Abstract
Background
The rapidly rising incidence of papillary thyroid cancer may be due to overdiagnosis of a reservoir of subclinical disease. To conclude that overdiagnosis is occurring, evidence for an association between access to health care and the incidence of cancer is necessary.
Methods
We used Surveillance, Epidemiology, and End Results (SEER) data to examine U.S. papillary thyroid cancer incidence trends in Medicare-age and non–Medicare-age cohorts over three decades. We performed an ecologic analysis across 497 U.S. counties, examining the association of nine county-level socioeconomic markers of health care access and the incidence of papillary thyroid cancer.
Results
Papillary thyroid cancer incidence is rising most rapidly in Americans over age 65 years (annual percentage change, 8.8%), who have broad health insurance coverage through Medicare. Among those under 65, in whom health insurance coverage is not universal, the rate of increase has been slower (annual percentage change, 6.4%). Over three decades, the mortality rate from thyroid cancer has not changed. Across U.S. counties, incidence ranged widely, from 0 to 29.7 per 100,000. County papillary thyroid cancer incidence was significantly correlated with all nine sociodemographic markers of health care access: it was positively correlated with rates of college education, white-collar employment, and family income; and negatively correlated with the percentage of residents who were uninsured, in poverty, unemployed, of nonwhite ethnicity, non-English speaking, and lacking high school education.
Conclusion
Markers for higher levels of health care access, both sociodemographic and age-based, are associated with higher papillary thyroid cancer incidence rates. More papillary thyroid cancers are diagnosed among populations with wider access to healthcare. Despite the threefold increase in incidence over three decades, the mortality rate remains unchanged. Together with the large subclinical reservoir of occult papillary thyroid cancers, these data provide supportive evidence for the widespread overdiagnosis of this entity.
Introduction
Thyroid cancer is currently the third fastest rising cancer diagnosis in the United States. Estimates in the last decade placed the annual rate of increase at 3%, resulting in a doubling of thyroid cancer incidence in 30 years (1–4). Similar patterns of increase have been reported in Canada, Australia, and Western Europe (5–8). The causes of this so-called “thyroid cancer epidemic” are not completely understood (9).
The rising papillary thyroid cancer incidence rate may represent either a true increase in the occurrence of disease or an increasing number of diagnoses due to escalating levels of diagnostic scrutiny (1–3,10). With more widespread use of ultrasonography and fine-needle aspiration biopsy and with many radiographic “incidentalomas” discovered on nonthyroid imaging, a larger number of clinically occult, small thyroid nodules are being detected and investigated (1,9,11). These incidentalomas may exemplify the epidemiologic term “overdiagnosis,” which postulates that the rising number of diagnoses reflects more effective detection of a subclinical reservoir of cancers, which would not have caused symptoms or death, if left undetected (12).
There are two prerequisites for concluding that overdiagnosis of a disease is occurring: there must be (i) a large reservoir of occult disease and (ii) increasing health care activities leading to the detection of the disease reservoir (12). There is strong evidence for the first condition, with the prevalence of occult papillary thyroid cancer at autopsy estimated as high as 8%–35% (13–15), but evidence for the second condition is limited. Our objective is to examine the strength of the association between health care activities and the incidence of papillary thyroid cancer.
We hypothesize that markers of increased access to health care will have a positive association with the incidence of papillary thyroid cancer. We test this hypothesis in two ways. First, we compare the trend in papillary cancer incidence over three decades, in two cohorts of patients with differing health insurance access: those age 65 years and older, who have near-universal (>95%) health care coverage through Medicare (16), and those under 65 years old, who have less certain health insurance coverage and among whom 18% are currently uninsured (17). We hypothesize that in recent years, incidence would increase faster in the Medicare-age cohort than in the non–Medicare-age cohort.
Second, we perform an ecologic analysis to determine the influence of county-level markers of health care access on papillary thyroid cancer incidence. We use nine widely accepted socioeconomic variables as markers of county-level healthcare access (18–25). We hypothesize that counties with higher levels of access to care have a higher incidence of papillary thyroid cancer. Here, we report that the incidence of papillary thyroid cancer is increasing more rapidly in the Medicare-age population and that markers of wider health care access are associated with a higher incidence of papillary thyroid cancer in U.S. counties.
Methods
Data sources
Data on thyroid cancer incidence, patient age, and county of residence are from the National Cancer Institute (NCI)'s Surveillance, Epidemiology, and End Results (SEER) program. Started in 1973, SEER has grown to capture 28% of the United States population. To form its socioeconomically representative cross-section of the U.S. population, SEER currently captures all cancers diagnosed in 18 geographic regions (26,27).
SEER collects details on demographics, tumor characteristics, therapy, and survival of cancer patients. Strict quality control is an integral part of the SEER program (26,28–30). Because SEER is a de-identified dataset, the NCI does not require institutional review board oversight; a data use agreement was signed. The SEER 18 and SEER 9 datasets were accessed using SEERStat, release 7.1.0 (released July 2012; NCI Division of Cancer Control and Population Sciences, Bethesda, MD). County-level socioeconomic data were obtained from the U.S. Census 2000 and Small Area Health Insurance Estimates programs (2005) (31,32). The nine variables used as indicators of health care access have been widely used in analyses of cancer incidence and sociodemographic markers (17–24): percentages of county population that are uninsured, below poverty, unemployed, employed in white collar occupations, of nonwhite ethnicity, non-English speaking (defined by the Census as “linguistic isolation”), without a high school education, with at least a bachelor's degree, and mean county-level family income.
Definitions
Papillary thyroid carcinomas were defined as tumors arising in the thyroid gland with papillary histology codes 8050, 8052, 8130, 8260, 8340–8344, 8450, 8452 (33). Incidence rates were calculated per 100,000 population, age-adjusted to the 2000 United States Census population (34). The Medicare-age cohort was defined as patients age 65 years or older at the time of cancer diagnosis; the non–Medicare-age cohort comprised patients under 65 years old.
Analysis
Papillary thyroid cancer incidence rates were calculated for Medicare-age and non–Medicare-age patients in the SEER 9 dataset, from 1973 to 2009 (the most recent year for which data are available). During these years, the percentage of Americans lacking health insurance has not appreciably changed (17). Because thyroid nodules and papillary thyroid cancer are more prevalent in older persons, Joinpoint log-linear regression analysis was used to identify inflection points in the incidence trend lines, and to compare annual percentage change. Joinpoint version 3.5.2 (NCI Surveillance Research, Bethesda, MD) was used to identify inflection points and to compare incidence trends using a permuted comparability test, in which the null hypothesis was that the regression lines for incidence in two cohorts are coincident or parallel.
For the ecologic analysis, county papillary thyroid cancer incidence in 2000–2005 was the dependent variable and the nine markers of county-level socioeconomic status were explanatory variables. We restricted the analysis to incidence data from 2000 to 2005 to maintain fidelity with the 2000 U.S. Census Data and Small Area Health Insurance Estimates Program data (31,32) and to minimize the effects of migration over time. We included only the 443 counties with a population >40,000. County-level data were expressed as mean values weighted by county population, with 5th and 95th percentile values. The nine socioeconomic variables were analyzed in univariate analysis, using Pearson correlation weighted by county population, and in multivariable regression. Because the variability of papillary thyroid cancer incidence rates is heteroscedastic, varying inversely with county population, a generalized least-squares regression model weighted by county population was used. All variables were entered into the regression model, to determine overall strength of the association, and to calculate the overall r2 of the model.
To examine small area variation within states, a generalized linear mixed model was fitted to the rates with a log link and random effects for county (35). The correlation between counties was specified according to the distance between county centroids. Annual rates were combined for this analysis, and variables were included for year only. An autoregressive structure over time among repeated county rates was also specified. The empirical Bayes estimates for county random effects were plotted to obtain smoothed maps for assessing small area variation without including variability due to population sizes. These analyses were performed using SPSS 19 (IBM Corp., Armonk, NY) and PROC GLIMMIX in SAS 9.2 (SAS Institute, Cary, NC).
Results
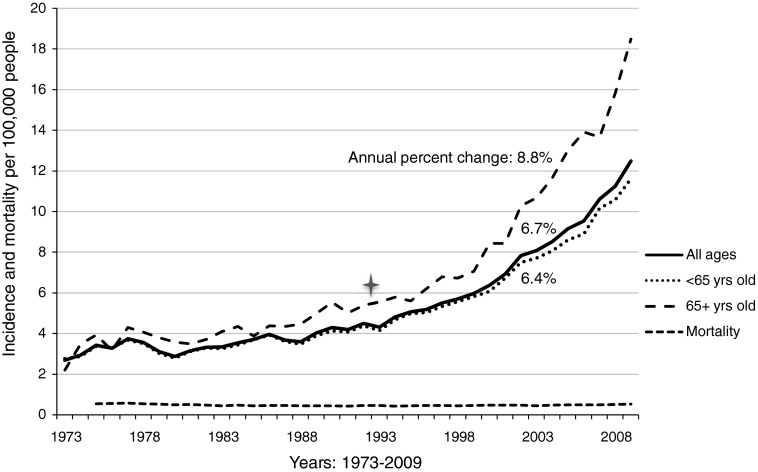
Over 36 years, the incidence of papillary thyroid cancer in the United States increased to 3.6 times the 1973 rate—from 3.5 per 100,000 to 12.5 per 100,000 in 2009 (p<0.001; Fig. 1). During this time period, the majority of the increased incidence was attributable to cancers below palpable size: 65.1% of the increase was comprised of tumors <2.0 cm in size. The majority of this increase occurred after 1993, when the incidence was 4.3 per 100,000. The annual percent change between 1993 and 2009 was 6.7%. Mortality has remained unchanged since data were first reported in 1975, near 0.5 per 100,000 (in 2009 [95% confidence interval (CI) 0.50–0.55]; annual percentage change since 1975, −0.11% [CI −0.24 to 0.018]) (36).
FIG. 1.
Trends in incidence and mortality of papillary thyroid cancer, by patient age at diagnosis. Incidence data are from the Surveillance, Epidemiology and End Results (SEER) Program, SEER 9 Regs Research Data. Mortality data are from the National Center for Health Statistics. Incidence and mortality data are age-adjusted to year 2000 census, and reported per 100,000 people. Annual percent change calculation is for years 1993–2009, calculated in Joinpoint 3.5.2 (April 2011; Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute).
Papillary thyroid cancer incidence trends stratified by Medicare-eligible age
Before the early 1990s, the incidence rate of papillary thyroid cancer among persons of Medicare-eligible age (4–6 per 100,000) was marginally higher than among persons under 65 years old (3–5 per 100,000). However, in recent decades, incidence rates have diverged, with Joinpoint regression identifying an inflection point at 1993. In the Medicare-age cohort, papillary thyroid cancer incidence has increased more rapidly than in the population as a whole (from 1993 to 2009, annual percentage change 8.8%, p<0.001). In 2009, the incidence in Medicare-age patients was 18.5 per 100,000, 67% higher than the nationwide incidence rate.
In the non–Medicare-age cohort, incidence more closely tracked the overall trend, increasing at an annual percent change of 6.4% between 1993 and 2009, a slower increase than in the population as a whole (p<0.001). In 2009, the incidence in non–Medicare-age patients was 11.6 per 100,000 (Fig. 1).
Variation stratified by county and geographic area
Between 2000 and 2009, in the 18 geographic registries in SEER, incidence ranged widely from 5.9 per 100,000 among Alaska Natives to 12.0 per 100,000 in Connecticut—a twofold difference.
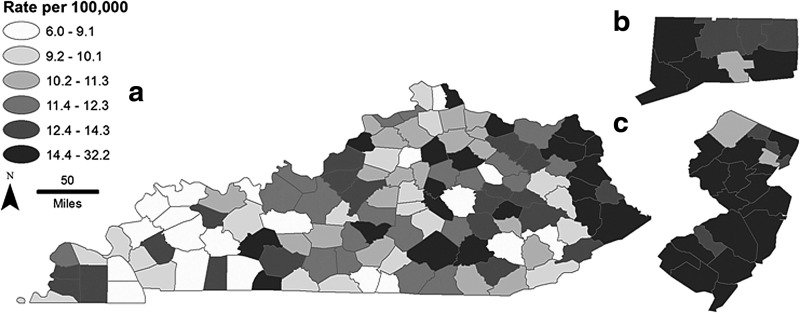
Among the 497 counties included in SEER, 10 counties had zero incident cases, including three counties with population greater than 40,000 (Howard County, IA; Martin County, KY; Trimble County, KY). The counties with population greater than 40,000 and the highest incidence rates were Los Alamos County, NM (29.7 per 100,000); Lucas County, IA (25.8 per 100,000); and Modoc County, CA (20.4 per 100,000). Figure 2 demonstrates the wide variability in incidence, even within geographically close areas within smaller states. Incidence data and mean county-level data (weighted by county population) for socioeconomic variables are summarized in Table 1.
FIG. 2.
Incidence of papillary thyroid cancer in 2009, by county, in Kentucky (a), Connecticut (b), and New Jersey (c). Incidence data are from the SEER Program. Rates were smoothed by geographic distance using a generalized linear mixed model. Representative states were chosen to demonstrate the variability of thyroid cancer incidence within geographically close areas.
Table 1.
County-Level Thyroid Cancer Incidence and Socioeconomic Data, 2000–2005
| Average | 5th percentile | 95th percentile | |
|---|---|---|---|
| Median county population (n=497) | 139,035 | 12,837 | 6,396,100 |
| Measures of incidence (per 100,000) | |||
| Incidence of PTC, all ages | 7.39 | 1.50 | 13.16 |
| Incidence of PTC, <65 years | 4.96 | 0.00 | 10.20 |
| Measures of socioeconomic status | |||
| % uninsured | 15.90 | 7.48 | 25.90 |
| % below poverty | 9.79 | 3.82 | 25.36 |
| % with less than high school education | 20.61 | 9.94 | 42.44 |
| % with at least bachelor's degree | 26.11 | 7.47 | 34.65 |
| Median family income | 53,679 | 26,136 | 66,808 |
| % unemployed | 6.91 | 3.82 | 25.36 |
| % white collar employment | 35.99 | 21.12 | 42.34 |
| % with non-English primary language | 6.46 | 0.00 | 9.45 |
| % of nonwhite ethnicity | 21.27 | 0.51 | 57.31 |
Data are presented as weighted means, except for those indicated as median values.
PTC, papillary thyroid cancer.
All nine measures of county-level health care access were significantly correlated with the incidence of papillary thyroid cancer on univariate analysis (Table 2). Incidence was positively correlated with county-level mean family income (p=0.001), county population with at least a bachelor's degree (p=0.001), and county population employed in white collar occupations (p=0.003). Papillary thyroid cancer incidence was inversely correlated with county unemployment rate (p=0.003), poverty rate (p<0.001), and population that was non-English speaking (p=0.016), without high school education (p<0.001), of nonwhite ethnicity (p<0.001), and uninsured (p<0.001). Thus, areas with higher income and education were more likely to have higher incidence rates, while areas with more unemployment, poverty, and non-English speakers were more likely to have lower rates of papillary thyroid cancer incidence.
Table 2.
Correlations Between County Health Care Access and County-Level Incidence of Papillary Thyroid Cancer
| |
Dependent variable |
|||||
|---|---|---|---|---|---|---|
| |
Incidence of papillary thyroid cancer (all ages) |
Incidence of papillary thyroid cancer (age <65 years) |
||||
| Explanatory variable | Correlation | p value (univariate) | p value (multivariable) | Correlation | p value (univariate) | p value (multivariable) |
| Bachelor's degree | 0.15 | 0.001 | 0.11 | 0.09 | 0.03 | 0.17 |
| Family income | 0.15 | 0.001 | 0.12 | 0.06 | 0.12 | 0.03 |
| White collar employment | 0.13 | 0.003 | 0.40 | 0.05 | 0.14 | 0.04 |
| English not primary language | −0.10 | 0.016 | 0.18 | −0.07 | 0.07 | <0.001 |
| Unemployment rate | −0.13 | 0.003 | 0.98 | −0.04 | 0.22 | 0.03 |
| No high school education | −0.23 | <0.001 | 0.76 | −0.23 | <0.001 | 0.012 |
| Uninsured | −0.25 | <0.001 | 0.02 | −0.26 | <0.001 | <0.001 |
| Nonwhite ethnicity | −0.25 | <0.001 | <0.001 | −0.29 | <0.001 | <0.001 |
| Poverty rate | −0.27 | <0.001 | 0.22 | −0.25 | <0.001 | 0.83 |
Values represent the Pearson correlation coefficient and p values, for both univariate and multivariable analyses. Significant values are presented in boldface.
When analysis was limited to the non–Medicare-age population, several additional factors became independently significant on multivariable analysis: family income (p=0.03), unemployment rate (p=0.03), and population with white collar employment (p=0.04), non-English speaking (p<0.001), and without high school education (p=0.012).
When the regression model was limited to the non–Medicare-age population, these nine markers of health care access together explained 25% of the variability in county-level papillary thyroid cancer incidence (r=0.50, r2=0.25, F=15.32, standard error of estimate=1630, p<0.001). When the regression model was expanded to include the Medicare-age population, only 14% of the variability in county-level incidence was explained by these nine markers (r=0.38, r2=0.14, F=7.94, standard error=1912, p<0.001). This attenuated model is consistent with the leveling effect of near-universal health care access in the Medicare-age population, diminishing the ability of these nine markers to estimate the level of access to health care, once patients turn 65.
Discussion
Between 1973 and 2009, the incidence of papillary thyroid cancer more than tripled. Over the past two decades, the overall incidence rate has been increasing by >6% per year. Among patients with near-universal Medicare health care coverage at age 65, the annual rate of increase is higher, nearly 9% per year. Although thyroid cancer was marginally more prevalent among older persons before the 1990s, the incidence of thyroid cancer has accelerated at a faster rate in the Medicare-age cohort over the past two decades. Across the U.S. counties captured by the SEER cancer registry, markers of access to health care are strongly correlated with the incidence of papillary thyroid cancer. Incidence tends to be highest in counties with higher levels of income and with greater percentages of residents with white-collar employment and bachelor's degrees. Incidence rates tend to be lowest in counties with higher percentages of residents who are unemployed, uninsured, of nonwhite ethnicity, non-English speaking, in poverty, and without a high school education. Together, these findings illustrate an association between access to health care and the incidence of papillary thyroid cancer.
Seven years ago, we reported that the incidence of differentiated thyroid cancer had doubled between 1973 and 2002. We proposed that overdiagnosis may be the chief cause of this phenomenon (2). We and others had also previously observed that the incidence of thyroid cancer appeared to be rising fastest in more affluent regions of the country, and speculated that this may be attributable to wider access to healthcare (4,37). Consistent with this hypothesis, we and others had also reported differences in thyroid cancer incidence between ethnic groups, with incidence rates highest among non-Hispanic white individuals, again raising the possibility that thyroid cancer incidence may be correlated with access to health care. However, the variation in thyroid cancer incidence by ethnicity was attenuated in cases of nonpapillary histology, arguing against the presence of differences in diagnostic scrutiny (4,38). Therefore, the strength of the association between health care access and the incidence of thyroid cancer in the United States had been unclear.
The data in the present study now demonstrate that the rising incidence of differentiated thyroid cancer has continued unabated, and that the incidence of thyroid cancer is strongly associated with multidimensional measures of access to health care. These data therefore provide further support for the hypothesis of overdiagnosis.
Overdiagnosis is the identification of a disease which, if left undetected, would not cause symptoms or death for that patient during his or her lifetime. Before concluding that this phenomenon is occurring, two conditions must be satisfied. First, there must be evidence for a large reservoir of subclinical disease. Second, there must be a strong association between health care activity and the detection of the reservoir of subclinical cancers. There is robust evidence for a subclinical reservoir of papillary thyroid cancer. A meta-analysis of 24 autopsy series revealed a mean prevalence of occult papillary thyroid cancer of 7.6% (15). In two independent autopsy studies in which normal-appearing thyroid glands were thinly sectioned at 2–3 mm intervals, occult papillary thyroid cancers were identified in 33.3% and 35.6% of subjects (13,14). At these prevalence rates, the estimated subclinical reservoir in the United States is between 25 and 100 million Americans.
To date, there has been no direct evidence to satisfy the second condition for overdiagnosis: an association between health care activity and the incidence of papillary thyroid cancer. Here, we used a natural experiment design in a population-based U.S. registry to demonstrate a robust association between markers of health care access and the rate of papillary thyroid cancer diagnosis. A statistical model based on nine markers of access to care explained as much as 25% of the variability in the county-level incidence of papillary thyroid cancer. The model was most statistically robust when including only people under age 65, but was attenuated when Medicare-eligible persons (age 65 and older) were included. In the United States, at age 65, near-universal health care coverage provided by Medicare diminishes the ability to estimate the level of access to care with markers such as unemployment rate, poverty rate, income, and education. These findings are consistent with the hypothesis that papillary thyroid cancer diagnosis is highly dependent on access to health care.
Interestingly, the association between health care access and overdiagnosis has been shown in other cancers, such as prostate cancer, a disease known to be prone to overdiagnosis (39). Prostate cancer incidence has been robustly correlated with markers of access to care in multiple studies: regions with higher income and educational attainment have higher prostate cancer incidence, attributable to increased use of prostate-specific antigen testing (19,40–42). Because thyroid cancer is not a disease recommended for screening by the U.S. Preventive Services Task Force, a study specifically examining thyroid screening and thyroid cancer diagnoses is not possible.
Certainly, the association between access to care and papillary thyroid cancer incidence cannot rule out a coexistent true increase in the occurrence of thyroid cancer. It is possible that more thyroid cancers are developing, and that areas with increased access to care have been more successful at diagnosing these cases. However, in a scenario of increasing cancer incidence, thyroid cancer mortality rates would be expected to rise. Despite a 3.6-fold increase in papillary thyroid cancer incidence, nationwide papillary thyroid cancer mortality has not changed in 34 years, making this explanation less likely. Similar mortality data have been reported by others (10). Furthermore, a plausible biological explanation for an increase in papillary thyroid cancer cases is lacking. High levels of population exposure to the one known risk factor, ionizing radiation, have decreased over the past 50 years. In the United States, nuclear tests have not been performed since 1961 (43), and radiotherapy for benign conditions of the head and neck has not been routine since the late 1950s (44). Today, the main source of radiation exposure in the United States is background exposure to radon and thoron, followed by medical x-rays and computed tomography (CT) scans (45). CT scan radiation doses are much lower than these historical sources, with a very low estimated excess attributable cancer risk of <0.01%–0.05% over a lifetime (46). Airplane travel results in radiation exposure, but at a dose several orders of magnitude below a CT scan (<0.1 mSv compared to 100 mSv for a full-body CT scan). Therefore, there is no biologically credible explanation that seems able to account for the tripling in papillary thyroid cancer incidence over the past 30 years.
Our study has important limitations, related to the fact that the available measures of health care access are necessarily crude and indirect. First, county-level measures of health care access are used as surrogates for more ideal measures, such as the number of practitioner-performed screening physical examinations or imaging studies of the neck and thyroid. Unfortunately, U.S. billing data, the ideal source for a large cohort, do not reliably capture incidences of physical examination of the neck or symptoms prompting neck imaging, making it impossible to test this association directly. Most importantly, billing databases, by their very nature, do not capture patients with other (or no) health insurance, and therefore do not allow the analysis of varying levels of access to care. For these reasons, a population-based registry is ideally suited for ecologic studies such as this one. A second caveat is that county levels of access to health care do not capture the individual experience of residents—many who live in affluent counties are unemployed, are of nonwhite ethnicity, or have less than a high school education. Given these limitations, the statistical tests we performed would tend to underestimate any association between health care access and the incidence of thyroid cancer.
In conclusion, these data demonstrate an association between levels of health care activity and the number of papillary thyroid cancers diagnosed in the United States. Together with the well-known large subclinical reservoir of disease, these results now provide evidence that overdiagnosis explains much of the thyroid cancer “epidemic.” Current trends suggest that in coming years many more of these occult cancers will be detected and many more patients will undergo treatment for papillary thyroid cancer. The additional treatment resulting from overdiagnosis is by definition of no benefit and only of potential harm, making thyroid cancer overdiagnosis a growing public health concern.
Acknowledgments
We thank the GeoSpatial Resource at The Dartmouth-Hitchcock Norris Cotton Center for assistance in map creation, with key work done by Heather Carlos. L.G.T.M. received funding from a National Institutes of Health grant (T32 CA009685). T.D.T. received funding from the National Institutes of Health (National Cancer Institute RC2 CA148259, and Cancer Center Support Grant 5P30 CA023108).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Davies L. Ouellette M. Hunter M. Welch HG. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope. 2010;120:2446–2451. doi: 10.1002/lary.21076. [DOI] [PubMed] [Google Scholar]
- 2.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Morris LG. Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010;200:454–461. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris LG. Sikora AG. Myssiorek D. DeLacure MD. The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol. 2008;15:1169–1176. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 5.Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997) Thyroid. 2002;12:141–149. doi: 10.1089/105072502753522374. [DOI] [PubMed] [Google Scholar]
- 6.Colonna M. Grosclaude P. Remontet L. Schvartz C. Mace-Lesech J. Velten M. Guizard A. Tretarre B. Buemi AV. Arveux P. Esteve J. Incidence of thyroid cancer in adults recorded by French cancer registries (1978–1997) Eur J Cancer. 2002;38:1762–1768. doi: 10.1016/s0959-8049(02)00110-7. [DOI] [PubMed] [Google Scholar]
- 7.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S. Semenciw R. Ugnat AM. Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85:1335–1339. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenhardt L. Grosclaude P. Cherie-Challine L. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 11.Grodski S. Brown T. Sidhu S. Gill A. Robinson B. Learoyd D. Sywak M. Reeve T. Delbridge L. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144:1038–1043. doi: 10.1016/j.surg.2008.08.023. discussion 1043. [DOI] [PubMed] [Google Scholar]
- 12.Welch HG. Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 13.Harach HR. Franssila KO. Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Tanriover O. Comunoglu N. Eren B. Comunoglu C. Turkmen N. Dogan M. Gundogmus UN. Occult papillary thyroid carcinoma: prevalence at autopsy in Turkish people. Eur J Cancer Prev. 2011;20:308–312. doi: 10.1097/CEJ.0b013e32834473dc. [DOI] [PubMed] [Google Scholar]
- 15.Valle LA. Kloos RT. The prevalence of occult medullary thyroid carcinoma at autopsy. J Clin Endocrinol Metab. 96:E109–113. doi: 10.1210/jc.2010-0959. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum M. Patchias EM. Measuring coverage for seniors in Medicare Part A and estimating the cost of making it universal. J Health Polit Policy Law. 2010;35:49–62. doi: 10.1215/03616878-2009-040. [DOI] [PubMed] [Google Scholar]
- 17.DeNavas-Walt C. Proctor BD. Smith JC. U.S. Government Printing Office; Washington, D.C: 2012. Income, Poverty, Health Insurance Coverage in the United States: 2011., Vol P60-243. [Google Scholar]
- 18.Clarke CA. Moy LM. Swetter SM. Zadnick J. Cockburn MG. Interaction of area-level socioeconomic status and UV radiation on melanoma occurrence in California. Cancer Epidemiol Biomarkers Prev. 2010;19:2727–2733. doi: 10.1158/1055-9965.EPI-10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackillop WJ. Zhang-Salomons J. Boyd CJ. Groome PA. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000;89:901–912. doi: 10.1002/1097-0142(20000815)89:4<901::aid-cncr25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Clegg LX. Reichman ME. Miller BA. Hankey BF. Singh GK. Lin YD. Goodman MT. Lynch CF. Schwartz SM. Chen VW. Bernstein L. Gomez SL. Graff JJ. Lin CC. Johnson NJ. Edwards BK. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the Surveillance, Epidemiology, and End Results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao Y. Jemal A. Zhang X. Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995–2004 (United States) Cancer Causes Control. 2009;20:1855–1863. doi: 10.1007/s10552-009-9379-y. [DOI] [PubMed] [Google Scholar]
- 22.Harper S. Lynch J. Meersman SC. Breen N. Davis WW. Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005) Cancer Epidemiol Biomarkers Prev. 2009;18:121–131. doi: 10.1158/1055-9965.EPI-08-0679. [DOI] [PubMed] [Google Scholar]
- 23.Klassen AC. Curriero FC. Hong JH. Williams C. Kulldorff M. Meissner HI. Alberg A. Ensminger M. The role of area-level influences on prostate cancer grade and stage at diagnosis. Prev Med. 2004;39:441–448. doi: 10.1016/j.ypmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Schootman M. Lian M. Deshpande AD. Baker EA. Pruitt SL. Aft R. Jeffe DB. Temporal trends in geographic disparities in small-area breast cancer incidence and mortality, 1988 to 2005. Cancer Epidemiol Biomarkers Prev. 2010;19:1122–1131. doi: 10.1158/1055-9965.EPI-09-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh GK. Miller BA. Hankey BF. Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 26.Clegg LX. Reichman ME. Hankey BF. Miller BA. Lin YD. Johnson NJ. Schwartz SM. Bernstein L. Chen VW. Goodman MT. Gomez SL. Graff JJ. Lynch CF. Lin CC. Edwards BK. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute. SEER. Surveillance, Epidemiology, and End Results program data, 1973–2009. http://seer.cancer.gov/data. [Feb 1;2013 ]. http://seer.cancer.gov/data
- 28.Clegg LX. Feuer EJ. Midthune DN. Fay MP. Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 29.Clegg LX. Gail MH. Feuer EJ. Estimating the variance of disease-prevalence estimates from population-based registries. Biometrics. 2002;58:684–688. doi: 10.1111/j.0006-341x.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 30.Zippin C. Lum D. Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.United States Census Bureau. Census 2000 Gateway. www.census.gov/main/www/cen2000.html. [Feb 1;2013 ]. www.census.gov/main/www/cen2000.html
- 32.International classification of diseases for oncology. In: Fritz A, editor; Percy C, editor; Jack A, editor; Shanmugaratnam K, editor; Sobin L, editor; Parkin DM, editor; Whelan S, editor. 3rd. World Health Organization; Geneva: 2000. [Google Scholar]
- 33.O'Hara B. Experimental health insurance estimates for low-income and demographic groups by state. Health Serv Res. 2008;43(5p1):1693–1707. doi: 10.1111/j.1475-6773.2008.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gigli A. Mariotto A. Clegg LX. Tavilla A. Corazziari I. Capocaccia R. Hachey M. Steve S. Estimating the variance of cancer prevalence from population-based registries. Stat Methods Med Res. 2006;15:235–253. doi: 10.1191/0962280206sm427oa. [DOI] [PubMed] [Google Scholar]
- 35.Breslow NE. Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- 36.Surveillance Research Program 2012 Age adjusted U.S. mortality rates and 95% confidence intervals by cancer site. National Cancer Institute; Bethesda, MD: [Feb 1;2013 ]. [Google Scholar]
- 37.Li N. Du XL. Reitzel LR. Xu L. Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, end results registry, 1980–2008. Thyroid. 23:103–110. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aschebrook-Kilfoy B. Ward MH. Sabra MM. Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etzioni R. Penson DF. Legler JM. di Tommaso D. Boer R. Gann PH. Feuer EJ. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 40.Cheng I. Witte JS. McClure LA. Shema SJ. Cockburn MG. John EM. Clarke CA. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control. 2009;20:1431–1440. doi: 10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L. Cozen W. Bernstein L. Ross RK. Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst. 2001;93:705–709. doi: 10.1093/jnci/93.9.705. [DOI] [PubMed] [Google Scholar]
- 42.Steenland K. Rodriguez C. Mondul A. Calle EE. Thun M. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004;15:939–945. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 43.Information for physicians on irradiation related thyroid cancer. CA Cancer J Clin. 1976;26:150–159. doi: 10.3322/canjclin.26.3.150. [DOI] [PubMed] [Google Scholar]
- 44.Ron E. Saftlas AF. Head and neck radiation carcinogenesis: epidemiologic evidence. Otolaryngol Head Neck Surg. 1996;115:403–408. doi: 10.1177/019459989611500507. [DOI] [PubMed] [Google Scholar]
- 45.Sinnott B. Ron E. Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31:756–773. doi: 10.1210/er.2010-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenner DJ. Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]