Abstract
Aims: The potential receptor for hydrogen sulfide (H2S) remains unknown. Results: H2S could directly activate vascular endothelial growth factor receptor 2 (VEGFR2) and that a small interfering RNA (siRNA)-mediated knockdown of VEGFR2 inhibited H2S-induced migration of human vascular endothelial cells. H2S promoted angiogenesis in Matrigel plug assay in mice and this effect was attenuated by a VEGF receptor inhibitor. Using tandem mass spectrometry (MS), we identified a new disulfide complex located between Cys1045 and Cys1024 within VEGFR2 that was labile to H2S-mediated modification. Kinase activity of the mutant VEGFR2 (C1045A) devoid of the Cys1045–Cys1024 disulfide bond was significantly higher than wild-type VEGFR2. Transfection with vectors expressing VEGFR2 (C1045A) caused a significant increase in cell migration, while the migration-promoting effect of H2S disappeared in the cells transfected with VEGFR2 (C1045A). Therefore, the Cys1045–Cys1024 disulfide bond serves as an intrinsic inhibitory motif and functions as a molecular switch for H2S. The formation of the Cys1045–Cys1024 disulfide bond disrupted the integrity of the active conformation of VEGFR2. Breaking the Cys1045–Cys1024 disulfide bond recovered the active conformation of VEGFR2. This motif was prone to a nucleophilic attack by H2S via an interaction of their frontier molecular orbitals. siRNA-mediated knockdown of cystathionine γ-lyase attenuated migration of vascular endothelial cells induced by VEGF or moderate hypoxia. Innovation and Conclusion: The study provides the first piece of evidence of a molecular switch in H2S-targeting receptor protein kinase in H2S-induced angiogenesis and that may be applicable to additional kinases containing functionally important disulfide bonds in mediating various H2S actions. Antioxid. Redox Signal. 19, 448–464.
Introduction
Initial studies conducted by Hosoki et al. in 1997 suggested a biological role for hydrogen sulfide (H2S) in mammals in which H2S was shown to promote the relaxation of vascular smooth muscles (13). This pioneering study spurned further research that began to reveal an important physiological role for H2S in the cardiovascular system (3, 9, 14, 21, 25, 28–31, 32). Although numerous studies have established H2S as an important regulator of mammalian physiological processes, little is known about the molecular mechanisms underlying its actions. More specifically, do mammalian cells and tissues express a receptor for H2S (12)?
Innovation.
The present study provides the first piece of evidence for hydrogen sulfide (H2S)-targeting receptor protein kinase and reveals a new intrinsic inhibitory S–S bond in vascular endothelial growth factor receptor 2 that serves as a molecular switch for H2S-induced modification and factional regulation. This may be applicable to additional kinases containing functionally important S–S bonds in mediating various H2S actions.
Recently, we reported that H2S is proangiogenic in vascular endothelial cells (3), this finding leading us to hypothesize that a receptor could be involved in H2S-mediated signaling in mammals (12). H2S-induced cell migration and tube formation of vascular endothelial cells were dependent on RAC-alpha serine/threonine-protein kinase (Akt) phosphorylation (3). Interestingly, we also observed Akt phosphorylation in H2S-induced protection against cardiomyocyte apoptosis (31). In this context, Akt activation appeared to be a common occurrence in a number of cell types stimulated with H2S. This leads us to believe that Akt or its upstream regulators may serve as receptors of H2S. In vascular endothelial cells, Akt plays a pivotal role in cell growth and migration by transducing intracellular signals of the vascular endothelial growth factor (VEGF) (17). The type 2 receptor of VEGF, a receptor tyrosine kinase named VEGFR2, mediates most of the biological effects of VEGF (23). Upon binding of VEGF, VEGFR2 dimerizes leading to trans-autophosphorylation of a series of tyrosine residues, including Tyr951, Tyr996, Tyr1054, Tyr1059, Tyr1175, and Tyr1214. Our aim of the current study was to attempt to characterize the receptor of H2S in this signaling system in vascular endothelial cells. This study also aims to investigate how H2S interacts with its molecular switch and explore how the molecular switch could be turned on and off.
Results
VEGFR2 functions as direct target molecule for H2S in vascular endothelial cells and also in Matrigel plug assay in mice
H2S treatment was found to specifically induce the phosphorylation of VEGFR2 at Tyrosine 1175 (Tyr1175) in vascular endothelial cells. In contrast, VEGF treatment induced phosphorylation of all VEGFR2 tyrosine sites (Fig. 1H; Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). The time-dependent increase in phosphorylation status of VEGFR2 (Tyr1175) induced by H2S (Supplementary Fig. S3A) corresponded with the downstream phosphorylation of phosphoinositide 3-kinase (PI3K) (p85, Tyr458 and p55, Tyr199) (Supplementary Fig. S3B), Akt (Ser473) (Supplementary Fig. S3C), and focal adhesion kinase (Tyr576 and 577) (Supplementary Fig. S3D). We also confirmed that VEGFR2 (Fig. 2D), but not Akt (Fig. 2F, Supplementary Fig S1J) are directly activated by H2S in a cell-free system. Since Akt could be further activated in positive control experiments using mitogen activated kinase-like protein (MAPK) and phosphoinositide-dependent kinase 1 (PDK-1) and we failed to find a positive control for PI3K in this cell-free system (Fig. 2E), we cannot exclude any possible direct reaction between H2S and PI3K. Indeed, these results further support the idea that VEGFR2 is a direct target molecule of H2S.
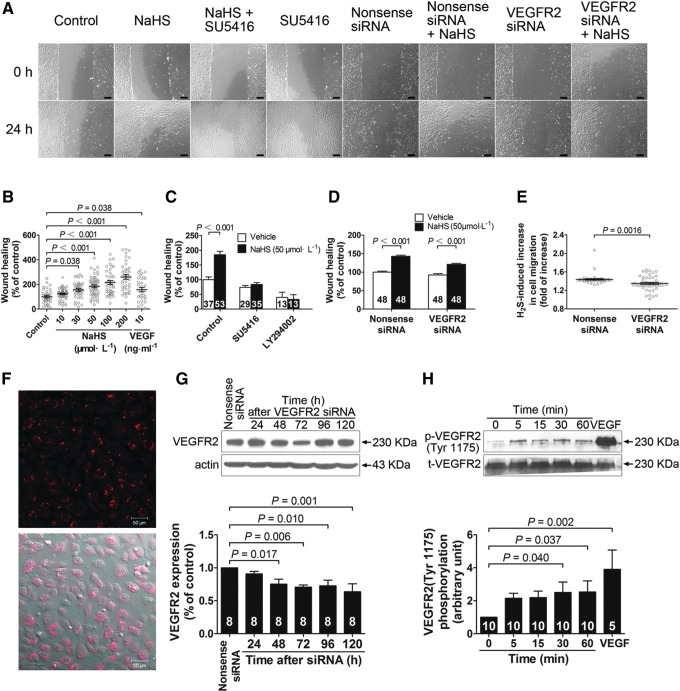
FIG. 1.
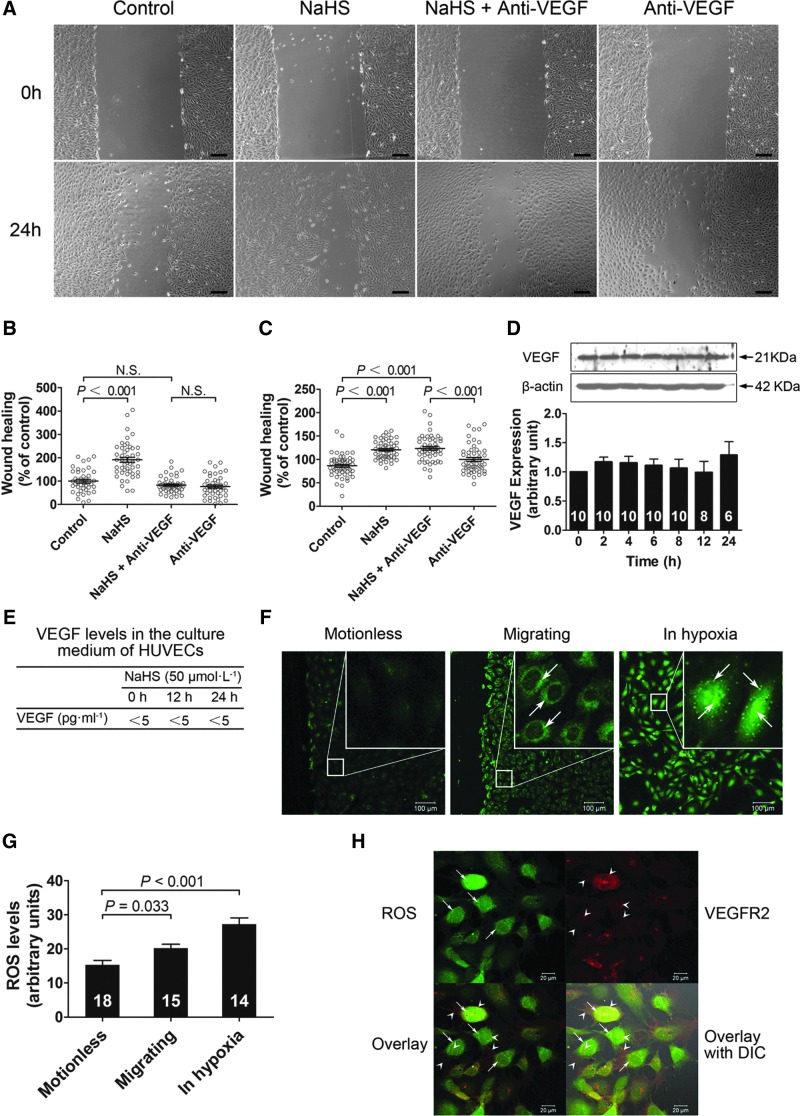
Role of VEGFR2 in hydrogen sulfide-induced promotion of vascular endothelial cell migration. Hydrogen sulfide caused a concentration-dependent promotion of cell migration (A, B), which was abolished by the VEGF receptor inhibitor SU5416 or PI3K inhibitor LY294002 (C). siRNA transfection in the cells resulted in a transfection efficiency of ∼95% (F) and a time-dependent decrease in VEGFR2 protein expression (G). Hydrogen sulfide promoted cell migration in both the nonsense siRNA and the VEGFR2 siRNA groups (A, D) and this effect was inhibited in cells with VEGFR2 siRNA knockdown (E). Hydrogen sulfide increased the phosphorylation of the Tyr1175 site of VEGFR2 in human vascular endothelial cells (H). Bar=200 μm in A. VEGFR2, vascular endothelial growth factor receptor 2; PI3K, phosphoinositide 3-kinase; SiRNA, small interfering RNA.
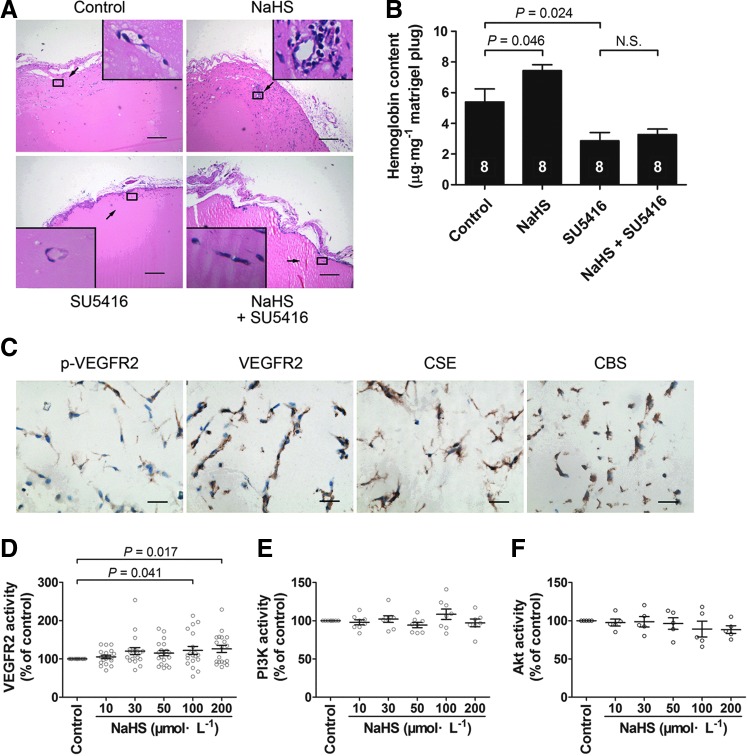
FIG. 2.
VEGFR2 mediates hydrogen sulfide-induced angiogenesis in vivo using Matrigel plug assay in mice. (A) Representative micrographs of hematoxylin–eosin stained gel sections from mice administered with vehicle, NaHS (50 μmol·kg−1·day−1), the VEGF receptor inhibitor SU5416 (12.5 mg·kg−1·day−1), and NaHS (50 μmol·kg−1·day−1) plus SU5416 (12.5 mg·kg−1·day−1), respectively. (B) Hemoglobin content measurement with the tetramethylbenzidine method. (C) Matrigel sections stained with antibodies against phospho-VEGFR2 antibody, VEGFR2 antibody, CSE antibody, and CBS, respectively. n=8 in each group. In a cell-free system, hydrogen sulfide directly activated VEGFR2 (D), but showed no effect on activity of PI3K (E) and Akt (F). Bar=200 μm in A and Bar=25 μm in C. CSE, cystathionine γ-lyase; CBS, cystathionine β-synthase.
In a separate series of experiments, small interfering RNAs (siRNA) specific for human VEGFR2 was used to transfect vascular endothelial cells yielding a transfection efficiency of 95% (Fig. 1F). This siRNA for VEGFR2 caused a partial knockdown in protein expression of VEGFR2 in vascular endothelial cells at 48, 72, 96, and 120 h after siRNA transfection (Fig. 1G). However, high-efficiency lentiviral-mediated VEGFR2 knockdown caused significant apoptosis of vascular endothelial cells and cell viability was too low to perform further experiments, such as wound-healing assay (data not shown). While H2S still showed some capacity to promote cell migration in cells transfected with nonsense siRNA and in cells transfected with siRNA specific for VEGFR2 (with partial knockdown of the receptors) (Fig. 1A and D), a significant decrease in H2S-induced cell migration in the VEGFR2 siRNA cells was observed as compared to the cells transfected with nonsense siRNA (Fig. 1E).
We used Matrigel plug assay in mice to assess the role of VEGFR2 in H2S-induced angiogenesis in vivo. Exogenous H2S administration promoted angiogenesis as evidenced by a significant increase in the hemoglobin content in the Matrigel at a dose of 50 μmol·kg−1·day−1 NaHS as compared with that of vehicle-treated control (Fig. 2B). This H2S effect was inhibited by coadministration of the VEGF receptor inhibitor SU5416 (12.5 mg·kg−1·day−1). Immunohistochemical staining also showed VEGFR2, including its phosphorylated form in the new vessels (Fig. 2C). These data suggest that VEGF receptors are also required in H2S-induced angiogenesis in vivo.
Breaking of the disulfide bond is the only chemical modification induced by H2S
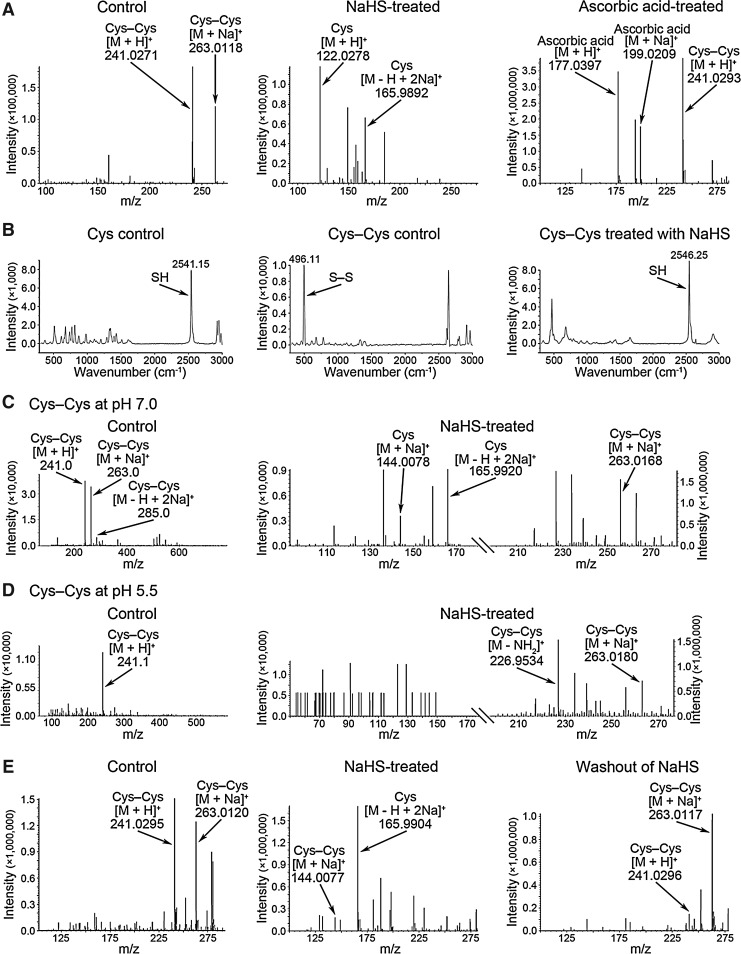
Electrospray ionization MS (ESI-MS) analysis indicated that H2S did not cause any chemical modification of individual amino acids contained within the tertiary structure of VEGFR2 (Supplementary Fig. S4 and Supplementary Fig. S5). As a further test, we also designed model peptides according to the sequences of the activation loop (CDFGLARDIYKDPDYVRKGDAR), the catalytic loop (IHRDLAARNI), and the nucleotide binding loop (LGRGAFGQ) of VEGFR2 and no chemical modifications were found upon incubation with H2S (Supplementary Fig. S6 C-E). Since direct modification of amino acid residues within VEGFR2 was not found, we next determined if H2S could promote the cleavage of disulfide (S–S) bonds using the model chemical, cystine (Cys–Cys). As predicted, the reaction of cystine with H2S yielded cysteine (Cys) (Fig. 3A; see Supplementary Data for details). This observation was also supported by additional studies using Raman spectroscopy that also confirmed the breaking of the S–S bond (Fig. 3B; see Supplementary Data for details).
FIG. 3.
ESI-MS spectra and Raman bands showing hydrogen sulfide-induced cleavage of the S–S bond in the model chemical of Cys–Cys. (A) ESI-MS spectra of Cys–Cys in the absence or presence of NaHS or ascorbic acid. (B) Raman spectroscopy showing the bands for the S–S bond and the SH group. (C, D) ESI-MS spectra showing the effect of hydrogen sulfide on the S–S bond at pH values of 7.0 or 5.5. (E) ESI-MS spectra showing a recovery of the S–S bond after washout of hydrogen sulfide. ESI-MS, Electrospray ionization mass spectrometry.
H2S in solution is composed of a mixture of H2S gas and the HS− anion that are in a dynamic equilibrium. This equilibrium is pH sensitive with acidification reducing the concentration of the HS− anion and increasing that of H2S gas. Therefore, the S–S bond breaking effects of H2S were re-examined using a range of pH values. Cleavage of the S–S bond occurred at pH values ≥7.0, while that at pH values ≤5.5 was abolished (Fig. 3C and D, Supplementary Table S1). This finding suggests that the S–S bond breaking ability is largely due to the HS− anion and not H2S gas. We also found that H2S-induced S–S bond breaking was recoverable following a washout period to remove residual H2S (Fig. 3E). This finding indicates that the breaking of the S–S bond is a reversible chemical process akin to that described for several redox-sensitive proteins. Interestingly, ascorbic acid, a potent reducing agent, was not able to break the S–S bond at a concentration that showed equal reducing potency as H2S (Fig. 3A). This observation suggests that the mechanism underlying H2S-induced S–S bond breakage is not solely due to its reducing nature. In a series of additional experiments, H2S was also found to break the S–S bond located within small molecular weight peptides (Supplementary Fig. S6A), a process that was also recoverable following a washout period to remove H2S (Supplementary Fig. S6B).
The HS− anion acts as a nucleophile during the breaking of the S–S bond; a two-step reaction based on the interaction of the frontier molecular orbitals
Quantum chemical calculations and ESI-MS were performed to investigate how the HS− anion breaks the S–S bond. The results revealed a two-step reaction:
Reaction 1: HS−+C3H6NO2–S–S–C3H6NO2→C3H6NO2–S−+HS–S–C3H6NO2
Reaction 2: HS−+HS–S–C3H6NO2→C3H6NO2–S−+HS–SH
Each of the reactions was initiated by the nucleophilic attack of the HS− anion on the S–S bond. HS− attacked the S–S bond via an interaction with its frontier molecular orbitals; the highest occupied molecular orbital (HOMO) of the nucleophile HS− and the lowest unoccupied molecular orbital of the electrophile under attack (Fig. 4A and Fig. 5A–F for reaction 1; Fig. 4B and Fig. 5G–L for reaction 2). The specificity of the nucleophilic attack of HS− toward the S–S bond was based on their electric charges (the sulfur of HS− is electronegative and the sulfurs of the S–S are electropositive; see Supplementary Table S3) and on the energy and the geometry of the molecular orbitals of the reactants and intermediates of the reactions (Fig. 5; see Supplementary Data for details).
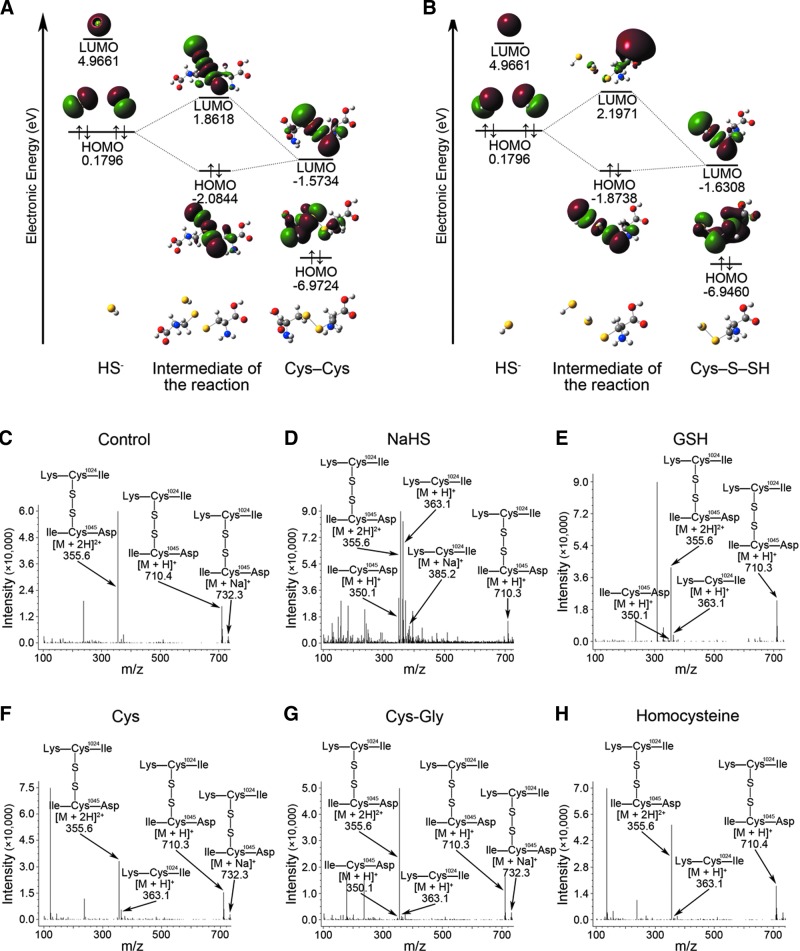
FIG. 4.
Quantum chemical mechanisms underlying hydrogen sulfide-induced breaking of the S–S bond with the model chemical Cys–Cys and comparison of the S–S bond cleaving effect of H2S with a group of common biological thiols. The specificity of the interaction between HS− and the S–S bond is based on the energy and geometry of the frontier molecular orbitals involved in reaction 1 (A) and reaction 2 (B) which are required to break an S–S bond. The HOMO of HS− reacts with the LUMO of Cys–Cys resulting in the intermediate of reaction 1 (A). In the consequent reaction 2, the HOMO of HS− reacts with the LUMO of Cys–S–SH (B). MS analysis of the S–S bond cleaving effect of NaHS (D), GSH (E), Cys (F), Cys-Gly (G), and homocysteine (H). The control sample is treated with vehicle (C). H2S is most potent in breaking the S–S bond in comparison with these biological thiols. HOMO, highest occupied molecular orbitals; LUMO, lowest unoccupied molecular orbitals; GSH, glutathione; Cys, cysteine; Cys-Gly, cysteinylglycine.
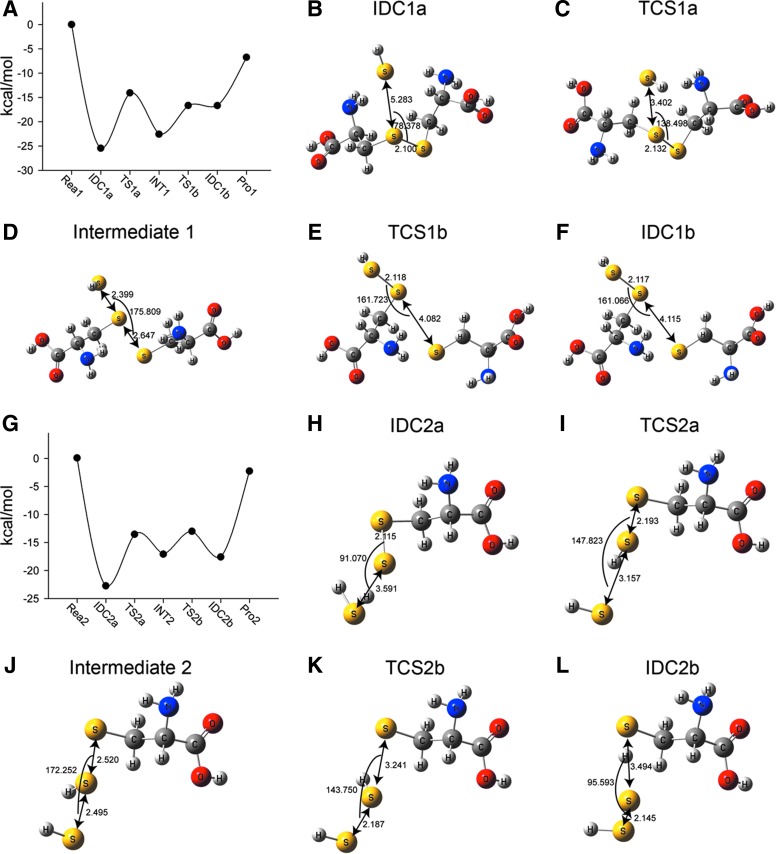
FIG. 5.
The energy and geometries of the two-step nucleophilic attack of HS− to break an S–S bond. Potential-energy surface (A) and optimized key geometries (B–F) for the nucleophilic attack of HS− on Cys–S–S–Cys (reaction 1). (B) the ion–dipole complex 1a (IDC1a); (C) transition state 1a (TS1a); (D) intermediate 1; (E) transition state 1b (TS1b); (F) ion–dipole complexe 1b (IDC1b). Potential-energy surface (G) and optimized key geometries (H–L) for the nucleophilic attack of HS− on Cys–S–SH (reaction 2). (H) the ion–dipole complex 2a (IDC2a); (I) transition state 2a (TS2a); (J) intermediate 2; (K) transition state 2b (TS2b); (L) ion–dipole complex 2b (IDC2b).
These theoretical calculations were supported by our ESI-MS experiments where Cys–S–SH was identified transiently before its subsequent disappearance nearing completion of the reactions (Supplementary Fig. S9). These data support the idea that Cys–S–SH was a product of reaction 1 and served as a reactant for reaction 2.
H2S is most efficient in reducing S–S bonds among common biological thiols
H2S was compared with a range of biological thiols, including glutathione, Cys, cysteinylglycine, and homocysteine at concentrations with equal reducing potency. Comparison of ion intensity ratios between the cleaved ion ([M+H]+ m/z 363.1) and the peptide containing the Cys1045-Cys1024 S–S bond ([M+2H]2+ m/z 355.6 plus [M+H]+ m/z 710.3 and [M+Na]+ m/z 732.3) showed that H2S was the most potent reducing factor in breaking S–S bonds as compared with these biological thiols (Fig. 4C–H). This observation suggests that cleavage of S–S bonds is specific to H2S among biological thiols.
VEGFR2 contains a novel S–S bond between Cys1045 and Cys1024 that can be cleaved by H2S
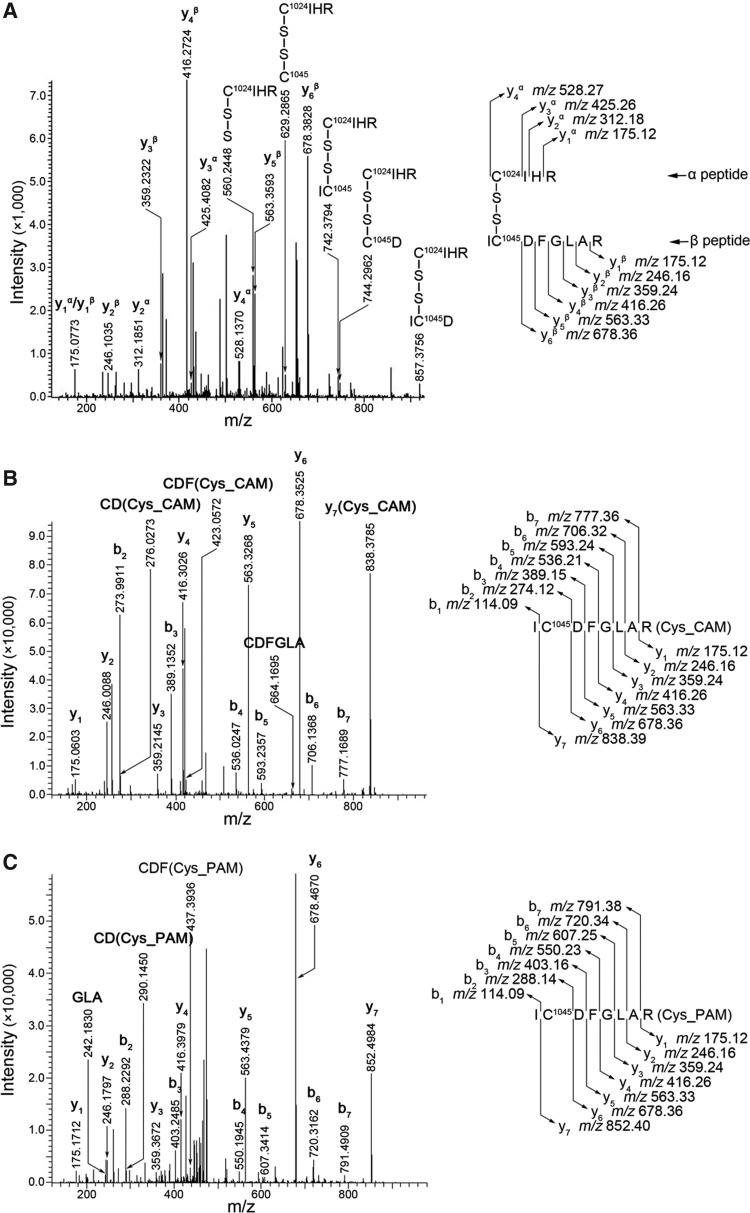
ESI collision-induced dissociation (CID)-MS-MS analysis of VEGFR2 revealed a novel S–S bond located between Cys1045 and Cys1024 within its structure. Fig. 6A shows a precursor ion molecule of [M+3H]3+ m/z 473.90 that yielded a series of CID fragments, which matched with the CID-induced y ions of two trypsin-digested peptide ions (designated as the α and the β peptide), that is, C1024IHR (y1α – y4α) and IC1045DFGLAR (y1β – y6β). This illustrates that these two peptides are joined together by a covalent bond. An additional series of CID-induced y ions were also identified that contained the Cys1024 residue within the polypeptide chains, including the α peptide bound with an additional sulfur atom ([M+H]+ m/z 560.24), the α peptide bound with an additional Cys residue ([M+H]+ m/z 629.29), the α peptide bound with a Cys residue where an isoleucine residue (which is neighboring Cys1045 on the N-terminal side) was bound ([M+H]+ m/z 742.38), the α peptide bound with a Cys residue where an aspartic acid residue (which is neighboring Cys1045 on the C-terminal side) was bound ([M+H]+ m/z 744.30), and the α peptide bound with a Cys residue where an isoleucine residue and an aspartic acid residue (which are neighboring Cys1045 on the N-terminal side and the C-terminal side, respectively), were bound ([M+H]+ m/z 857.38). These data confirmed that the covalent bond was localized between the two Cys residues (Cys1045 and Cys1024). Treatment of these peptides with dithiothreitol (a well-established S–S bond breaker) induced the breaking of the S–S bond between Cys1045 and Cys1024 (Fig. 6B). Interestingly, we also found that the S–S bond between Cys1045 and Cys1024 could also be broken by treatment with H2S (Fig. 6C).
FIG. 6.
ESI-CID-MS-MS spectra of VEGFR2. (A) CID spectra of [M+3H]3+ m/z 473.90 from a tryptic digest of VEGFR2 in the absence of NaHS showing an S–S bond between Cys1045 and Cys1024. (B) CID spectra of [M+2H]2+ m/z 476.24 from a tryptic digest of VEGFR2 in the presence of dithiothreitol showing the β peptide containing Cys1045. (C) CID spectra of [M+2H]2+ m/z 483.25 from a tryptic digest of VEGFR2 in the presence of NaHS showing the β peptide containing Cys1045. CID, collision-induced dissociation; Cys_CAM, Carboxyamidomethyl cysteine; Cys_PAM, propionamide cysteine.
The Cys1045-Cys1024 S–S bond has a role in regulating the conformation of the kinase core of VEGFR2
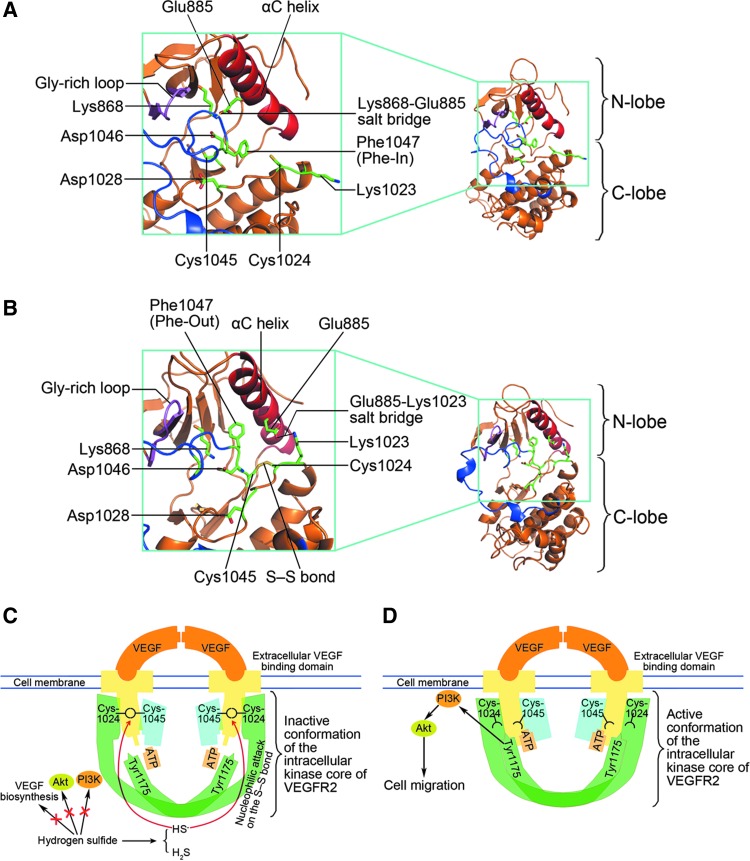
The active conformation of VEGFR2 requires the Cys1045-Cys1024 S–S bond to be broken; this allowing for contact between Glu885 and Lys868 via a highly conserved salt bridge (Fig. 7A). This interaction is crucial for positioning and stabilization of Lys868 where the α and β phosphates of adenosine triphosphate (ATP) are coordinated in phosphotransfer reactions (16, 24). When the Cys1045-Cys1024 S–S bond is present, the αC helix (where Glu885 resides) is shifted resulting in a flip of Glu885 from its ion contact with Lys868 (being 14 Å away) leading to the formation of a new salt bridge of 2.84 Å between Glu885 and the adjacent Lys1023 residue (Fig. 7B). Interestingly, the arginine-glycine-aspartic acid (DFG) motif is also displaced in the presence of the Cys1045-Cys1024 S–S bond. This was characterized by an anticlockwise ∼90° rotation of the Phe1047 from the Phe-In conformation (19) observed at 42780 ps (Fig. 7B; Supplementary Fig. S7). In contrast, the allosteric effect of the Cys1045-Cys1024 S–S bond on the conformation of the invariant catalytic residue (Asp1028), the activation loop, and the glycine-rich loop was not significant (Fig. 7A and B; Supplementary Fig. S7B). These data illustrate that the Cys1045-Cys1024 S–S bond disrupts the integrity of the catalytic core of VEGFR2, blocking the precise positioning of the ATP phosphates.
FIG. 7.
Molecular dynamics simulation showing conformation of the kinase core of VEGFR2. (A) There is a conserved salt bridge between Lys868 and Glu885 in VEGFR2 without the Cys1045-Cys1024 S–S bond. The DFG motif is in the Phe-In conformation. (B) The Lys868-Glu885 salt bridge is disrupted and the DFG motif rotates anticlockwise from the Phe-In conformation in VEGFR2 with the Cys1045-Cys1024 S–S bond. (C, D) Schematic illustration of the main finds of the present study. Hydrogen sulfide directly acts on VEGFR2, but not on PI3K or on Akt in promoting vascular endothelial cell migration. There is a disulfide bond between Cys1045 and Cys1024 serving as an intrinsic inhibitory motif that disrupts the integrity of the VEGFR2 kinase core, which is essential for precise coordination of ATP for the phosphotransfer reaction (C). Hydrogen sulfide yields HS−, which breaks the Cys1045-Cys1024 disulfide bond and thus recovers the integrity of the VEGFR2 kinase core resulting in an increase in VEGFR2 kinase activity (D). Consequently, VEGFR2 (at the Tyr1175 site), PI3K, and Akt are phosphorylated and cell migration is promoted. Although the migration-promoting effects of hydrogen sulfide were not mediated by an increase in VEGF biosynthesis, extracellular VEGF binding (to dimerize VEGFR2) is a premise for hydrogen sulfide-induced VEGFR2 activation.
Mutation at the Cys1045 site results in an increase in the kinase activity of VEGFR2 and H2S does not activate mutant VEGFR2
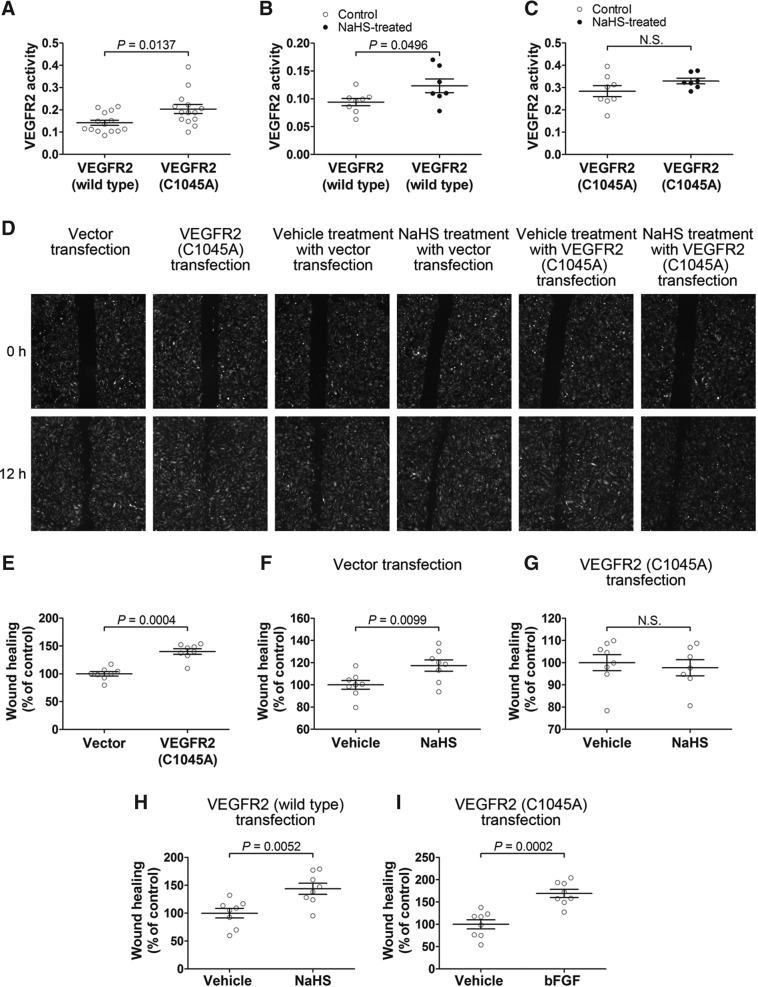
Mutational analysis was conducted in which Cys1045 was replaced with an alanine (C1045A) to prevent the formation of the Cys1045-Cys1024 S–S bond within VEGFR2. As shown in Fig. 8A, the C1045A mutation caused a significant increase in VEGFR2 kinase activity. Moreover, H2S was not able to activate the mutant VEGFR2 (C1045A) (Fig. 8C). In control studies using wild-type VEGFR2, H2S was still able to activate VEGFR2 (Fig. 8B).
FIG. 8.
Effects of Cys1045 mutation on kinase activity of VEGFR2 and the effects of overexpression of VEGFR2 (C1045A) or wild-type VEGFR2 on the migration-promoting effect of hydrogen sulfide in vascular endothelial cells. (A) A single amino acid mutation at Cys1045 of VEGFR2 results in a significant increase in kinase activity. (B, C) Hydrogen sulfide does not activate the mutant VEGFR2 (C1045A) (C), but activates the wild-type VEGFR2 (B). (D) Micrographs of wound-healing experiments using human vascular endothelial cells transfected with lentivirus vectors expressing mutant VEGFR2 (C1045A) or control vectors in the presence or absence of hydrogen sulfide. (E) Cell migration was significantly increased in human vascular endothelial cells transfected with the vectors expressing VEGFR2 (C1045A) as compared with the cells transfected with control vectors. Hydrogen sulfide shows a migration-promoting effect in the cells transfected with control vectors (F) and in the cells transfected with VEGFR2 (wild-type) transfection (H). The migration of the cells transfected with mutant VEGFR2 (C1045A) could not be further increased by hydrogen sulfide (G), but was able to be promoted by FGF (I).
Expression of mutant VEGFR2 (C1045A) promotes migration of vascular endothelial cells and the migration-promoting effect of H2S disappears in the cells expressing VEGFR2 (C1045A)
Mutant VEGFR2 (C1045A) devoid of the Cys1045-Cys1024 disulfide bond was transfected and expressed in human vascular endothelial cells using a lentivirus vector. In wound-healing experiments, cell migration was significantly increased in human vascular endothelial cells transfected with the vectors expressing VEGFR2 (C1045A) as compared with the cells transfected with control vectors (Fig. 8D and E). However, although H2S still exerted a migration-promoting effect in the cells transfected with control vectors (Fig. 8D and F) and in the cells transfected with wild-type VEGFR2 (Fig. 8H), this H2S effect disappeared in the cells transfected with mutant VEGFR2 (C1045A) (Fig. 8D and G), while fibroblast growth factor 2 (basic) (bFGF) (10 ng·ml−1) still showed a promoting effect on cell migration (Fig. 8I). These data not only indicate that the Cys1045-Cys1024 disulfide bond serves as an intrinsic inhibitory motif, but also demonstrate that this disulfide complex functions as a target structure in VEGFR2 to mediate H2S-induced cell migration.
Basal extracellular VEGF binding is required in H2S-induced migration of vascular endothelial cells
In the vascular endothelial cells, the H2S effects were dependent on basal VEGF binding on the extracellular domain of VEGFR2, since the migration-promoting effect of H2S was blunted by the neutralizing antibodies administered 24 h before (Fig. 9A and B). However, this effect was not blocked by the VEGF neutralizing antibodies administered 0.5 h before (Fig. 9C). Moreover, H2S did not cause an increase in the expression of VEGF in the cells (Fig. 9D). Meanwhile, there was no detectable increase in VEGF levels in the cell culture medium (Fig. 9E), excluding a pathway that H2S promotes cell migration via an increase in VEGF levels.
FIG. 9.
Role of VEGF and ROS in the migration-promoting effect of hydrogen sulfide. (A, B) Represent micrograph (A) and datum graphs (B) of the wound-healing experiments where the cells were treated with the VEGF neutralizing antibodies 24 h before NaHS treatment. (C) Effects of hydrogen sulfide on wound healing in the presence or absence of VEGF neutralizing antibodies administered 0.5 h before NaHS treatment. (D, E) Effects of hydrogen sulfide on VEGF expression in the vascular endothelial cells (D) and in the culture medium (E). (F) Micrographs showing ROS levels in motionless vascular endothelial cells, migrating cell (5 h after wound healing), and cells cultured under hypoxic conditions. (G) Bar graphs showing statistical analysis of ROS levels in motionless cells, migrating cells, and cells cultured under hypoxic conditions. (H) Micrographs showing colocalization of ROS signals and VEGFR2 in vascular endothelial cells during migration 5 h after wound healing. ROS, reactive oxygen species; DIC, differential interference contrast. Bar=200 μm in A.
Reactive oxygen species levels are increased in migrating vascular endothelial cells
Intracellular reactive oxygen species (ROS) were identified in both migrating- and motionless vascular endothelial cells (Fig. 9F, thin arrows). Quantification of ROS showed that total intracellular ROS levels were significantly increased in migrating cells as compared with that of motionless cells (Fig. 9F and G, thin arrows). This observation illustrates that a transient oxidizing intracellular environment may occur during cell migration. Interestingly, in motionless vascular endothelial cells cultured under hypoxic conditions, intracellular ROS levels were also significantly increased (Fig. 9F and G, thin arrows). Immunofluorescent double staining showed that ROS (thin arrows) were identified in the vascular endothelial cells expressing VEGFR2 (arrow heads) (Fig. 9H). This indicates that VEGFR2 is subjected to a transient intracellular oxidizing environment during cell migration.
Endogenous H2S is required for VEGF-induced migration of vascular endothelial cells
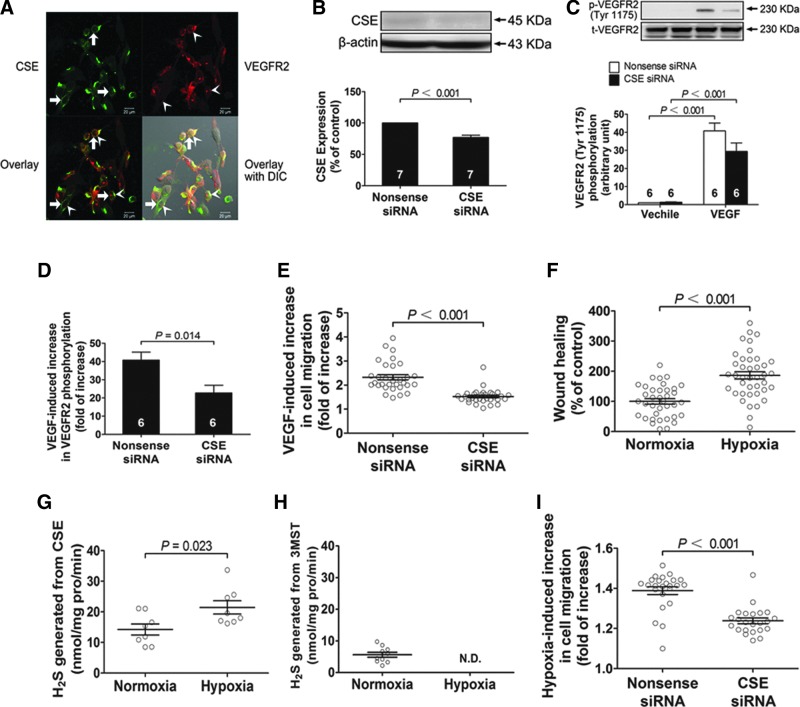
The H2S generating enzyme, cystathionine-γ-lyase (CSE), was mainly localized at subcellular areas at the membrane of vascular endothelial cells (Fig. 10A, thick arrows). Double staining showed that CSE was colocalized with VEGFR2 (arrow heads) (Fig. 10A). CSE was also identified in the new vessels penetrated into the Matrigel in mice (Fig. 2C). The data suggest that endogenous H2S might be generated in the vascular endothelial cells where VEGFR2 resides.
FIG. 10.
Endogenous H2S is required in VEGF induced cell migration. (A) Micrographs showing cellular distribution of the H2S generating enzyme CSE (thick arrows) and colocalization of CSE and VEGFR2 (arrow heads) in vascular endothelial cells. (B) Transfection of CSE siRNA caused a decrease of CSE protein expression in vascular endothelial cells. (C, D) VEGF-induced phosphorylation of Tyr1175 on VEGFR2 was inhibited by transfection of CSE siRNA. (E) The migration-promoting effect of VEGF upon vascular endothelial cells was significantly decreased by CSE RNA interference. (F) Moderate hypoxia could significantly promote the migration of vascular endothelial cells. (G) The activity of H2S producing enzyme CSE was elevated by moderate hypoxia. (H) The activity of H2S producing enzyme 3MST was inhibited under moderate hypoxia conditions. (I) siRNA-mediated knockdown of CSE attenuated hypoxia-induced increase in the migration of vascular endothelial cells.
siRNA-mediated knockdown of CSE attenuated VEGF-induced migration of vascular endothelial cells (Fig. 10B and E) and also attenuated VEGF-induced phosphorylation of VEGFR2 at the Tyr1175 site (Fig. 10B, C and D). The results illustrate endogenous H2S is required for VEGF to promote migration of vascular endothelial cells.
Endogenous H2S is required in hypoxia-induced migration of vascular endothelial cells
Wound-healing assay showed that moderate hypoxia, which was created by depriving 20% dissolving oxygen from the culture medium caused a significant promotion on migration of vascular endothelial cells (Fig. 10F). Activity of the H2S generating enzyme, CSE, was increased in the cells cultured under moderate hypoxic conditions as compared with the cells cultured under normoxic conditions (Fig. 10G). H2S generated from 3-mercaptopyruvate sulfurtransferase (3MST) was also found at a rate of 5.62 nmol·mg·pro−1·min−1 in the vascular endothelial cells cultured under normoxic conditions. Under moderate hypoxic conditions, H2S produced from 3MST was not detectable and CSE became the dominant source of H2S production (Fig. 10G and H). Moreover, siRNA-mediated knockdown of CSE attenuated hypoxia-induced increase in the migration of vascular endothelial cells (Fig. 10I). These results suggest that endogenous H2S mediates hypoxia-induced migration of vascular endothelial cells.
Discussion
The proangiogenic activity of VEGF on vascular endothelial cells is thought to be mediated via VEGFR2 following dimerization (23). Here we show that VEGFR2 was directly activated by H2S suggesting that VEGFR2 acts as a direct target molecule for H2S in vascular endothelial cells. The physiological relevance of this effect was further clarified by siRNA-mediated knockdown of VEGFR2 in human vascular endothelial cells. We found that the ability of H2S to promote migration was inhibited in VEGFR2 knockdown cells as compared with that of the control cells. In these experiments using the cells with siRNA-mediated knockdown of VEGFR2, VEGFR2 were decreased, however, were not completely knocked out. This may explain why H2S-induced promotion on cell migration was inhibited, but was not completely abolished in these cells with siRNA-mediated partial knockdown of VEGFR2. This is the first direct evidence for the existence of H2S-targeting receptor protein kinase in mammals. This idea is further supported by in vivo experiments where the proangiogenic effect of H2S was attenuated by a VEGF receptor inhibitor.
In further investigations exploring how H2S acts upon VEGFR2, we identified a new S–S bond between Cys1045 and Cys1024 in VEGFR2. The existence of this disulfide bond in VEGFR2 has not been reported to date. CID-MS-MS analysis indicated that the Cys1045-Cys1024 S–S bond was the only chemical motif present within VEGFR2 that was labile to modification by H2S.
The mechanisms underlying the interaction between H2S and its target molecule may be different from that of a typical ligand–receptor interaction where the ligand docks with the pockets/cavities within its receptor (2). Indeed, H2S is too small a molecule to have a conformation large enough to dock with any potential pockets/cavities by conformational matching. The specificity of H2S for its receptor is based on a mechanism beyond conformational docking. This idea is supported by our MS and Raman spectroscopy experiments as well as our theoretical analysis of quantum chemistry, where we identified the HS− anion as the reactive nucleophile that attacks the S–S bond within VEGFR2. The energy and geometry of the frontier molecular orbitals of HS− and the S–S bond provides the specificity to the proposed reaction mechanism. In this context, any proteins containing an S–S bond may serve as a potential target for H2S. We showed here that the Cys1045-Cys1024 S–S bond serves as an intrinsic inhibitory motif as determined by functional analysis of mutant VEGFR2 (C1045A). The VEGFR2 protein–tyrosine kinase core is characterized as having a bilobed architecture (16). Some highly conserved motifs, such as the αC helix, the salt bridge between Lys868 (Lys1030 in the insulin receptor) and Glu885 (Glu1047 in the insulin receptor; Supplementary Fig. S8), the orientation of Phe1047 (Phe1151 in the insulin receptor; Supplementary Fig. S8) of the DFG motif (in the active Phe-In or inactive Phe-out conformation; Supplementary Fig. S8), are essential for the interconversion of both inactive and active states (16, 24). Our molecular dynamics simulations show that the integrity of the active conformation of the VEGFR2 kinase core was disrupted in the presence of the Cys1045-Cys1024 S–S bond. This explains why the Cys1045 mutation caused a significant increase in VEGFR2 activity. Moreover, since the increased activity of this mutation was unaffected by H2S treatment, it further confirms that this structural motif is important in H2S-VEGFR2 interactions and signal transduction. Indeed, we have presented evidence indicating that this interaction proceeds via a two-step reaction using cystine as a model disulfide. In this reaction, H2S breaks the S–S bond yielding Cys–SH and HS–SH. Although we could confirm the presence of Cys–SH using ESI-MS spectrometry, HS–SH was not detected. It is feasible to suggest that HS–SH is an unstable intermediate that quickly dissociates to H2S and S (10).
Mustafa et al. reported an H2S-induced S-sulfhydration of the –SH group of the Cys residues in a series of proteins, including GAPDH (18). We did not identify such an S-sulfhydration of the Cys residues contained in VEGFR2 by ESI-MS spectrometry. It remains to be further investigated whether the Cys residues contained in different protein molecules reside in different chemical and conformational microenvironments, which may be crucial for possible S-sulfhydration modification of these residues.
The molecular switch for H2S is located on the intracellular domain of VEGFR2. Extracellular H2S may diffuse through cell membrane and subsequently dissociates into H+ and HS−, which can break the intracellular S–S by nucleophilic attack. To date, there is no information about how far H2S can diffuse in the cytoplasm and how fast H2S can be degradated by oxidation in a cell. Nevertheless, the intracellular kinase domain of VEGFR2 is localized just to inboard cell membrane and may be susceptible to the H2S molecules diffusing from the extracellular medium. On the other hand, it remains unknown whether the endogenous H2S generated in different subcellular compartments might have a distinct physiological role.
It is worth noting that there are additional biological thiols that may also reduce S–S bonds. We found here that H2S is the most potent reducing factor in cleaving the S–S bonds as compared with other biological thiols. This finding suggests that the cleavage of S–S bonds is specific to H2S, among the known biological thiols. Our quantum chemical analysis showed that the nucleophilic attack of HS− on the sulfur atoms of the S–S bond determines the specificity and efficiency of H2S at promoting cleavage of S–S bonds. This nucleophilic attack is based on the behavior of the frontier molecular orbitals of the atoms involved in the reaction.
The migration-promoting effect of H2S was abolished by pretreatment with a neutralizing antibody against VEGF administered 24 h in advance of H2S treatment. Interestingly, no effect on migration was observed when the neutralizing antibody was administered 0.5 h in advance of treatment. This finding illustrates that basal extracellular VEGF binding to VEGFR2 is required in H2S-mediated VEGFR2 signaling. Since the likelihood of VEGFR2 monomers on cell membrane to collide with each other in a living cell is very low, the monomers activated by H2S must dimerize. This may explain why VEGF plays a functional role in H2S-induced dimerization of VEGFR2. This is in accordance with Abe and Kimura who report that H2S enhances the activity of N-methyl-D-aspartic acid (NMDA) receptors and this effect requires binding of NMDA to NMDA receptors (1). H2S alone is not sufficient to activate its direct target protein molecules. However, this fact does not weaken the physiological importance of H2S. We have also found that VEGF failed to promote cell migration without endogenous H2S. In this context, the H2S-targeting protein molecules are a family of H2S-dependent proteins rather than simply a classical receptor of H2S.
We found here that ROS levels were significantly increased in vascular endothelial cells during the process of cell migration or under hypoxic conditions. These data suggest that a transient intracellular oxidizing event occurs during certain physiological/pathophysiological conditions. Therefore, the receptors would be oxidized in migrating cells where H2S could reduce the oxidized form of VEGFR2. This idea was further supported by immunofluorescent double staining of the migrating cells where increased ROS signals were identified in the cells expressing VEGFR2.
Moreover, the present study also showed that the H2S generating enzyme, CSE is colocalized with VEGFR2 in vascular endothelial cells. Cellular localization of the H2S generating enzymes is controversial. Shibuya et al. show that 3MST is localized to the endothelium of the thoracic rat. Neither CSE nor cystathionine β-synthase (CBS) is identified in the endothelium of the thoracic aorta in rat (26). In addition, Olson et al. reported that 3MST and CBS, but not CSE, are localized to cultured endothelial cells isolated from the bovine pulmonary artery (20). In contrast, CSE has been expressed in the endothelium in mouse, human, and bovine and in cultured endothelial cells isolated from human and bovine vessels (15, 21). Expression of different H2S generating enzymes may be species/tissue specific. We identified CSE expression in human umbilical endothelial cells and in the endothelial cells in Matrigel in mice in the present study and further showed that CSE had a pivotal role in regulating cell migration using vascular endothelial cells with siRNA-mediated knockdown of CSE. The results suggest that basal endogenous H2S is required for VEGF to activate VEGFR2 and promote cell migration. Since the molecular switch for H2S, the Cys1045-Cys1024 S–S bond, is located in the intracellular domain of VEGFR2 and CSE is also localized to subcellular areas adjacent to the cell membrane, endogenously generated H2S is very close to its target motif.
In addition to the pivotal role of endogenous H2S in basal conditions, we further found that generation of endogenous H2S produced by CSE, not by 3MST, was increased to mediate hypoxia-induced migration of vascular endothelial cells. This phenomenon suggests an inverse relationship between the CSE/H2S system and O2 levels. This is in line with Olson et al. who show an inverse relationship between H2S and O2 levels in the lung tissue of a cow and sea lion (20). These data suggest that the H2S generating system functions as an O2 sensor and may thereby be involved in the pathophysiological regulation/compensation of the cells in response to hypoxia. On the other hand, the differential regulation of CSE and 3MST under hypoxic conditions suggests that different H2S generating enzymes may have distinct physiological roles under various physiological and pathophysiological conditions, and provides fertile ground for future works to investigate the mechanisms underlying the proangiogenic role of endogenous H2S in chronic ischemic diseases.
Cys residues in proteins, including tyrosine kinase receptors, some other enzymes, and channels are primary targets for oxidative stress. The thiols in Cys can be oxidized by ROS to form disulfide bonds, resulting in dysfunction of redox-sensitive proteins (4, 5). Vitagenes, a group of genes encoding proteins, such as heat shock proteins, glutathione, and thioredoxin/thioredoxin reductase system with antioxidant and antiapoptosis properties, have an important role in redox homeostasis (6, 7). We found here that H2S could enhance the activity of VEGFR2 by reducing the Cys1045-Cys1024 disulfide bond. This gives rise to a hypothesis that the H2S producing enzymes and H2S may participate in the vitagene network in the regulation of redox-sensitive proteins that contain disulfide bond(s) as molecular switch. Indeed, different components of the vitagene network, including the H2S producing enzymes and H2S, may have distinct distribution patterns and thereby act differentially on their target proteins in various tissues and cells.
As summarized in Fig. 7C and D, VEGFR2 functions as an H2S-targeting receptor protein kinase in promoting human vascular endothelial cell migration. The characterized intrinsic inhibitory S–S bond between Cys1045 and Cys1024 in VEGFR2 serves as a specific molecular switch for H2S-induced modification that regulates VEGFR2 function. Moreover, VEGFR2 does not respond to VEGF without basal endogenous H2S and may be thus termed as “H2S-dependent receptor protein kinase.” This work also gives rise to the possibility that any protein molecules that contain a functionally important S–S bond may serve as potential target protein for H2S.
Materials and Methods
Cell culture, Western blot analysis, and measurement of VEGF levels and the total antioxidative capacity were performed according to the methods previously described (3) that are described in detail in the Supplementary Data.
Measurement of VEGF levels in cell culture medium
VEGF levels in the culture medium were measured by Enzyme-Linked Immunosorbent Assay (ELISA) using a commercially available kit (R&D Systems) according to the manufacturer's instructions (see Supplementary Data).
Assay of the kinase activity of VEGFR2, PI3K, and Akt in a cell-free system
The kinase activity of pure recombinant VEGFR-2, PI3K, and Akt was individually assessed in the presence or absence of NaHS at concentrations of 10, 30, 50, 100, and 200 μM in a cell-free system containing none of any other intracellular signaling elements.
VEGFR-2 activity was measured using the VEGF receptor kinase assay kit (Cell Signaling Technology). PI3K activity was assayed using the PI3-Kinase ELISA kit (Echelon eBiosciences). Akt activity was measured using an Akt assay kit (Cell Signaling Technology) (see Supplementary Data).
Spectrometry studies
MS analyses of model chemicals were performed using a SHIMADZU mass spectrometer (LCMS-IT-TOF). VEGFR2 was digested with trypsin and on-line isolation was performed using high-performance liquid chromatography (Michrom Bioresources). Sample digests were analyzed using tandem MS with a LTQ Orbitrap XL mass spectrometer (Thermo Electron). The Raman S–S stretching band was collected on a HORIBA Jobin Yvon LabRam-1B Raman spectrometer (see Supplementary Data).
Construction of mutant VEGFR2 (C1045A)
Mutant VEGFR2 (C1045A) without the Cys1045-Cys1024 S–S bond was provided by SignalChem according to a custom-development program.
Overexpression of human VEGFR2 (C1045A) mutant and wild-type human VEGFR2
VEGFR2 (NM_002253) (789-end) mutated at Cys1045 to Ala1045 or wild-type VEGFR2 (789-end) was cloned into pGC-FU, the lentiviral vector expression plasmid (GeneChem). Human umbilical vein endothelial cells (HUVECs) grown at 70%–80% confluence were infected with the C1045A mutant lentivirus, wild-type VEGFR2 lentivirus, or control lentivirus. The multiplicity of infection (MOI) of lentivirus trasfection was 5. The cells were passaged for further experiments after infection for 3 days.
ROS measurement, immunofluorescence, and confocal microscopy
2,7-dichlorofluorescin diacetate (Sigma) was used to measure intracellular ROS levels. Colocalization of CSE and VEGFR2 in vascular endothelial cells was determined with double-staining immunofluorescence in HUVECs and observed using a laser scanning microscope (Leica TCS SP5) (see Supplementary Data).
siRNA-mediated knockdown of VEGFR2 and CSE
An siRNA specific for human VEGFR2 (sense, 5′-CAUGUUCUCUAAUAGCACAtt-3′; antisense, 5′-UGUGCUAUUAGAGAACAUGgt-3′), an siRNA specific for human CSE (sense, 5′-GCAUCUGAAUUUGGAUUAAtt-3′; antisense, 5′-UUAAUCCAAAUUCAGAUGCca-3′), and scrambled siRNA as a negative control were obtained from Ambion. HUVECs were transfected by a transfection reagent (siPORT NeoFX; Ambion), and then cultured for 48 h for the consequent wound-healing experiments (see Supplementary Data).
Angiogenesis in vivo: Matrigel plug assay
C57 BL/6 female mice were anesthetized and injected subcutaneously with 400 μl Matrigel. Vehicle, NaHS (50 μmol·kg−1·day−1), or SU5416 (12.5 mg·kg−1·day−1) were injected intraperitoneally for 7 days. Then, mice were euthanized and the Matrigel plugs were collected. Angiogenesis in Matrigel in mice was assessed by morphological analysis and quantification of the hemoglobin content. Matrigel plugs were paraffin embedded and sections (5 μm) were stained with hematoxylin–eosin or for further immunostaining using antibodies against phospho-VEGFR2, VEGFR2, CSE, and CBS with the ABC kits (Santa Cruz Biotechnology). Hemoglobin quantification was performed by the tetramethylbenzidine method (22). The investigation was approved by the ethic committee of Fudan University Shanghai Medical College.
Molecular dynamics simulations of H2S-induced regulation of VEGFR2 conformation
The molecular dynamics simulations were performed using the GROMACS package version 4.0.7 (11) with the Optimized Potentials for Liquid Simulations (OPLS) force field (www.gromacs.org). The VEGFR2 model used in the present study was configured based on the X-ray crystalline structure of VEGFR2 at 2.40 Å resolution taken from the Protein Data Bank, entry code 1VR2 (see supplementary Data).
Quantum chemistry analysis for the mechanisms underlying H2S-induced S–S bond breaking
For quantum chemistry analysis, ab initio molecular orbital calculations were carried out for density functional theory studies with the Gaussian 09 program using the Becke three-parameter hybrid functional combined with the Lee-Yang-Parr correlation functional (see Supplementary Data).
Measurement of the activity of CSE and 3MST
Vascular endothelial cells were cultured under nomoxic or hypoxic conditions for 24 h before measurement of H2S production. To measure the CSE activity, the enzyme substrate L-cysteine (10 mM) and the cofactor pyridoxal-5′-phosphate (2 mM) were added to the cells for an incubation of 4 h. To measure 3MST activity, L-cysteine (5 mM) and α-ketoglutarate (1 mM) were added to the cells for an incubation of 4 h according to the methods described by Shibuya et al. who show that Cys aminotransferase generates 3-mercaptopyruvate (3MP) in the presence of Cys and α-ketoglutarate, while 3MP serves as a substrate for 3MST to produce H2S (26, 27). H2S concentrations in the culture medium were measured using a H2S-sensitive electrode (World Precision Instruments) (8). The H2S concentrations were calculated against the calibration curves of standard H2S solutions. The amount of H2S produced per microgram cell protein per minute was calculated as the activity of these H2S-generating enzymes.
Statistical analysis
Results are expressed as mean±standard error. Statistical analyses were performed using SPSS Statistics 17.0. Differences among three or more groups were analyzed by one-way analysis of variance. Two-group cases were analyzed with the Student's t-test. Significance was established at the p<0.05 level.
Supplementary Material
Abbreviations Used
- 3MP
3-mercaptopyruvate
- 3MST
3-mercaptopyruvate sulfurtransferase
- CBS
cystathionine β-synthase
- CID
collision-induced dissociation
- CSE
cystathionine γ-lyase
- Cys
cysteine
- DCFH-DA
2,7-dichlorofluorescin diacetate
- ELISA
enzyme-linked immunosorbent assay
- ESI-MS
electrospray ionization mass spectrometry
- FAK
focal adhesion kinase
- H2S
hydrogen sulfide
- HOMO
the highest occupied molecular orbital
- HUVECs
human umbilical vein endothelial cells
- LUMO
the lowest unoccupied molecular orbital
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- SE
standard error
- siRNA
small interfering RNAs
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology (2010CB912601, 2009ZX09303 - 006) of China and the National Natural Science Foundation of China (81230003, 30825016, 30971064, and 81000045). We thank Dr. Peter Rose for his critical comments on the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abe K. Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongrand P. Ligand-receptor interactions. Rep Prog Phys. 1999;62:921–968. [Google Scholar]
- 3.Cai WJ. Wang MJ. Moore PK. Jin HM. Yao T. Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. doi: 10.1016/j.cardiores.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese V. Cornelius C. Cuzzocrea S. Iavicoli I. Rizzarelli E. Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese V. Cornelius C. Dinkova-Kostova AT. Calabrese EJ. Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese V. Cornelius C. Dinkova-Kostova AT. Iavicoli I. Di Paola R. Koverech A. Cuzzocrea S. Rizzarelli E. Calabrese EJ. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochimica et Biophysica Acta. 2012;1822:753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese V. Cornelius C. Stella AM. Calabrese EJ. Cellular stress responses, mitostress and carnitine insufficiencies as critical determinants in aging and neurodegenerative disorders: role of hormesis and vitagenes. Neurochem Res. 2010;35:1880–1915. doi: 10.1007/s11064-010-0307-z. [DOI] [PubMed] [Google Scholar]
- 8.Doeller JE. Isbell TS. Benavides G. Koenitzer J. Patel H. Patel RP. Lancaster JR., Jr. Darley-Usmar VM. Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao XY. Scalia R. Kiss L. Szabo C. Kimura H. Chow CW. Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargrove J. Persulfide generated from L-cysteine inactivates tyrosine aminotransferase—requirement for a protein with cysteine oxidase activity and gamma-cystathionase. J Biol Chem. 1988;263:17262–17269. [PubMed] [Google Scholar]
- 11.Hess B. Kutzner C. Van Der Spoel D. Lindahl E. Gromacs 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 12.Hoefer IE. Something is rotten in the state of angiogenesis—H2S as gaseous stimulator of angiogenesis. Cardiovasc Res. 2007;76:1–2. doi: 10.1016/j.cardiores.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Hosoki R. Matsuki N. Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 14.Johansen D. Ytrehus K. Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—evidence for a role of KATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H. Shibuya N. Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal. 2012;17:45–57. doi: 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mctigue M. Wickersham J. Pinko C. Showalter R. Parast C. Tempczyk-Russell A. Gehring M. Mroczkowski B. Kan C. Villafranca J. Appelt K. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure. 1999;7:319–330. doi: 10.1016/s0969-2126(99)80042-2. [DOI] [PubMed] [Google Scholar]
- 17.Morales-Ruiz M. Fulton D. Sowa G. Languino LR. Fujio Y. Walsh K. Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa AK. Gadalla MM. Sen N. Kim S. Mu WT. Gazi SK. Barrow RK. Yang GD. Wang R. Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:72. doi: 10.1126/scisignal.2000464. ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidle S. Cancer Drug Design and Discovery. New York: Academic Press; 2007. p. 233. [Google Scholar]
- 20.Olson KR. Whitfield NL. Bearden SE. Leger JS. Nilson E. Gao Y. Madden JA. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–R60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papapetropoulos A. Pyriochou A. Altaany Z. Yang G. Marazioti A. Zhou Z. Jeschke M. Branski L. Herndon D. Wang R. Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CC. Morel JCM. Amin MA. Connors MA. Harlow LA. Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 23.Robinson C. Stringer S. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 24.Roskoski R. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y. Chen Y. Zhu Y. Huang G. Moore P. Huang S. Yao T. Zhu Y. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–H2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y. Cao Y. Wang W. Ma S. Yao T. Zhu Y. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79:632–641. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- 29.Wang M. Cai W. Li N. Ding Y. Chen Y. Zhu Y. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010;12:1065–1077. doi: 10.1089/ars.2009.2945. [DOI] [PubMed] [Google Scholar]
- 30.Yang GD. Sun XF. Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and Caspase-3. FASEB J. 2004;18:1782–1784. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 31.Yao LL. Huang XW. Wang YG. Cao YX. Zhang CC. Zhu YC. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3 beta-dependent opening of mPTP. Am J Physiol Heart Circ Physiol. 2010;298:H1310–H1319. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 32.Zhao WM. Zhang J. Lu YJ. Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.