Abstract
Background
Thyroid cancer has increased globally, with a prominent increase in small, papillary thyroid cancers (PTC). The Korean population has a high iodine intake, high prevalence of BRAF V600E mutations, and family histories of thyroid cancer. We examined the clinicopathological characteristics and outcomes of thyroid cancers in Korean patients over four decades.
Methods
The medical records of 4500 thyroid cancer patients, between 1962 and 2009 at a single center, including 3147 PTC patients, were reviewed.
Results
The mean age of the patients was 46.8±13.2 years; women accounted for 82.9% of the patients, and the median follow-up duration was 4.8 years (mean 7.0±5.8 years, range 1–43 years). The number of patients visiting the clinic increased from 411 during 1962–1990 to 2900 during 2000–2009. Age at diagnosis increased from 39.6±12.9 to 48.6±12.4 years. The male to female ratio increased from 1:6 to 1:4.5. The proportion of small (<1 cm) tumors increased from 6.1% to 43.1%, and the proportion of cancers with lymph node (LN) involvement or extrathyroidal extension (ETE) decreased from 76.4% to 44.4% and from 65.5% to 54.8% respectively. Although there were decreases in the proportion of LN involvement and ETE, these decreasing rates were not proportional to the expected rates based on the decreased proportion of large tumors. The overall recurrence and mortality rates were 13.3% and 1.4%. The five-year recurrence rate significantly decreased (from 11% to 5.9%), and the five-year mortality also improved (from 1.5% to 0.2%).
Conclusions
The incidence of thyroid cancer has rapidly increased, with a decrease in tumors of large size, LN involvement, and ETE, although the decreasing rates of LN involvement and ETE were not as prominent as decreasing rates of large size tumors. The mortality and recurrence rates have also decreased. Future long-term follow-up of patients diagnosed in the most recent decade is needed to confirm the prognostic characteristics of Korean PTC patients.
Introduction
Among endocrine tumors, thyroid cancer is the most frequent cancer, and its incidence has increased strikingly in many countries (1–8). In particular, the rates of papillary thyroid cancers (PTC) with small tumors have increased (3,6,9). The increased rate of thyroid cancer is often attributed to the early detection of tumors resulting from diligent health screening (1,5,10,11), because changes in the pathological characteristics have also become more favorable with less frequent lymph node (LN) involvement, extrathyroidal extension (ETE), and distant metastases (7). However, several studies have reported controversial results regarding the pathological changes (5,12), implying that the increasing prevalence of thyroid cancer has resulted from other causal factors, such as hormonal or environmental influences and exposure to radiation (13–16).
The incidence of thyroid cancer in Korea has increased rapidly. The age-adjusted incidence rates per 100,000 persons increased from 2.1 and 10.4 in 1999 to 15.4 and 79.6 in 2009 in men and women respectively (17). This incidence is higher in Korea than in other countries, where the incidence range was 0.5–3.5 cases per 100,000 males and 1.5–12.1 cases per 100,000 females between 1998 and 2002 (3). Although there is no clear explanation for the increased prevalence in Korea, several characteristics may contribute to the difference. The Korean population has traditionally had iodine-rich diets (18), the rates of patients with BRAF V600E mutations are higher (58–81%) (19–21) than the average rate (49.2%) in other countries (22), and the prevalence of a family history of thyroid cancer is two times higher than in other countries (23). An understanding of the clinicopathological characteristics of the Korean population and how changes in these characteristics have influenced the incidence of thyroid cancer over long periods is important.
In this study, we retrospectively reviewed the medical records of thyroid cancer patients who underwent thyroid surgery between 1962 and 2009 at the Seoul National University Hospital, and analyzed the clinicopathological characteristics and outcomes.
Materials and Methods
Subjects
The medical records of 4666 patients, diagnosed with thyroid cancer (ICD10 code C73) between 1962 and 2009 at the Seoul National University Hospital Thyroid Clinic, were reviewed. After excluding 161 patients who had benign thyroid disease or did not undergo subsequent examination or treatment, 4500 patients were included in the final analyses of the changes in the clinical characteristics of thyroid cancer patients at this clinic.
As the outcomes are quite different among patients with different histological types of cancers, only the outcomes for PTC, which was the major histological type, were analyzed. Of the 4074 patients with confirmed PTC, only those who underwent thyroid surgery with or without radioactive iodine (RAI) remnant ablation and who were followed for at least 12 months were included. Finally, 3147 patients were analyzed for outcomes and risk factors.
Treatment and follow-up strategy
The patients underwent one of three different types of thyroid surgery: lobectomy, subtotal thyroidectomy, or total thyroidectomy. Empirical dissection of lymph nodes at the central or anterior neck region began in 2003, and its frequency gradually increased. It has been performed routinely for PTC tumors >1 cm since 2007.
Postoperative RAI remnant ablation therapy was conducted using 131I in patients who had regional LN involvement, gross extrathyroidal invasion, or distant metastasis. Diagnostic iodine whole body scans (WBS) were not routinely performed. Instead, patients were initially treated with therapeutic iodine ablation. Patients without pathologically aggressive characteristics were treated repeatedly with 30 mCi of RAI, until postablation WBS did not demonstrate iodine uptake. For patients with an aggressive pathology, including residual tumor lesions, gross invasions, lateral neck node metastases, or aggressive histological types predicting poor outcomes, higher doses of RAI of 100–200 mCi were used selectively.
After initial surgery, regular monitoring of clinical examinations, serum thyroglobulin (Tg) and anti-Tg antibody (Tg-Ab) levels, simple chest radiographs, and periodic neck ultrasonography were carried out. Regular monitoring of Tg levels was started in the mid-1990s, and neck ultrasonography was performed at the beginning of 2000. Plain chest radiography had been routinely performed in our hospital because of its low price and a lower radiation exposure. However, during the last decade, our patients were no longer examined with chest radiography for routine recurrence screening because there was no evidence of a useful role in the management of thyroid cancer patients (24). For patients with a moderate to high risk for recurrence, patients underwent thyrotropin (TSH) suppression with the aim of an undetectable level of serum TSH for 5–10 years. Thereafter, in patients without evidence of recurrence, the dose of levothyroxine was adjusted to maintain a normal serum TSH level. For low risk patients, serum TSH levels were kept close to the lower margin of the reference range.
Biochemical tests
Over the long period covered by this study, biochemical test methodologies changed several times. Measurement of serum thyroglobulin was performed using a commercial immunoradiometric assay (IRMA) kit (Diasorin, Saluggia, Italy) until 2004, when it was replaced by a specific, high-sensitivity IRMA (Tg-plus; BRAHMS Diagnostica GmbH, Berlin, Germany). Antibodies against thyroglobulin have been measured using specific radioimmunoassay (RIA) kits (anti-Tg; BRAHMS Diagnostica GmbH) since July 1997. Measurements of serum TSH, using a commercially available kit (Daiichi Radioisotope Labs, Tokyo, Japan), started in August 1998. The kit was replaced by the Liaison® TSH kit (Diasorin) in February 2007. Serum total thyroxine (T4) was measured by a commercial RIA kit (Monobind, Costa Mesa, CA), and eventually replaced by the measurement of free T4 using GammaCoat™ Free T4 (Diasorin), and free T4 has been measured using a different commercial kit (RIA-gnost® FT4; CIS bio International, Gif-Sur-Yvette, Cedex, France) since March 2003.
Definitions of recurrence
Recurrence was defined as situations in which patients were pathologically confirmed to have recurrent disease by cytology, using fine needle aspiration, or specimens from surgical excision. Even though pathologic confirmation was not made, patients with highly suspicious lesions on imaging studies such as WBS, computed tomography, magnetic resonance imaging, or positron emission tomography were defined as having tumor recurrence. In our study, isolated elevations of serum Tg or anti-Tg-Ab were not defined as recurrence because monitoring of serum Tg and anti-Tg-Ab had not been routinely performed prior to the mid-1990s in our hospital.
Statistics
Continuous outcomes were analyzed using independent t-tests between groups of two and one-way analysis of variance (ANOVA) among groups of three of more. Dichotomous outcomes were analyzed using the chi-square test for trend and logistic regression analysis. Analyses of outcomes, known to vary broadly depending on histological types, were confined to patients with PTC. To analyze the changes in outcomes, the cumulative recurrence and mortality rates were calculated by life table analyses. All statistical analyses were performed using SPSS V19.0 (IBM SPSS, New York, NY). Statistical significance was defined as p<0.05.
Results
Patient numbers and histological types of thyroid cancer
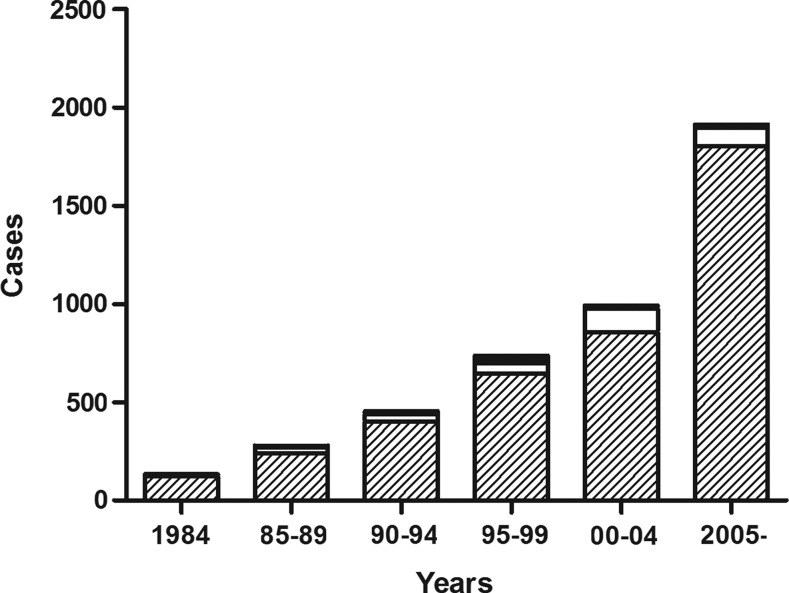
The number of patients visiting the clinic increased rapidly, with particularly prominent increases in the number of PTC patients (Fig. 1 and Table 1). Although cases of follicular thyroid cancer also increased in absolute numbers, their proportionate occurrence decreased slightly (p=0.054). The incidence of medullary thyroid cancer increased between 1990 and 1999, but then it decreased significantly starting in 2000. The numbers of cases of poorly differentiated cancers, including anaplastic thyroid cancer, increased, but then they decreased proportionately over time. The proportion of patients who underwent total thyroidectomy, including near total thyroidectomy, increased, and RAI remnant ablation was performed in more patients over time (Table 1).
FIG. 1.
The number of patients with thyroid cancer, grouped by time periods, with respect to histological types (papillary:  ; follicular: □; medullary: ■).
; follicular: □; medullary: ■).
Table 1.
General Clinical Characteristics of Thyroid Cancers, Grouped by Time Period
| Pre-1990 | 1990–1999 | Post-1999 | Total | |
|---|---|---|---|---|
| Number | 411 | 1189 | 2900 | 4500 |
| Age at diagnosis (y)* | 39.6±12.9 | 44.8±14.2 | 48.6±12.4 | 46.8±13.2 |
| Sex (male/female), n (%)** | 60/351 (14.6/85.4) | 185/1004 (15.6/84.4) | 523/2377 (18.0/82.0) | 768/3732 (17.1/82.9) |
| Median follow-up duration (y) | 18.9 (1–43) | 11.0 (1–20) | 3.3 (1–10) | 4.8 (1–43) |
| Pathologic type, n (%) | ||||
| Papillary* | 362 (88.1) | 1047 (88.1) | 2654 (91.5) | 4063 (90.3) |
| Follicular | 40 (9.7) | 93 (7.8) | 212 (7.3) | 345 (7.7) |
| Medullary* | 5 (1.2) | 26 (2.2) | 12 (0.4) | 43 (0.9) |
| Poorly differentiated cancer (including anaplastic cancer) | 0 (0) | 15 (1.2) | 16 (0.6) | 31 (0.7) |
| Othersa | 4 (1.0) | 8 (0.7) | 6 (0.2) | 18 (0.4) |
| Surgery, n (%) | ||||
| Total thyroidectomy* | 88 (21.4) | 560 (47.1) | 2682 (92.3) | 3330 (73.9) |
| Subtotal thyroidectomy* | 136 (33.1) | 308 (25.9) | 78 (2.7) | 522 (11.6) |
| Lobectomy* | 172 (41.9) | 287 (24.1) | 113 (3.9) | 572 (12.7) |
| Othersb | 9 (2.2) | 26 (2.2) | 20 (0.7) | 55 (1.2) |
| Missing | 6 (1.4) | 8 (0.7) | 12 (0.4) | 26 (0.6) |
| RAI remnant ablation, n (%)* | 132 (39.3) | 547 (51.4) | 1679 (59.3) | 2358 (55.7) |
Values are indicated by number with (%) or mean±standard deviation (SD).
Other pathologic types included lymphoma, carcinoma, etc.
Other types of surgery were mass excision, debulking surgery, and tumorectomy.
p<0.001; **p<0.05.
RAI, radioactive iodine; Pre-, before (excluding); Post-, after (excluding).
Clinical characteristics of thyroid cancer
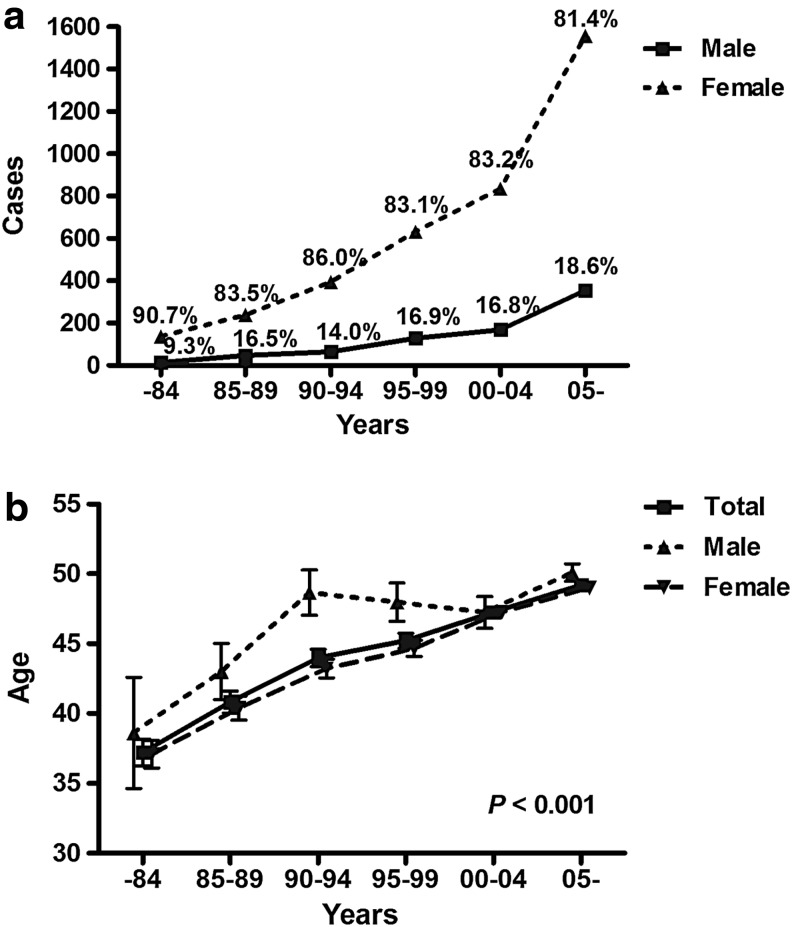
During the study period, the mean age of patients with thyroid cancer was 46.8±13.2 years, with 768 men (17.1%) and 3732 women (82.9%). Figure 2 shows the increasing number of patients of both sexes, with an increasing proportion of men, from 9.3% to 18.6% (p=0.001; Fig. 2a). The age at diagnosis also increased linearly from 37.2±11.8 years before 1984 to 49.2±11.9 years after 2005, for both sexes (p<0.001 for both; Fig. 2b).
FIG. 2.
Trends of sex distribution and mean ages at diagnosis, grouped by time periods. (a) Each number on the lines indicates the proportion of males ( ) or females (
) or females ( ), grouped by time periods. (b) Each point indicates mean age and bars crossing a line indicate standard deviations for males (
), grouped by time periods. (b) Each point indicates mean age and bars crossing a line indicate standard deviations for males ( ), females (
), females (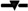 ), and both sexes (
), and both sexes ( ).
).
Pathological characteristics of thyroid cancer
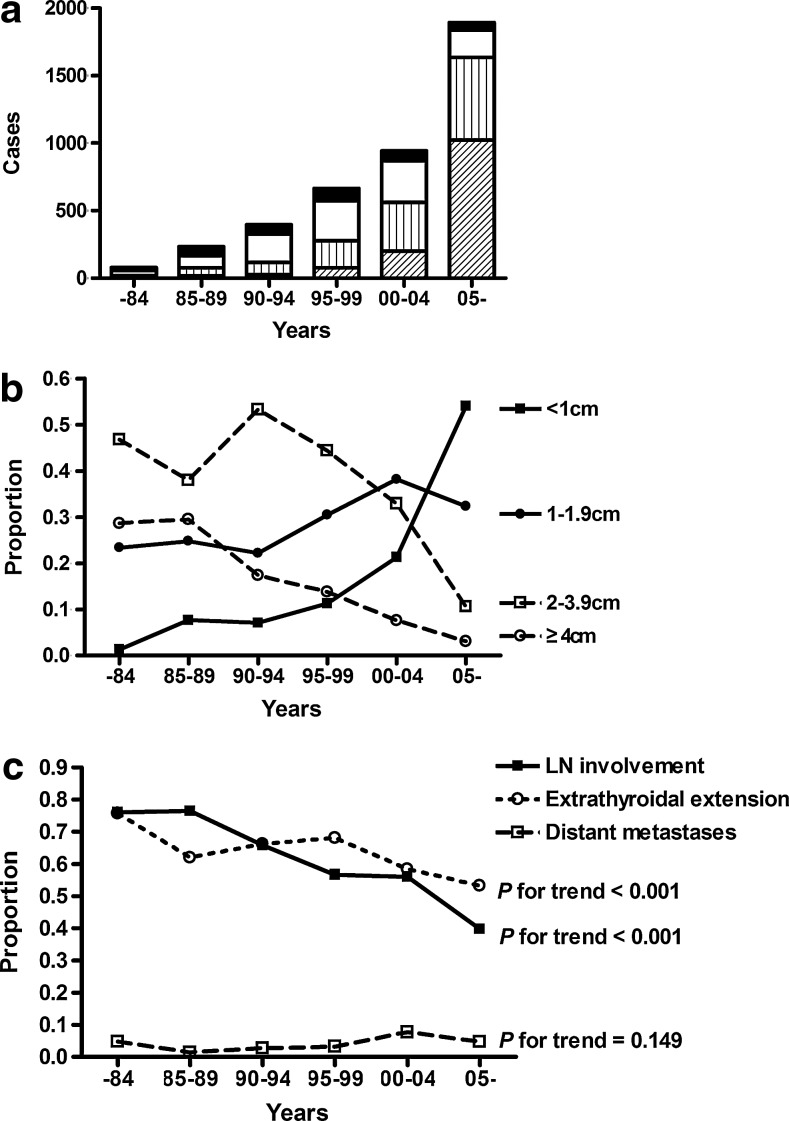
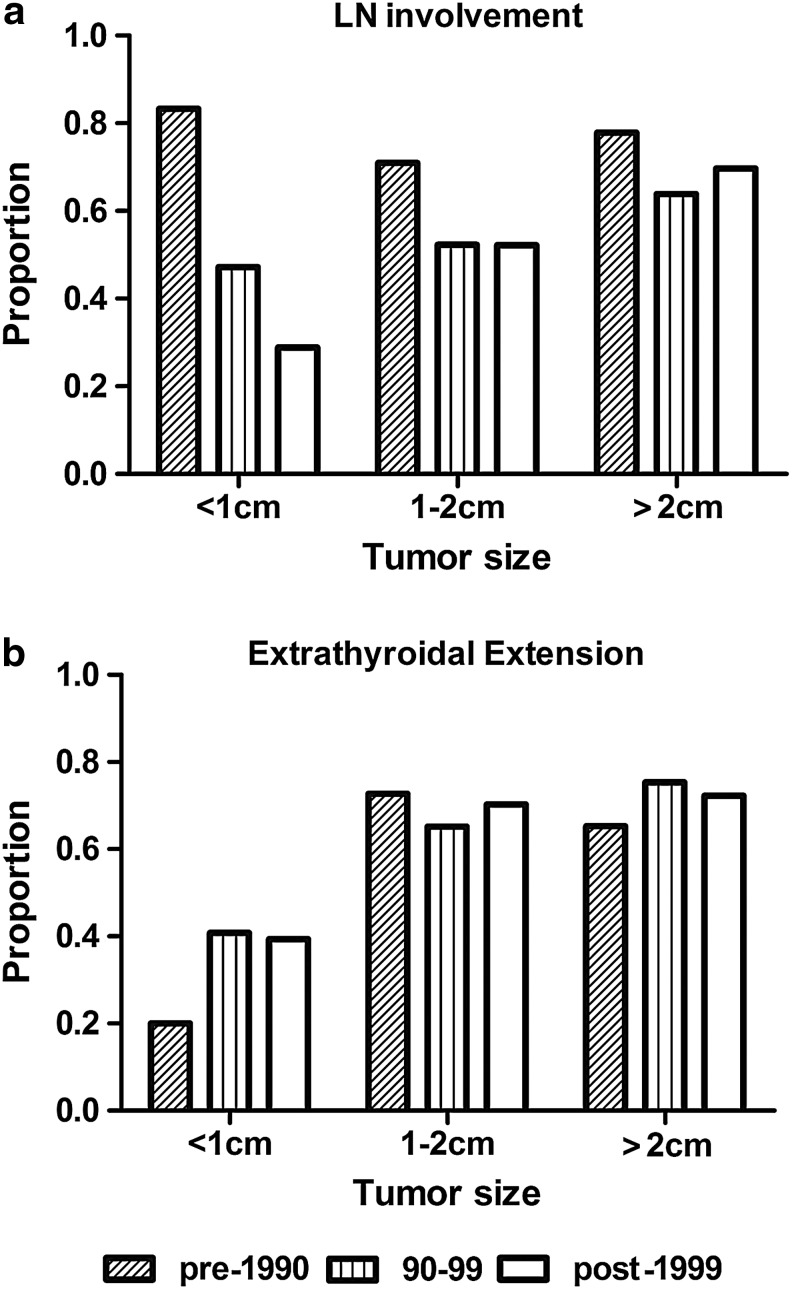
The number and proportion of small tumors (<2 cm) increased over time, especially for papillary thyroid microcarcinomas (PTMC, <1 cm), from 9% before 1990 to 54% after 2005 (Fig. 3a). Although the absolute number of patients with tumors >2 cm also increased until 2004, the number then decreased after 2004 (Fig. 3b). Comparing the pre-1984 period and the period starting in 2005, the proportion of cases with LN involvement showed a consistent decrease from 76.1% to 39.1%, and the proportion of ETEs also decreased from 75.7% to 53.2% (Fig. 3c). However, despite these reductions, these rates did not decline to the extent that the rates of tumors >2 cm did, from 77.1% to 10.4% during the same period. In the analyses evaluating the proportion of LN involvement in each group classified by tumor size, the group with a tumor size <1 cm showed a decreasing trend in the proportion of LN involvement. However, the group with a tumor size of 1–2 cm did not show a decreasing trend, similar to the group with a tumor size >2 cm (Fig. 4a). Moreover, the proportion of ETE also did not show improving trends, regardless of tumor size (Fig. 4b).
FIG. 3.
Changes of pathological features of thyroid cancer, grouped by time periods. (a) The number of patients, grouped by time periods, with specific sized tumors (<1 cm:  ; 1–1.9 cm:
; 1–1.9 cm:  ; 2–3.9 cm: □; ≥4 cm: ■). (b) The changes in the proportions of each tumor size, <1 cm (
; 2–3.9 cm: □; ≥4 cm: ■). (b) The changes in the proportions of each tumor size, <1 cm ( ), 1–1.9 cm (
), 1–1.9 cm ( ), 2–3.9 cm (
), 2–3.9 cm ( ), and ≥4 cm (
), and ≥4 cm (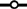 ). (c) The changes in the proportion of each pathologic characteristic: lymph node (LN) involvement (
). (c) The changes in the proportion of each pathologic characteristic: lymph node (LN) involvement ( ), extrathyroidal extension (ETE;
), extrathyroidal extension (ETE; 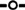 ), and distant metastases (
), and distant metastases ( ).
).
FIG. 4.
Changes of the proportion of LN involvement and ETE, according to tumor size. (a) The changes in the proportion of LN involvement with times, grouped by tumor size, (b) and the changes in the proportion of ETE with times, grouped by tumor size (pre-1990:  ; 1990–1999:
; 1990–1999:  ; post-1999: □).
; post-1999: □).
Changes of recurrence and mortality rates
During a median follow-up period of 5.1 years (mean 7.0±5.8 years, range 1–48 years), 13.3% of the patients experienced a recurrence, and 1.4% of the patients died from thyroid cancer. The cumulative recurrence rate increased continuously, with rates of 18% at 10 years and 31% at 20 years. The cancer-specific cumulative mortality rate also increased, with rates of 1.4% at 10 years and 6% at 20 years. Comparing the prognoses between time periods, the five-year recurrence rates and cancer-specific mortality rates declined significantly from 10.6% to 5.9% and from 1.5% to 0.2% respectively. Owing to the limited number of patients experiencing recurrence and mortality, the 10-year recurrence and cancer-specific mortality rates showed similar trends, without statistical significance (Table 2).
Table 2.
Comparison of the 5- and 10-Year Recurrence Rates and Cancer-Specific Mortality Rates in Patients with Papillary Thyroid Cancers, Grouped by Time Period
| Pre-1990 | 1990–1999 | Post-1999 | p | |
|---|---|---|---|---|
| Recurrence | ||||
| Overall | 101 (36.3) | 166 (19.7) | 157 (7.7) | <0.001 |
| 5 years | 29 (10.6) | 60 (7.1) | 200 (5.9) | 0.005 |
| 10 years | 53 (19.4) | 128 (15.3) | — | 0.061 |
| Mortality | ||||
| Overall | 17 (6.1) | 22 (2.6) | 5 (0.2) | <0.001 |
| 5 years | 4 (1.5) | 9 (1.1) | 4 (0.2) | <0.001 |
| 10 years | 13 (4.8) | 21 (2.5) | — | 0.060 |
Values are expressed by the number of cases and (%). Starting in 2000, the 10-year recurrence and mortality rates were not calculated because of insufficient follow-up duration.
Discussion
This study demonstrates an increase of thyroid cancer in Korean patients, with a concomitant decrease in patients with LN involvement and ETE. Additionally, the recurrence and mortality rates also declined with time. However, several clinicopathological differences were found between the study populations in other countries and our study population.
First, the ratios of PTC tumors among thyroid tumors were higher in Korea than in other countries. In contrast, the ratio of follicular thyroid cancer was lower in this Korean study than in other countries (1,3–5). The high prevalence of PTC may be attributed to regional characteristics within Korea, including an iodine-rich diet (18,25), reported to be associated with the development of PTC (26–29). Furthermore, Hashimoto's thyroiditis, which is a major type of autoimmune thyroiditis known to be associated with elevated iodine intake (30), was also reported to be associated with PTC (31,32), suggesting a link between iodine intake and PTC. However, the causal relationship remains unclear and requires further experimental and epidemiological studies.
Second, the ratio of men afflicted with thyroid cancer progressively increased, probably due to increasing health examinations (5,26,33). In Korea, the number of participants in health examinations has increased more rapidly in men (from 44.5% to 59%) than in women (from 55.6% to 59.6%) (34,35). Additionally, male participants were more likely to have risk factors for cardiovascular disease, such as hypertension or smoking, leading to evaluation of their vascular status via neck ultrasonography (36). However, we could not examine the association between increased health examinations and clinical changes in thyroid cancer, an aspect that warrants further study.
Third, in the first decade, our results showed a lower proportion of PTMC and higher proportions of LN involvement and ETE than results from other countries, followed by an increasing rate of PTMC and decreasing rates of LN involvement and ETE (Table 3). Interestingly, in the most recent decade, the proportions of LN involvement and ETE in our study were still higher than that reported in other countries, but there was a decrease in tumor size and increasing rates of PTMC similar to that found in other studies (7,26). Although these observations might suggest more aggressive characteristics of thyroid cancer in patients in Korea, this remains uncertain because there were considerable changes in the diagnostic tools and the management strategies over time, and our study had a number of cases without any information about nodal (27.0%) or invasion status (14.9%). To achieve greater certainty in the characterization of the aggressiveness of thyroid cancer in Korean patients, further studies with long-term follow-up are needed.
Table 3.
Comparisons of Clinicopathological Characteristics Outcomes Between the Current Study and Previous Ones
| |
Current study |
Other Korean (42) |
Hong Kong (33) |
USA (5,43) |
Germany (26) |
Italy (7) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-1990 | 1990–1999 | Post-1999 | 1995–2000 | 2001–2006 | Pre-1981 | 1981–1990 | 1991–2000 | 1973–2005 | 1981–1985 | 1986–1990 | 1991–1995 | 1969–1989 | 1990–2004 | |
| No. of subjects | 411 | 1189 | 2900 | 1102 | 3544 | 156 | 459 | 733 | 2400 | 476 | 1215 | 2972 | ||
| Age (y) | 40±13 | 45±14 | 49±12 | 44±18 | 45±17 | 47±16 | 46 (median) | 42±16 | 43±14 | |||||
| <45 years (%) | 65.3 | 50.9 | 37.4 | 40 | 27 | 32 | 37 | 25 | ||||||
| 46–55 years (%) | 22.7 | 23.4 | 31.9 | 49 | 59 | 36 | 40 | 45 | ||||||
| >55 years (%) | 12.0 | 25.7 | 30.7 | 11 | 14 | 32 | 23 | 30 | ||||||
| Female (%) | 85.4 | 84.4 | 82 | 87.3 | 86.5 | 82 | 80 | 80 | 73 | 77.7 | 74.8 | |||
| PTC (%) | 88.1 | 88.1 | 91.5 | 86 | 92 | 61 | 78 | 88 | 88 | 53.2 | 64.9 | 71.3 | 80.5 | 91 |
| FTC (%) | 9.7 | 7.8 | 7.3 | 39 | 24 | 12 | 9 | 35.5 | 28.7 | 21.4 | 19.5 | 9 | ||
| Size (cm) | 3.0±1.9 | 2.5±1.8 | 1.4±1.2 | 3.5 | 2.8 | 2.5 | ||||||||
| <2 cm (%) | 30.5 | 37.1 | 77.4 | 56 | 81 | |||||||||
| 2–4 cm (%) | 40.2 | 47.7 | 18 | 36 | 16 | |||||||||
| >4 cm (%) | 29.3 | 15.2 | 4.6 | 8 | 3 | |||||||||
| PTMC (%) | 6.1 | 9.7 | 43.1 | 19 | 47 | 5.1 | 16.1 | 21.7 | 20 | 29 | 28 | 7.9 | 28.7 | |
| LN involvement (%) | 76.4 | 59.7 | 44.4 | 32.7 | 31.6 | 24.8 | 25a | 10 | 12 | 19 | 34.2 | 22.4 | ||
| ETE (%) | 65.5 | 67.5 | 54.8 | 23.7 | 28.5 | 42.7 | 18.3 | 18.3 | ||||||
| Distant metastases (%) | 2.2 | 3.1 | 5.6 | 9 | 6.1 | 5.3 | 5a | 7 | 7 | 7 | 5.4 | 2 | ||
| Recurrence (%) | ||||||||||||||
| Overall | 36.0 | 29.5 | 7.6 | |||||||||||
| 5 years | 11.0 | 7.0 | 5.9 | 14.1 (local)/9.7 (distant) | ||||||||||
| 10 years | 20.1 | 15.2 | - | 6 | 8 | 10 | ||||||||
| Survival (%) | ||||||||||||||
| Overall | 93.9 | 97.4 | 99.8 | 91.4 | 98.7 | |||||||||
| 5 years | 98.5 | 98.9 | 99.8 | 94.3 | 97.2a | |||||||||
| 10 years | 95.2 | 96.5 | - | 86 | 94 | 96 | ||||||||
Values are expressed in mean±SD or percentage.
Values are from the report for 2001–2007 from 17 SEER geographic areas (43).
FTC, follicular thyroid cancer; PTMC, papillary thyroid microcarcinoma; LN, lymph node; ETE, extrathyroidal extension; RFS, recurrence free survival.
Several studies have suggested that there are different genetic characteristics in Korean patients with thyroid cancer. One is the higher prevalence of the BRAF V600E mutation compared to other countries (22,37), a molecular alteration that has been associated with aggressive characteristics. However, based on the inconsistent results from previous studies (38–40), the impact of BRAF mutations remains, at least in part, controversial. Another aspect is the higher prevalence of patients with familial nonmedullary thyroid cancer, which suggests a genetic predisposition in Korean PTC patients (23,41). However, the poor outcome of familial nonmedullary thyroid cancer remains a topic of debate, and there are few studies about the genetic characteristics in Korean thyroid cancer patients.
As shown in Table 3, the recurrence rate in Korea was higher than that in Germany during the same period, while our results were similar to those in Hong Kong (5,7,26,33,42,43). These regional differences might be associated with the epidemiologic background of iodine intake or chronic thyroiditis (44–47). However, the association between thyroid cancer and iodine intake or thyroiditis remains unclear (48–50). Table 3 also shows that the disease-free survival rates increased over time in this study, a finding that is consistent with previous studies (7,17). As mentioned above, these improvements of prognostic outcomes of thyroid cancer may be associated with improved diagnostic tools and optimized management strategies during the most recent decade.
The current study is limited by its retrospective nature involving old records, the selection of cases without missing data, and the exclusion of patients with missing data regarding pathologies or treatments. For example, our data cannot distinguish minimal ETE from gross ETE because the pathologic reports did not classify ETE into minimal extension and gross extension in the past. In addition, this study was conducted with patients at only one tertiary referral hospital. Consequently, it is possible that there is a selection bias for patients with more severe disease status, making it hard to expand our results to the general Korean population.
In summary, the proportions of patients of both sexes, as well as female patients with PTC, were higher in this study from Korea compared to other countries. The number of patients with small size tumors has significantly increased, and those with LN involvement or ETE has decreased. The decreasing rates of LN involvement and ETE were gradual during the study period, and they contrast with the increasing rate of small size tumors. Although the prognostic outcomes have improved, further studies with long-term follow-up are needed to characterize the biological behavior of thyroid cancer in Korean patients.
Acknowledgments
This study was partially supported by the Research Grant Number CB-2011-03-01 of the Korean Foundation for Cancer Research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Olaleye O. Ekrikpo U. Moorthy R. Lyne O. Wiseberg J. Black M. Mitchell D. Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur Arch Otorhinolaryngol. 2010;268:899–906. doi: 10.1007/s00405-010-1416-7. [DOI] [PubMed] [Google Scholar]
- 2.Machens A. Dralle H. Decreasing tumor size of thyroid cancer in Germany: institutional experience 1995–2009. Eur J Endocrinol. 2010;163:111–119. doi: 10.1530/EJE-10-0203. [DOI] [PubMed] [Google Scholar]
- 3.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 6.Aschebrook-Kilfoy B. Ward MH. Sabra MM. Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elisei R. Molinaro E. Agate L. Bottici V. Masserini L. Ceccarelli C. Lippi F. Grasso L. Basolo F. Bevilacqua G. Miccoli P. Di Coscio G. Vitti P. Pacini F. Pinchera A. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab. 2010;95:1516–1527. doi: 10.1210/jc.2009-1536. [DOI] [PubMed] [Google Scholar]
- 8.Liu S. Semenciw R. Ugnat AM. Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85:1335–1339. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes DT. Haymart MR. Miller BS. Gauger PG. Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–236. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds RM. Weir J. Stockton DL. Brewster DH. Sandeep TC. Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 2005;62:156–162. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 11.Leenhardt L. Grosclaude P. Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 12.Morris LG. Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010;200:454–461. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess JR. Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16:47–53. doi: 10.1089/thy.2006.16.47. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y. Lagarde F. Tsuda N. Funamoto S. Preston DL. Koyama K. Mabuchi K. Ron E. Kodama K. Tokuoka S. Papillary microcarcinoma of the thyroid among atomic bomb survivors: tumor characteristics and radiation risk. Cancer. 2010;116:1646–1655. doi: 10.1002/cncr.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rego-Iraeta A. Perez-Mendez LF. Mantinan B. Garcia-Mayor RV. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid. 2009;19:333–340. doi: 10.1089/thy.2008.0210. [DOI] [PubMed] [Google Scholar]
- 16.Moradi T. Nordqvist T. Allebeck P. Galanti MR. Risk of thyroid cancer among Iranian immigrants in Sweden. Cancer Causes Control. 2008;19:221–226. doi: 10.1007/s10552-007-9087-4. [DOI] [PubMed] [Google Scholar]
- 17.Jung KW. Park S. Kong HJ. Won YJ. Lee JY. Seo HG. Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JY. Moon SJ. Kim KR. Sohn CY. Oh JJ. Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J. 1998;39:355–362. doi: 10.3349/ymj.1998.39.4.355. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH. Suh KS. Kang DW. Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto's thyroiditis. Pathol Int. 2005;55:540–545. doi: 10.1111/j.1440-1827.2005.01866.x. [DOI] [PubMed] [Google Scholar]
- 20.Jo YS. Li S. Song JH. Kwon KH. Lee JC. Rha SY. Lee HJ. Sul JY. Kweon GR. Ro HK. Kim JM. Shong M. Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab. 2006;91:3667–3670. doi: 10.1210/jc.2005-2836. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH. Lee ES. Kim YS. Won NH. Chae YS. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology. 2006;38:201–204. doi: 10.1080/00313020600696264. [DOI] [PubMed] [Google Scholar]
- 22.Kim TH. Park YJ. Lim JA. Ahn HY. Lee EK. Lee YJ. Kim KW. Hahn SK. Youn YK. Kim KH. Cho BY. Park DJ. The association of the BRAF (V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 23.Park YJ. Ahn HY. Choi HS. Kim KW. Park DJ. Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012;22:356–362. doi: 10.1089/thy.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzen J. Beese M. Mester J. Brumma K. Beyer W. Clausen M. Chest X ray: routine indication in the follow-up of differentiated thyroid cancer? Nuklearmedizin. 1998;37:208–212. [PubMed] [Google Scholar]
- 25.Kim JY. Kim KR. Dietary iodine intake and urinary iodine excretion in patients with thyroid diseases. Yonsei Med J. 2000;41:22–28. doi: 10.3349/ymj.2000.41.1.22. [DOI] [PubMed] [Google Scholar]
- 26.Farahati J. Geling M. Mader U. Mortl M. Luster M. Muller JG. Flentje M. Reiners C. Changing trends of incidence and prognosis of thyroid carcinoma in lower Franconia, Germany, from 1981–1995. Thyroid. 2004;14:141–147. doi: 10.1089/105072504322880382. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson B. Coleman MP. Ron E. Adami HO. Iodine supplementation in Sweden and regional trends in thyroid cancer incidence by histopathologic type. Int J Cancer. 1996;65:13–19. doi: 10.1002/(SICI)1097-0215(19960103)65:1<13::AID-IJC3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Kolonel LN. Hankin JH. Wilkens LR. Fukunaga FH. Hinds MW. An epidemiologic study of thyroid cancer in Hawaii. Cancer Causes Control. 1990;1:223–234. doi: 10.1007/BF00117474. [DOI] [PubMed] [Google Scholar]
- 29.Blomberg M. Feldt-Rasmussen U. Andersen K. Kjaer S. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer. 2012;131:2360–2366. doi: 10.1002/ijc.27497. [DOI] [PubMed] [Google Scholar]
- 30.Bulow Pedersen I. Knudsen N. Carle A. Vejbjerg P. Jorgensen T. Perrild H. Ovesen L. Banke Rasmussen L. Laurberg P. A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 2011;75:120–126. doi: 10.1111/j.1365-2265.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim KW. Park YJ. Kim EH. Park SY. Park do J. Ahn SH. Jang HC. Cho BY. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011;33:691–695. doi: 10.1002/hed.21518. [DOI] [PubMed] [Google Scholar]
- 32.Gul K. Dirikoc A. Kiyak G. Ersoy PE. Ugras NS. Ersoy R. Cakir B. The association between thyroid carcinoma and Hashimoto's thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20:873–878. doi: 10.1089/thy.2009.0118. [DOI] [PubMed] [Google Scholar]
- 33.Chow SM. Law SC. Au SK. Mang O. Yau S. Yuen KT. Lau WH. Changes in clinical presentation, management and outcome in 1348 patients with differentiated thyroid carcinoma: experience in a single institute in Hong Kong, 1960–2000. Clin Oncol (R Coll Radiol) 2003;15:329–336. doi: 10.1016/s0936-6555(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 34.Chun EJ. Jang SN. Cho SI. Cho Y. Moon OR. Disparities in participation in health examination by socio-economic position among adult Seoul residents. J Prev Med Public Health. 2007;40:345–350. doi: 10.3961/jpmph.2007.40.5.345. [DOI] [PubMed] [Google Scholar]
- 35.Korea Centers for Disease Control and Prevention. Community Health Survey Report 2010.
- 36.Steele SR. Martin MJ. Mullenix PS. Azarow KS. Andersen CA. The significance of incidental thyroid abnormalities identified during carotid duplex ultrasonography. Arch Surg. 2005;140:981–985. doi: 10.1001/archsurg.140.10.981. [DOI] [PubMed] [Google Scholar]
- 37.Kim KH. Kang DW. Kim SH. Seong IO. Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004;45:818–821. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 38.Lee X. Gao M. Ji Y. Yu Y. Feng Y. Li Y. Zhang Y. Cheng W. Zhao W. Analysis of differential BRAF (V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol. 2009;16:240–245. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 39.Puxeddu E. Moretti S. Elisei R. Romei C. Pascucci R. Martinelli M. Marino C. Avenia N. Rossi ED. Fadda G. Cavaliere A. Ribacchi R. Falorni A. Pontecorvi A. Pacini F. Pinchera A. Santeusanio F. BRAF (V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 40.Lee KC. Li C. Schneider EB. Wang Y. Somervell H. Krafft M. Umbricht CB. Zeiger MA. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery. 2012;152:977–983. doi: 10.1016/j.surg.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchino S. Noguchi S. Kawamoto H. Yamashita H. Watanabe S. Shuto S. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002;26:897–902. doi: 10.1007/s00268-002-6615-y. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH. Kim TY. Ryu J-S. Gong G. Kim WB. Kim SC. Hong S. Shong YK. Trends analysis of characteristics of thyroid cancer patients in one medical center. J Kor Endocr Soc. 2008;23:35–43. [Google Scholar]
- 43.SEER Cancer Statistics Review, 1975–2008. In: Howlader N, editor; Noone AM, editor; Krapcho M, editor; Neyman N, editor; Aminou R, editor; Waldron W, editor; Altekruse SF, editor; Kodary CL, editor; Ruhl J, editor; Tatalovich Z, editor; Cho H, editor; Mariotto A, editor; Eisner MP, editor; Lewis DR, editor; Chen HS, editor; Feuer EJ, editor; Cronin KA, editor; Edwards BK, editor. National Cancer Institute; Bethesda, MD: 2011. (based on November 2010 SEER data submission). [Google Scholar]
- 44.Ott RA. McCall AR. McHenry C. Jarosz H. Armin A. Lawrence AM. Paloyan E. The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg. 1987;53:442–445. [PubMed] [Google Scholar]
- 45.Okayasu I. Fujiwara M. Hara Y. Tanaka Y. Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Verkooijen HM. Fioretta G. Pache JC. Franceschi S. Raymond L. Schubert H. Bouchardy C. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14:13–17. doi: 10.1023/a:1022593923603. [DOI] [PubMed] [Google Scholar]
- 47.Harach HR. Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 2008;19:209–220. doi: 10.1007/s12022-008-9038-y. [DOI] [PubMed] [Google Scholar]
- 48.Burgess JR. Dwyer T. McArdle K. Tucker P. Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978–1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab. 2000;85:1513–1517. doi: 10.1210/jcem.85.4.6554. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs GL. Gonda G. Vadasz G. Ludmany E. Uhrin K. Gorombey Z. Kovacs L. Hubina E. Bodo M. Goth MI. Szabolcs I. Epidemiology of thyroid microcarcinoma found in autopsy series conducted in areas of different iodine intake. Thyroid. 2005;15:152–157. doi: 10.1089/thy.2005.15.152. [DOI] [PubMed] [Google Scholar]
- 50.McLeod MK. East ME. Burney RE. Harness JK. Thompson NW. Hashimoto's thyroiditis revisited: the association with thyroid cancer remains obscure. World J Surg. 1988;12:509–516. doi: 10.1007/BF01655435. [DOI] [PubMed] [Google Scholar]