–2.43), respectively. The log-rank test indicated that the difference was statistically significant (p=0.000). Overall, there were 99 incident cases of persistent hyperbilirubinemia of grades 3–4 for 6053.44 person-years (IR=16.4 per 1000 person-years; 95% CI: 13.4–19.9). In the ATV exposed group, 95 events occurred for 1207.42 person-years (IR=78.7 per 1000 person-years; 95% CI: 64.3–96.2) and for the unexposed group, four events occurred for 4846.02 person-years (IR=0.8 per 1000 person-years; 95% CI: 0.3–2.2).
Determinants of hyperbilirubinemia grades 3 and 4
Table 2 shows the results for the univariate and multivariate Cox proportionnal hazards regression model. ATV exposure increased substantially the risk of hyperbilirubinemia (adjusted HR=147.90, p=0.000) compared to other ARVs exposure. Other determinants associated with an increased risk were older age at baseline (adjusted HR=1.04, p=0.006; each additional year of age at baseline increase the risk by 4%), use of ritonavir (adjusted HR=5.18, p=0.000) and use of zidovudine (adjusted HR=2.62, p=0.035). No interaction (modification effect) has been observed with efavirenz use or ritonavir use (results not shown).
Table 2.
Determinants of Hyperbilirubinemia: Univariate and Multivariate Analysis Using Time-Dependent Cox Regression Model*
| |
|
Univariate |
Multivariate |
||
|---|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P | |
| Atazanavir exposure | Yes vs. no | 92.3 (33.93–251.20) | 0.000 | 147.90 (33.64–604.18) | 0.000 |
| Bilirubin at baselinea | Per mg/dL (continuous) | 2.90 (2.04–4.14) | 0.000 | 1.22 (0.82–1.80) | 0.332 |
| Age at baseline | Per years (continuous) | 1.04 (1.001–1.05) | 0.049 | 1.04 (1.01–1.08) | 0.006 |
| Sex | Female vs. male | 1.06 (0.38–2.84) | 0.913 | 2.70 (0.40–16.48) | 0.293 |
| Race | Black vs. other | 0.37 (0.05–2.67) | 0.326 | 0.40 (0.04–3.81) | 0.423 |
| Sexual orientation | Heterosexuel vs. homosexual | 0.40 (0.15–1.09) | 0.071 | 0.27 (0.05–1.71) | 0.128 |
| Monthly income | >1500$/month vs. ≤1500$/month | 0.74 (0.43–1.27) | 0.267 | 1.04 (0.55–2.32) | 0.734 |
| Type of employment | Full time vs. other | 1.14 (0.75–1.74) | 0.544 | 1.42 (0.81–2.48) | 0.217 |
| Viral load at baselineb | 0–400 (ref.) | 1.00 | |||
| (log10 copies/mL) | 401–10,000 | 1.79 (1.14–2.82) | 0.011 | 1.46 (0.88–2.44) | 0.143 |
| 10,001–99,999 | 0.58 (0.18–1.84) | 0.352 | 1.35 (0.39–4.59) | 0.635 | |
| 100,000 and + | 1.50 (0.60–3.73) | 0.386 | 3.22 (1.15–9.05) | 0.026 | |
| CD4 at baselinec | 0–200 (ref.) | 1.00 | |||
| 200–499 | 1.00 (0.54–1.86) | 0.989 | 1.81 (0.87–3.80) | 0.115 | |
| 500 and + | 0.87 (0.47–1.63) | 0.667 | 1.54 (0.71–3.37) | 0.275 | |
| ALT | Grade 3/4 vs. grade 0/1/2 | 1.29 (0.18–9.26) | 0.800 | 0.87 (0.09–8.45) | 0.903 |
| Use of efavirenz | Yes vs. no | 0.11 (0.04–0.30) | 0.000 | 2.53 (0.85–7.48) | 0.094 |
| Use of lamivudine | Yes vs. no | 0.63 (0.38–1.03) | 0.065 | 1.34 (0.73–2.48) | 0.344 |
| Use of lopinavir | Yes vs. no | 0.10 (0.03–0.32) | 0.000 | 1.78 (0.41–7.66) | 0.441 |
| Use of ritonavir | Yes vs. no | 9.98 (5.03–19.82) | 0.000 | 5.18 (2.33–11.48) | 0.000 |
| Use of zidovudine | Yes vs. no | 0.51 (0.26–0.99) | 0.048 | 2.62 (1.07–6.46) | 0.035 |
ALT, alanine transaminase; ATV, atazanavir; CI, confidence intervals; HR, hazard ratio; P, p value.
Out of 1150, 27 were removed because they had hyperbilirubinemia grades 3 or 4 at baseline. Outcome defined by two consecutive measures of hyperbilirubinemia grade 3 or 4 (see text for details). Data are not shown for AST because of insufficient grade 3 and 4 observation during follow-up.
Baseline defined at appointment at or after June 20 2003. bCategorization of continuous variables were based on clinical significance. cCategorization based on World Health Organisation guidelines.
Regression of hyperbilirubinemia of grades 3 and 4
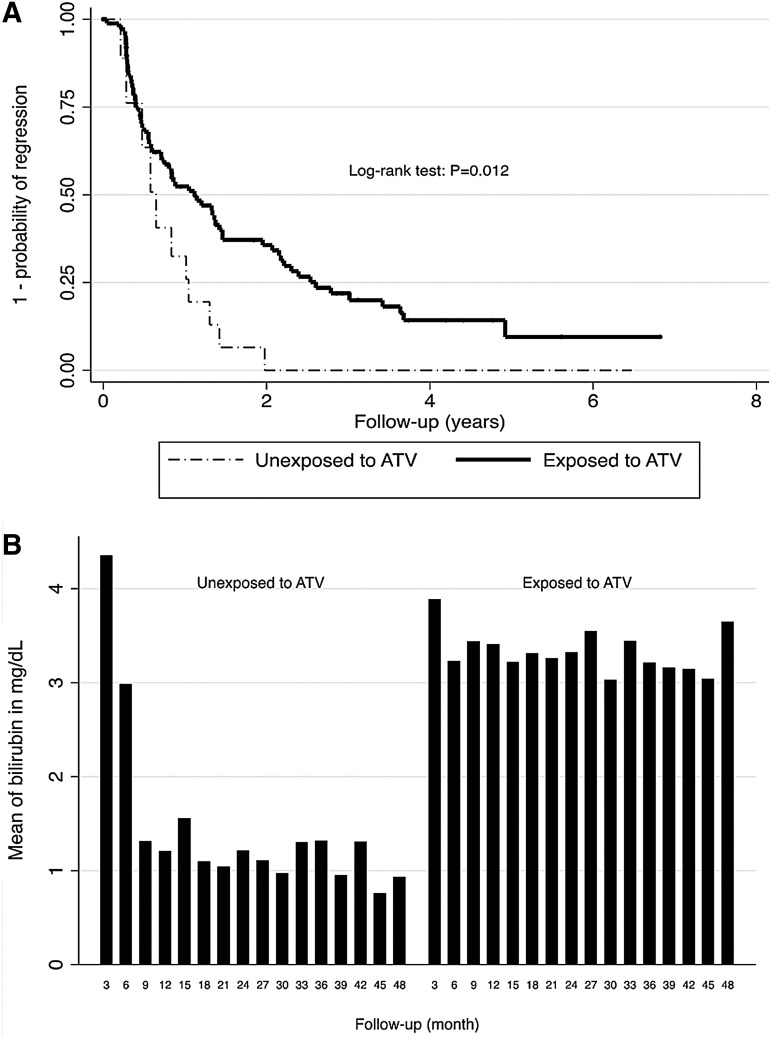
Figure 2A shows the probability of regression of patients with persistent hyperbilirubinemia grade 3/4 to lower grade (0, 1, or 2) stratified according to ARVs exposition. Among patients with persistent hyperbilirubinemia (grade 3 or 4), 91% of exposed to ATV regressed to lower grade at some points during follow-up. The median time to regress was 1.12 years (95% CI: 0.71–1.46); which means that 50% of the ATV users patients regress to a lower grade after 1.12 years, even if they were still exposed to ATV. However, the outcome was highly fluctuant because most patients who regressed have re-progressed to higher grade (3 or 4) later on. Figure 2B shows that the mean level of birilubin following the first event of persistent hyperbilirubinemia stayed relatively high and constant through years of exposure to ATV. Interestingly, 100% of patients with hyperbilirubinemia (grade 3 or 4) who stopped ATV drugs regressed to lower grade (Fig. 2A) after 1.98 years, with a median time of 0.64 year (95% CI: 0.22–1.05).
FIG. 2.
(A) Probability of regression of patients with persistent hyperbilirubinemia grade 3 or 4 to lower grade, stratified by atazanavir exposure. (B) Mean level of bilirubin every 3 months following the development of persistent hyperblirubinemia; 98 patient had persistent hyperbilirubinemia grade 3 or 4 defined as two consecutive measures of hyperbilirubinemia >2.5 X ULN and thus were included in this analysis. ATV, atazanavir; P, p value; ULN, upper limit normal.
Association of hyperbilirubinemia at adverse health outcome
Table 3 shows the odds ratios (OR) and their 95% CI for the association between the hyperbilirubinemia and composite health adverse outcome. There was no association between the development of adverse health outcome and the presence of hyperbilirubinemia (risk of adverse health outcome was similar in all grades as all adjusted ORs were around 1 for all grades 1 to 4 compared to grade 0).
Table 3.
Association Between Persistent Hyperbilirubinemia and Composite Health Adverse Outcome in HIV Patients: Univariate and Multivariate Analysis with GEE Model
| |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hyperbilirubinemia | OR (95% CI) | p | OR (95% CI) | p |
| Grade 0 (ref) | 1.00 | 1.00 | ||
| Grade 1 | 1.08 (1.00–1.15) | 0.041 | 1.02 (0.95–1.10) | 0.529 |
| Grade 2 | 1.23 (1.13–1.33) | 0.000 | 1.07 (0.97–1.17) | 0.195 |
| Grade 3 | 1.25 (1.12–1.39) | 0.000 | 1.04 (0.92–1.18) | 0.523 |
| Grade 4 | 1.20 (0.85–1.69) | 0.307 | 1.08 (0.76–1.54) | 0.671 |
CI, confidence intervals; GEE, generalized estimating equation; OR, odds ratio; P, p value.
Multivariate model included age, sex, race, sexual orientation, monthly income, type of employment, use of atazanavir, efavirenz, lamivudine, ritonavir, or zidovudine.
Discussion
In this study, we quantified the cumulative incidence of hyperbilirubinemia grade 3 or 4 among ATV users and non-users. We also analyzed the probability and time for regression to lower grade for patients who developed hyperbilirubinemia grade 3 or 4 stratified according to ATV use, as well as the determinants of hyperbilirubinemia and its clinical long-term impact among HIV-positive patients.
We observed a very high incidence of hyperbilirubinemia of grade 3 or 4 in ATV exposed patients. The incidence of hyperbilirubinemia of grade 3 or 4 was higher when we used the usual definition of outcome (failure considered to occur after one measure of grade 3 or 4) compare to persistent outcome (failure was considered to occur only after two consecutive measures of grade 3 or 4 separated by at least 3 months), 83.6% versus 46.8%, respectively after 8 years of follow-up. These long-term results indicated that most patients exposed to ATV developed hyperbilirubinemia of grade 3 or 4 at some point (83.6%), but also that this outcome fluctuated as only about half (46.8%) of patient developed persistent (at least 3 months) hyperbilirubinemia. Incidence reported by Torti et al. (estimated by using one measure of grade 3 or 4 as outcome) was 44.6% after a median time of 257 days.6 This is similar to our results as the cumulative incidence in ATV users after 1 year was 30.1%. However, our long-term follow-up cohort study shows that the development of hyperbilirubinemia was not only a short-term side effect. Most of the patients after 8 years of exposition developed hyperbilirubinemia. To our knowledge, our study is the cohort with the longest follow-up that has reported on the risk of hyperbilirubinemia and showed that most of the patients will develop hyperbilirubinemia at some point (some relatively rapidly after ATV exposure, whereas others after many years of exposition). The mean time to develop hyperbilirubinemia after ATV exposure was 3.3 years (%95 CI: 2.8–3.7).
Cox modeling permitted the analysis of the determinants of hyperbilirubinemia. Conservative control for confounding was applied. For example, hyperbilirubinemia of grade 3 or 4 at baseline was higher in ATV users compared to non-users (6.4% vs. 0.3%). The possible explanation we found for this difference at baseline was that ATV users were more likely to have been exposed to Indinavir (6.28% in ATV users vs. 2.26% in non-users) which is known to cause hyperbilirubinemia by inhibiting UGT enzymatic activity in 25% of patients.15 We controlled for total bilirubin at baseline in our multivariate regression models.
Unsurprisingly, the association between hyperbilirubinemia and ATV was very strong (adjusted HR=147.90, p=0.000). Other significant risk factors associated with hyperbilirubinemia were age at baseline, use of ritonavir, and use of zidovudine. Ritonavir, a protease inhibitor commonly used with ATV as a booster, is known to increase ATV plasma concentration and is correlated to hyperbilirubinemia, as such as other proteases inhibitors like lopinavir.10,16 Zidovudine, a nucleoside analog reverse-transcriptase inhibitor (NRTI), is not known to be associated to hyperbilirubinemia; further investigations would be necessary before we can conclude on this possible relationship. Efavirenz did not have a protective effect in our multivariate analysis contrarily to what has been found in a case report.8 However, the HR estimated for the group of nonnucleoside reverse transcriptase inhibitor (NNRTIs) in our multivariate analysis was protective although not statistically significant (adjusted HR=0.65, p=0.481) (data not shown). Potential for interaction of efavirenz and ritonavir with ATV was also analyzed in our study but was not found. Female patients were also found to have a higher likelihood of hyperbilirubinemia, although the results were not statistically significant. Conflicting results regarding sex have been described in the literature as some authors found a protective effect in women,6 whereas others described female sex as a factor associated with high ATV plasma concentrations and bilirubin level over 1.3 mg/dL.16
Our results confirmed that hyperbilirubinemia grade 3 or 4 is a very common side effect of ATV use. Our results also showed that the clinical trajectory regarding birilubin level of patients exposed to ATV fluctuated considerably, as most patients regressed to lower grade at some point during follow-up. However, regressions are virtually transient as the mean level of birilubin stayed relatively high after the development of hyperbilirubinemia. Importantly, hyperbilirubinemia was, as confirmed in the literature, reversible when ATV was stopped.2
One important question about the development of hyperbilirubinemia is to know whether it is associated with the development of adverse clinical outcome. Our results indicated that hyperbilirubinemia was not associated with adverse outcome such as high viral load, low CD4 cell count, or markers of hepatotoxicity (ALT/AST). McDonald et al.13 also found no association between hyperbilirubinemia and the development of adverse health outcome. These authors also showed that there was no difference in quality of life or adherence to treatment between HIV-infected patients with and without hyperbilirubinemia.
Our study has many strengths. To our knowledge, this cohort study provides data using the longest follow-up time in the evaluation of hyperbilirubinemia with a substantial sample of patients. Furthermore, this is a real-life cohort including older patients, making the results more generalizable to a clinic-based cohort of HIV-positive patients. Our study has however also some limitations. The results can not be generalized to all HIV-positive patients, given the low numbers of blacks and females patients in our study. Moreover, as the study was observational (not randomized), there is a potential for the presence of confounding by indication, although we applied a conservative strategy as explained above to control for potential confounding bias. Residual confounding due to lack of information such as medication adherence or baseline health status is possible. Also, it would have been interesting to analyze the role of other determinants such as co-morbidities, alcohol consumption, other non-ARV medications, including hepatotoxic and recreational drugs, but it was not possible as these variables were not available. Also, as our objective was to describe the clinical implication of ATV, we did not consider genetic mutations such as UGT1A1 in this study. Another limitation was the absence of information regarding the reason why ATV was interrupted, the icterus diagnosis, and the adherence to treatment. Finally, a large number of patients were excluded from the analysis because they did not have a visit at the clinic within the last 2 years and subsequently, important data evaluated in this study were missing. However, these patients were similar to the active patients in term of age, sex, and CD4 cell count (data not shown).
Hyperbilirubinemia was a very common side effect of ATV exposure, but fluctuation and regression to lower grade was also very common. Importantly, hyperbilirubinemia was not associated with the development of adverse health outcome. There is no clinical indication for a change in the ATV therapy based on the development of hyperbilirubinemia, apart from the signs of jaundice and scleral icterus, which are mostly esthetical symptoms.
Acknowledgments
We are grateful to Dr. Pierre Côté and all the physicians of the Clinique Médicale du Quartier Latin who collected and managed cohort data. We would like to thank Lucie Height for managing the database, and Louise Laporte for the quality of her data verification.
Funding sources: HT received a salary award (chercheur-boursier) from the Fonds de la recherche en santé du Québec and CL received a doctoral research award from the Canadian Institutes of Health Research (FRN: 96236).
Author Disclosure Statement
CL has no conflicts of interest to declare. J-GB has served as a consultant and on advisory boards, has received speaker fees from Abbott, Bristol-Myers Squibb Canada, GlaxoSmithKline Pharmaceuticals, ViiV Healthcare, Pfizer, Tibotec, Merck Canada, and Gilead Sciences, and is a member of institutions that have received research funding from Abbott, Bristol-Myers Squibb Canada, GlaxoSmithKline Pharmaceuticals, ViiV Healthcare, Boehringer Ingelheim, Pfizer, Roche, Tibotec, Merck Canada, and Gilead Sciences. SD has served as a consultant and on advisory boards, has received speaker fees from Abbott, Bristol-Myers Squibb Canada, Janssen, Merck Canada, and ViiV Healthcare, and has received grants to attend conferences from Abbott, Bristol-Myers Squibb Canada, Merck Canada and ViiV Healthcare. HT has served as a consultant and on advisory boards and has received speaker fees and travel assistance from Merck-Frosst Canada, Glaxo SmithKline Pharmaceuticals, Belgium, and Gilead Sciences.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Apr 12;2013 ]. p. F-1.http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 2.Zhang D. Chando TJ. Everett DW. Patten CJ. Dehal SS. Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 3.Barrios A. Rendon AL. Gallego O, et al. Predictors of virological response to atazanavir in protease inhibitor-experienced patients. HIV Clin Trials. 2004;5:201–205. doi: 10.1310/3HL3-HHBD-WKLR-XELL. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins T. Appearance-related side effects of HIV-1 treatment. AIDS Patient Care STDS. 2006;20:6–18. doi: 10.1089/apc.2006.20.6. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M. Grinsztejn B. Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 6.Torti C. Lapadula G. Antinori A, et al. Hyperbilirubinemia during atazanavir treatment in 2,404 patients in the Italian atazanavir expanded access program and MASTER Cohorts. Infection. 2009;37:244–249. doi: 10.1007/s15010-008-8010-6. [DOI] [PubMed] [Google Scholar]
- 7.Ribaudo HJ. Daar ES. Tierney C, et al. Impact of UGT1A1 Gilbert variant on discontinuation of ritonavir-boosted atazanavir in AIDS Clinical Trials Group Study A5202. J Infect Dis. 2013;207:420–425. doi: 10.1093/infdis/jis690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kummer O. Mossdorf E. Battegay M, et al. Treatment of an atazanivir associated grade 4 hyperbilirubinaemia with efavirenz. Gut. 2007;56:1477–1478. doi: 10.1136/gut.2007.126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park WB. Choe PG. Song KH, et al. Genetic factors influencing severe atazanavir-associated hyperbilirubinemia in a population with low UDP-glucuronosyltransferase 1A1*28 allele frequency. Clin Infect Dis. 51:101–106. doi: 10.1086/653427. 1. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Novoa S. Martin-Carbonero L. Barreiro P, et al. Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41–46. doi: 10.1097/QAD.0b013e328011d7c1. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Novoa S. Morello J. Barreiro P, et al. Switch from ritonavir-boosted to unboosted atazanavir guided by therapeutic drug monitoring. AIDS Res Hum Retroviruses. 2008;24:821–825. doi: 10.1089/aid.2007.0276. [DOI] [PubMed] [Google Scholar]
- 12.Squires KE. Young B. Dejesus E, et al. Similar efficacy and tolerability of atazanavir compared with atazanavir/ritonavir, each with abacavir/lamivudine after initial suppression with abacavir/lamivudine plus ritonavir-boosted atazanavir in HIV-infected patients. AIDS. 24:2019–2027. doi: 10.1097/QAD.0b013e32833bee1b. 24. [DOI] [PubMed] [Google Scholar]
- 13.McDonald C. Uy J. Hu W, et al. Clinical significance of hyperbilirubinemia among HIV-1-infected patients treated with atazanavir/ritonavir through 96 weeks in the CASTLE study. AIDS Patient Care STDS. 2012;26:259–264. doi: 10.1089/apc.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laprise C. Baril JG. Dufresne S. Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56:567–575. doi: 10.1093/cid/cis937. [DOI] [PubMed] [Google Scholar]
- 15.Zucker SD. Qin X. Rouster SD, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci USA. 2001;98:12671–12676. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez Novoa S. Barreiro P. Rendon A, et al. Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C–>T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42:291–295. doi: 10.1086/499056. [DOI] [PubMed] [Google Scholar]