Abstract
The targeted activation of nuclear factor erythroid-derived-2-like 2 (Nrf2) to alleviate symptoms of chronic kidney disease has recently garnered much attention. Unfortunately, the greatest clinical success to date, bardoxolone, failed in phase III clinical trial for unspecified safety reasons. The present letter to the editor discusses the clinical development of bardoxolone and explores potential reasons for the ultimate withdrawal from clinical trials. In particular, was the correct clinical indication pursued and would improved specificity have mitigated the safety concerns? Ultimately, it is concluded that the right clinical indication and heightened specificity will lead to successful Nrf2-based therapies. Therefore, the bardoxolone clinical results do not dampen enthusiasm for Nrf2-based therapies; rather it illuminates the clinical potential of the Nrf2 pathway as a drug target. Antioxid. Redox Signal. 19, 517–518.
Dear Editor:
The recent termination of the phase III clinical trial of the triterpenoid bardoxolone methyl (CDDO-Me) is disappointing. However, reaching this crucial drug development milestone along the arduous path from bench to bedside should be lauded as a success. Bardoxolone was rightly heralded as an exciting potential drug lead with a promise for treating many different complex diseases, including diabetic complications (4). In a randomized, placebo-controlled phase II trial, bardoxolone improved the estimated glomerular filtration rate in patients with advanced chronic kidney disease (CKD) and type 2 diabetes at 24 weeks and persisted 52 weeks (5). However, a follow-up randomized, double-blinded, placebo-controlled phase III trial enrolling over 2000 patients was terminated in November, 2012 due to undisclosed safety concerns expressed by the Independent Data Monitoring Committee (IDMC) (ClinicalTrials.gov Identifier: NCT01351675).
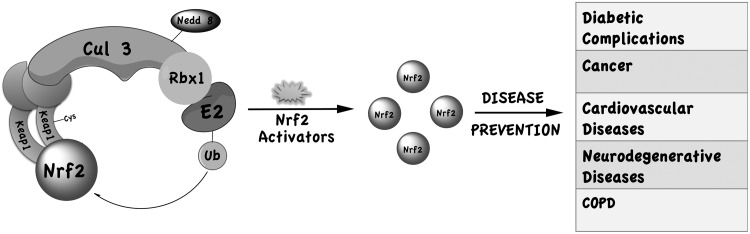
Despite this unfortunate failure, examination of the critical elements of this drug discovery effort is informative. One of the documented modes of action of these CDDO variants is to activate the transcription factor nuclear factor erythroid-derived-2-like 2 (Nrf2), which is normally activated during oxidative and xenobiotic stress, acting as the primary line of defense against such insults. Under nonstress conditions, Nrf2 levels are kept low by association with the Cul3-Keap1 E3 ubiquitin ligase that continuously ubiquitinatesNrf2, leading to Nrf2 degradation by the 26S proteasome. In response to oxidative insults or xenobiotic challenge, the E3 ubiquitin ligase is inactivated through modification of critical Keap1 cysteine residues, thereby allowing the accumulation of Nrf2. Free, cytosolic Nrf2 then translocates into the nucleus and, ultimately, coordinates enhanced expression of hundreds of antioxidant response element-bearing genes, encoding cytoprotective proteins, such as phase II detoxifying enzymes, drug transporters, and antioxidant enzymes (2, 8). Targeting Nrf2 activation with Nrf2 activators has been shown in experimental models to effectively prevent disease or inhibit the progression of diseases related to oxidative stress, including diabetes, cancer, cardiovascular diseases, neurodegenerative diseases, and chronic obstructive pulmonary disease (COPD) (Fig. 1). Numerous preclinical efforts have focused on developing Nrf2 activators for therapeutic use. In contrast, only a limited number of clinical trials have been initiated to test therapeutic or preventive performance of Nrf2 activators in humans. In addition to bardoxolone, the sulforaphane-rich broccoli sprout extract has been undergoing clinical evaluation (detailed information available at ClinicalTrials.gov).
FIG. 1.
Activation of the nuclear factor erythroid-derived-2-like 2 (Nrf2)-Keap1 defense pathway by Nrf2 activators confers protection against various diseases.
Why did bardoxolone fail and how can this inform future Nrf2-related drug discovery? The initial discovery of the electrophilic, triterpene class of molecules, like bardoxolone, and early mechanistic studies did not indicate activation of Nrf2 as the mode of action (1). It was not until 7 years later that Nrf2 began to be explored as an additional mode of action (3). Oral administration of bardoxolone potently induces the Nrf2-dependent response in multiple tissues (6). However, a proteomic analysis of the potential targets of CDDO-Im, a closely related variant of bardoxolone, revealed CDDO-Im interacts with 577 different proteins, including many different transcription factors (7). Although detailed studies of how CDDO derivatives modulate all of the non-Nrf2 pathways are lacking, it is likely that there are a large number of unintended side effects from these off-pathway targets. Because bardoxolone modulation of Nrf2 was a serendipitous discovery, future developmental efforts in the field of Nrf2 will benefit from a more targeted approach. A structure-based and targeted drug discovery has provided many clinical compounds and although none of these drugs is without some off-pathway interactions, these can be generally quite limited in scope (e.g., kinase and G-protein-coupled receptor-targeted therapies).
Additionally, is advanced CKD the most appropriate disease for Nrf2 activation therapeutics? Numerous studies using in vivo and in vitro disease models substantiate the significant role of Nrf2 in preventing, for example, diabetic complications, cancer, cardiovascular diseases, neurodegenerative diseases, and COPD (2, 8, 9). These findings led to the proposal that therapeutic activation of Nrf2 may be an avenue to prevent oxidation-related pathologies. It remains, however, that activation of Nrf2 has not been shown to reverse advanced pathological conditions, but to suppress further damage. It thus seems the indication under investigation in the bardoxolone clinical trial, late stage CKD, was doomed to fail from the onset. Not surprisingly, the phase II bardoxolone trials in patients with early stage CKD showed some benefit, as activation of Nrf2 would be predicted to alleviate further onset of diabetic renal complications.
So, where do we go from here? The case of bardoxolone provides an important framework for Nrf2-based therapy development. First, future drug development efforts should be geared to identify more specific Nrf2 activators to minimize toxicity, using a targeted approach. Second, Nrf2 activators should be tested for disease prevention or in patients with early stage diseases since activation of Nrf2 protects against progressive tissue damage rather than reversing pre-existing pathologies. Moving from bench to bedside remains the ultimate goal of modern day drug discovery; the effort with bardoxolone has moved Nrf2-based therapy discovery much closer to this goal.
Abbreviations Used
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- Nrf2
nuclear factor erythroid-derived-2-like 2
References
- 1.Honda T. Rounds BV. Gribble GW. Suh N. Wang Y. Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1998;8:2711–2714. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 2.Kensler TW. Wakabayashi N. Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 3.Liby K. Hock T. Yore MM. Suh N. Place AE. Risingsong R. Williams CR. Royce DB. Honda T. Honda Y. Gribble GW. Hill-Kapturczak N. Agarwal A. Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 4.Liby KT. Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64:972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pergola PE. Raskin P. Toto RD. Meyer CJ. Huff JW. Grossman EB. Krauth M. Ruiz S. Audhya P. Christ-Schmidt H. Wittes J. Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 6.Yates MS. Tauchi M. Katsuoka F. Flanders KC. Liby KT. Honda T. Gribble GW. Johnson DA. Johnson JA. Burton NC. Guilarte TR. Yamamoto M. Sporn MB. Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 7.Yore MM. Kettenbach AN. Sporn MB. Gerber SA. Liby KT. Proteomic analysis shows synthetic oleanane triterpenoid binds to mTOR. PLoS One. 2011;6:e22862. doi: 10.1371/journal.pone.0022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 9.Zheng H. Whitman SA. Wu W. Wondrak GT. Wong PK. Fang D. Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]