Abstract
Patients with recessive dystrophic epidermolysis bullosa (RDEB) have incurable skin fragility, blistering, and skin wounds due to mutations in the gene that codes for type VII collagen (C7) that mediates dermal–epidermal adherence in human skin. In this study, we evaluated if topically applied human recombinant C7 (rC7) could restore C7 at the dermal–epidermal junction (DEJ) and enhance wound healing. We found that rC7 applied topically onto murine skin wounds stably incorporated into the newly formed DEJ of healed wounds and accelerated wound closure by increasing re-epithelialization. Topical rC7 decreased the expression of fibrogenic transforming growth factor-β2 (TGF-β2) and increased the expression of anti-fibrogenic TGF-β3. These were accompanied by the reduced expression of connective tissue growth factor, fewer α smooth muscle actin (α-SMA)–positive myofibroblasts, and less deposition of collagen in the healed neodermis, consistent with less scar formation. In addition, using a mouse model in which skin from C7 knock out mice was grafted onto immunodeficient mice, we showed that applying rC7 onto RDEB grafts with wounds restored C7 and anchoring fibrils (AFs) at the DEJ of the grafts and corrected the dermal–epidermal separation. The topical application of rC7 may be useful for treating patients with RDEB and patients who have chronic skin wounds.
Introduction
Chronic cutaneous ulcers are an enormous health care problem consuming ~$20 billion per year in the United States.1 The precise mechanisms involved in the healing of skin wounds are not fully understood. Traditionally, the phases of wound healing have been divided into (i) clot formation and inflammation, (ii) re-epithelialization, (iii) granulation tissue formation by the processes of fibroplasia and neo-angiogenesis, and (iv) tissue remodeling.2 Except for topical platelet-derived growth factor (GF), there are no biological agents approved by the Federal Drug Administration for the enhancement of skin wound healing. Topical PDGF has been in use for several years, but is indicated only for diabetic skin wounds, and it has proven to have only modest efficacy.3–5
Patients with recessive dystrophic epidermolysis bullosa (RDEB) have inherited skin fragility resulting in widespread bullae and open erosive skin wounds that heal with scarring.6 Scarring results in many complications, such as a fibrotic fusion of the fingers and toes, esophageal stenoses, joint contractures, poor dentition, decreased ability to open the mouth, fixed tongue, and nutritional deficiencies. The chronic cycles of wounding and healing in patients with RDEB, coupled with fibrosis, are thought to be the reason that these patients develop aggressive skin squamous cell carcinomas that often result in premature demise.
RDEB is due to a gene defect in COL7A1 that codes for type VII collagen α chains.7–9 Type VII collagen (C7) is the major component of anchoring fibrils (AFs), large 700–900 nm structures that hold the epidermis and dermis of skin together.10,11 patients with RDEB have a paucity or complete absence of functional C7 and functional AFs in the dermal–epidermal junction (DEJ) of their skin, resulting in poor dermal–epidermal adherence. Structurally, C7 is a composed of three identical α chains, each consisting of a central collagenous triple-helical domain and two flanking amino- and carboxy-terminal non-collagenous domains NC1 and NC2, respectively.10–13 Within the extracellular space, C7 molecules form antiparallel dimers, which aggregate laterally to form AFs, wheat-stack shaped structures, that serve to anchor the epidermis onto the underlying dermis.
Like patients with chronic skin wounds, the current treatment of patients with RDEB is only supportive care because there is no consistently effective treatment. Recently, several clinical trials for RDEB have been initiated. Wang et al. demonstrated that the intradermal injection of allogeneic fibroblasts into patients with RDEB would result in increased endogenous mutated C7 in their skin, fewer new skin bullae and improved dermal–epidermal adherence.14 Wagner and coworkers have shown that bone marrow/stem cell transplantation into patients with RDEB results in increased C7 in the patients' skin and improved clinical outcomes.15 Although promising, neither therapy is consistently effective or without risk.
Our laboratory has developed in vitro and in vivo strategies geared to more direct RDEB therapy. We and others showed that C7-deficient RDEB skin cells (keratinocytes and dermal fibroblasts) when gene corrected to synthesize and secrete C7 (via infection with a minimal lentiviral vector or a phi C31 integrase), revert from the abnormal RDEB cellular phenotype and are normalized with regards to cell growth, cell motility, and cell matrix attachment.16,17 Using a mouse model with human RDEB skin equivalents generated with RDEB fibroblasts and keratinocytes and engrafted onto immunodeficient mice and a C7-knockout mouse model (Col7a1−/−, which recapitulates features of RDEB), we intradermally injected: (i) gene-corrected RDEB fibroblasts that could now synthesize and secrete C7,18 (ii) lentiviral vectors expressing C7,19 and (iii) C7 protein itself.20,21 All three strategies resulted in new C7 and AFs in the RDEB skin and a reversal of the poor dermal–epidermal adherence. We recently developed an alternative strategy using an intravenous approach with molecularly engineered RDEB fibroblasts over-expressing human C7.22 We demonstrated that intravenously injected fibroblasts homed to murine skin wounds, continuously delivered C7, which then incorporated into the healed wound's DEJ, formed stable AFs, and accelerated wound closure.
In the study described herein, we sought to determine if simply applying human recombinant C7 (rC7) topically would be useful in promoting wound healing. Using full-thickness wounds in athymic nude mice, we found that topically applied rC7 stably incorporated into the healing wounds' DEJ. Furthermore, topical application of rC7 to wounds promoted re-epithelialization and dramatically accelerated wound closure. Moreover, in a second animal model in which we grafted RDEB-like, C7-null skin from C7-knockout mice onto athymic nude mice, we found that topically applied rC7 incorporated into the DEJ of the RDEB skin grafts and corrected the grafts' poor dermal–epidermal adherence and AF defect. These studies provide the first evidence for the potential use of topically applied rC7 to improve skin wound healing and reverse the molecular and structural defects in RDEB.
Results
Topical rC7 incorporated into the regenerated BMZ
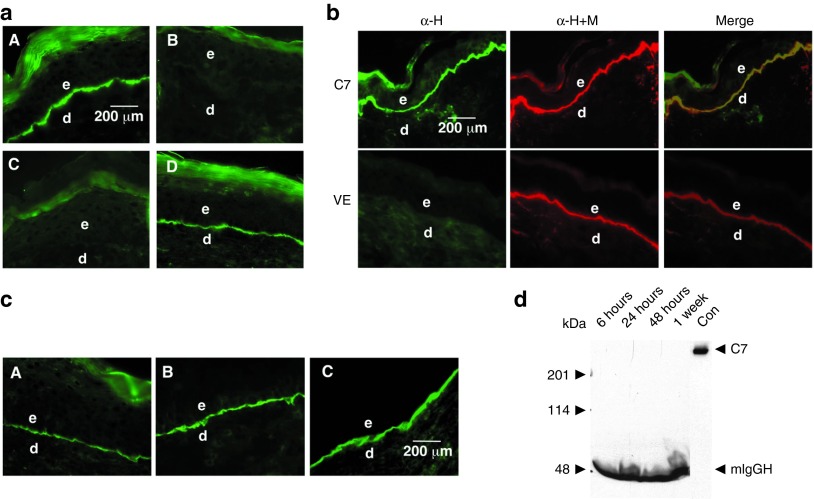
We purified milligram quantities of rC7 from conditioned media of gene-corrected RDEB fibroblasts as described earlier17,20 and used it to evaluate the feasibility of topical rC7 application for healing of skin wounds and RDEB treatment. We made 1.0 cm2 full-thickness excision wounds on the mid-back of athymic nude mice (n = 20) and topically applied 30 μg of rC7 in a 10% carboxymethylcellulose vehicle to the wound site. Skin biopsies were obtained from healed wounds at various time points after the topical application and subjected to immunostaining with an antibody specific for human C7. As shown in Figure 1a, the topically applied rC7 incorporated into the DEJ of the healed skin wounds as early as 2 weeks and was persistent for at least 2 months after a single application (panels A and D). In contrast, wounds treated with vehicle (n = 20) lacked human C7 at the DEJ (panel B). Interestingly, wounds treated with the NC1 domain of C7 (the amino-terminal half the C7 molecule) (n = 10 mice) did not have NC1 at their DEJ, suggesting that the ability of rC7 to incorporate into the DEJ requires full-length C7 (panel C). These experiments demonstrated that rC7 applied topically onto full thickness skin wounds was able to incorporate stably into the DEJ of the healed skin.
Figure 1.
Topically applied rC7 stably incorporated in the regenerated DEJ in the mouse skin. (a) Immunofluorescence staining of the mouse skin was performed with an antibody specific for human C7 at 2 weeks after the topical application of 30 µg of rC7, NC1, or vehicle alone. Note that the healed wounds treated with rC7 (n = 30 mice) demonstrated a linear pattern of C7 deposition at the DEJ (panel A). In contrast, no human C7 was detected in mice treated with vehicle alone (n = 20 mice) or NC1 (n = 10 mice) (panels B and C). Panel D shows the stable incorporation of human rC7 at the mouse DEJ at 8 weeks after the initial topical application of rC7. (b) Immunofluorescence staining of mouse skin was performed 2 weeks after the topical application of rC7. The skin sections were labeled with either a monoclonal antibody specific for human C7 (green, panel α-H) or a rabbit polyclonal antibody that recognizes both mouse and human C7 (red, panel α-M+H). Merged images demonstrate colocalization of topically applied human rC7 with endogenous mouse C7 at the mouse DEJ. The lower panel depicts staining of wounds treated with the vehicle (VE) alone. (c) Dose-dependent deposition of human rC7 at the mouse DEJ after topical rC7 application. Immunofluorescence staining of biopsy specimens were performed with an antibody specific for human C7 after the animal's wounds were treated with 8 µg (A), 16 µg (B), or 32 µg (C) of topical rC7, respectively. Scale bar: 200 µm. (d) Sera were taken from mice at the time indicated after topically applied with 30 µg rC7 and subjected to 4–15% SDS-PAGE followed by immunoblot analysis using an anti-NC1 antibody. Purified rC7 of 10 ng was run as a control (Con). The positions of full-length 290 kDa C7, 50 kDa mouse IgG heavy chain (mIgGH), and molecular weight markers are indicated. d, dermis; e, epidermis.
To confirm that the human C7 was correctly localized within the DEJ of the healing mouse skin, we colabeled the same vertical sections with a polyclonal antibody that recognizes both mouse and human C7 (α-H+M) and our human specific anti-C7 antibody (α-H). As shown in Figure 1b, these two antibodies showed perfect colocalization in the mouse's DEJ when the images were merged. Therefore, the topically applied rC7 incorporated to the same location of the DEJ as the mouse's endogenous C7. Note that wounds treated with the vehicle alone (VE in Figure 1b) showed the presence of mouse but not human C7.
After demonstrating the ability of the topical rC7 to incorporate into the mouse's DEJ, we wished to determine the minimal concentration of protein required for DEJ deposition. We applied 8, 16, and 32 µg of rC7 topically on the wounds. As shown in Figure 1c, there was a dose-dependent increase in the incorporation of rC7 into the mouse's DEJ.
Because rC7 was administered deeply into the wounds, it was important to determine if the topical rC7 was transported into the animals' circulation. To examine this issue, we performed immunoblot analysis on sera obtained from treated mice at 6 hours, 24 hours, 48 hours and 1 week after topical application of rC7. As shown in Figure 1d, 10 ng of rC7 running as a control was readily detected by our immunoblot analysis. However, we did not detect any rC7 in the blood stream.
Topical rC7 promoted wound healing via re-epithelialization of the epidermis
After having shown the incorporation of topically applied rC7 into the DEJ of healed wounds, we wished to determine if the topical application of rC7 accelerates the closure of skin wounds. Following full-thickness skin excision, we monitored wound closure over a 14-day period and found that rC7-treated wounds exhibited marked acceleration of wound closure compared with vehicle-treated wounds (Figure 2a,b).
Figure 2.
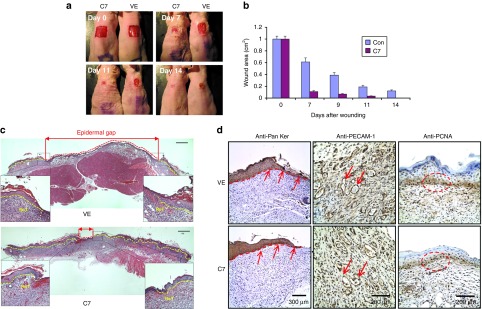
Topical application of rC7 promoted wound healing. A 1.0 cm2 (1 × 1 cm) square full-thickness excision wound was made on the mid-back of 8- to 10-week-old athymic nude mice, and rC7 (30 g) was applied topically once on day 0 (n = 20 mice per group). (a) Representative days 0, 7, 11, and 14 wounds are shown. (b) Mean ± SD open wound area measurements at days 0, 7, 9, 11, and 14 after wounding (n = 20 mice for each group). (c) On day 7, excisional biopsies of full-thickness wounded skin with a portion of unwounded skin were obtained from wounds treated with topical vehicle (VE) or rC7 (C7). The biopsy specimens were stained by H&E and photographed with a light microscope. Independently, photographed images with identical magnifications were reconstituted to show the unhealed areas of the wounds. Red dotted lines indicate the unhealed wound area. Yellow dotted lines mark the newly re-epithelialized epidermis. The fronts of newly re-epithelialized epidermis were enlarged, as shown in higher magnified images. Scale bars: 0.33 mm. (d) Immunohistochemistry analysis of biopsy specimens of day 7 full-thickness wounds treated with either vehicle (VE) or rC7 (C7) with anti-pan keratin (keratinocytes), anti-PECAM-1 (endothelial cells), and anti-PCNA antibodies. Ten randomly selected images per each condition from three independent experiments were analyzed for consensus. Representative images are shown. Scale bars: 0.3 mm (left column); 0.2 mm (middle and right columns). In the left column, arrows point to the keratin-labeled, re-epithelializing tongue (ReT); in the middle column, the arrows point to blood vessels; in the right column, the circles point to proliferating keratinocytes.
Re-epithelialization is a crucial part of wound healing and is primarily mediated by migrating keratinocytes. We previously showed that C7 strongly promotes human keratinocyte migration in vitro.23 To determine whether rC7-driven migration of the keratinocytes across the wound bed contributed to re-epithelialization and enhanced wound healing, as shown in Figure 2c, we completely excised the entire wounds plus some normal unwounded surrounding skin 7 days after treatment and subjected the tissue to H&E staining, microscopic analyses, and measurements. The percent of re-epithelialization across each wound was measured by light microscopy using a reticle to measure the proportion of each wound that was covered by a neo-epidermis in relation to the entire original open wound length. As shown in Figure 2c, wounds that were treated with rC7, compared with those treated with vehicle alone, exhibited a significant reduction in the epidermal gap, which is the length of the un-re-epithelialized open wound. Conversely, the rC7-treated wounds exhibited significantly more re-epithelialization than vehicle-treated wounds (Figure 2c, dotted yellow lines). The re-epithelializing tongue (ReT) can be clearly visualized in the enlarged images on both sides of the wounds.
To confirm the H&E results and also to analyze for the presence of keratinocytes and the presence of blood vessel formation in wounds treated with either rC7 or vehicle, we performed immunohistochemistry on the biopsy specimens with antibodies specific for keratinocytes and endothelial cells. As shown in Figure 2d, anti-pan keratin antibody staining clearly labeled the ReT (Figure 2d, red lines and arrows, left panels). There were no differences in the numbers of endothelial cells or blood vessels, as assessed by anti-PECAM-1 antibody staining (Figure 2d, arrows, middle panels), between vehicle- and rC7-treated wounds.
Re-epithelialization depends upon keratinocyte migration and cellular division. To evaluate if rC7 affects cellular proliferation, we conducted immunohistochemistry on biopsy specimens with an antibody specific to PCNA, a marker for cell proliferation. As shown in Figure 2d, there were no significant differences in the numbers of PCNA positive cells between vehicle- and rC7-treated wounds. We also compared the thickness of the epidermis (measured from basement membrane layer to the stratum corneum of the epidermis) in the healing wounds at days 7 and 19 after wounding (Table I). At each time point, no significant differences in epidermal thickness were observed in wounds treated with rC7 or vehicle. Taken together, these data indicate that the acceleration of wound healing by rC7 is likely mediated by promoting re-epithelialization rather than by increasing wound angiogenesis or cellular proliferation.
Table 1. Epidermal thickness of wounds in rC7- and vehicle-treated wounds.
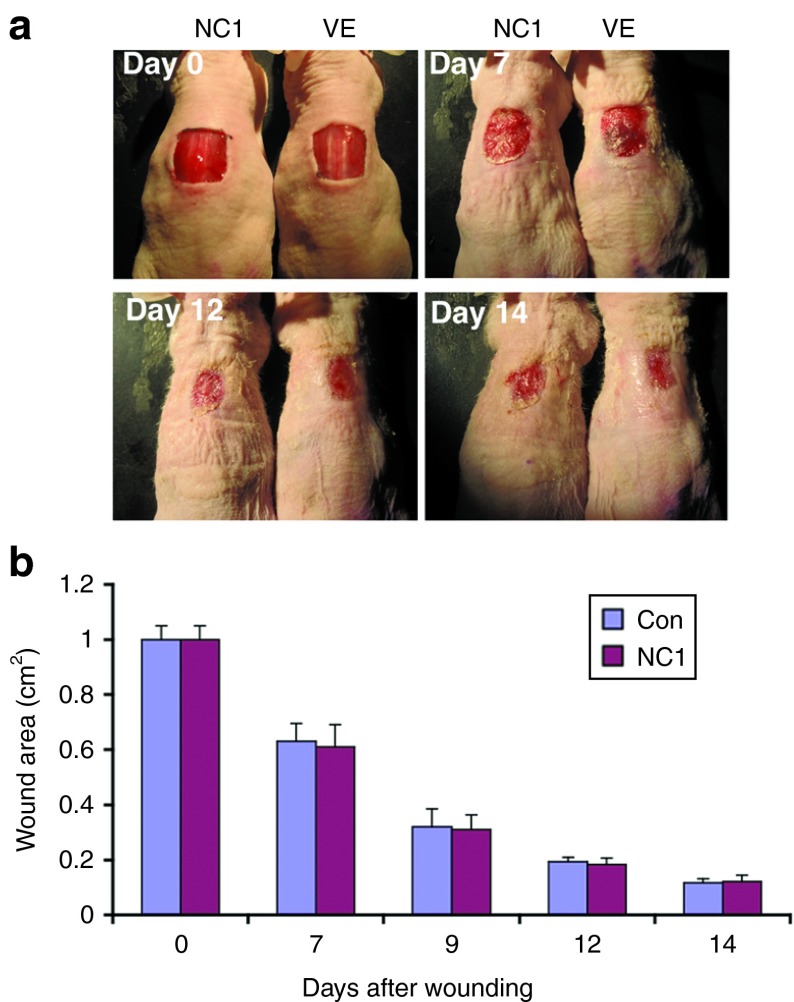
We previously showed that the non-collagenous NC1 domain of C7 failed to promote keratinocyte migration in vitro.23 To see if the ability of C7 to promote skin cell migration is critical for its ability to enhance skin wound closure in vivo, we performed wound healing experiments using topically applied NC1 domain at the same molar concentration as full-length rC7. As shown in Figure 3, the wounds of mice treated with topical NC1 exhibited similar wound healing rates as wounds treated with vehicle at days 9, 12, and 14 (Figure 3a,b). Collectively, these data suggest a direct correlation between the ability of C7 to promote keratinocyte migration and its ability to accelerate wound closure.
Figure 3.
Topical application of NC1 did not promote wound healing. A 1.0 cm2 (1 × 1 cm) square full-thickness excision wounds were made on the mid-back of 8- to 10-week-old athymic nude mice and purified NC1 domain (30 g) (n = 10 mice) or vehicle (VE) (n = 10 mice) were applied topically once on day 0. (a) Representative days 0, 7, 12, and 14 wounds are shown for wounds treated with NC1 or vehicle alone (VE). (b) Mean ± SD wound size measurements at days 0, 7, 9, 12, and 14 after wounding treated with NC1 (n = 10 mice for each group).
Topical rC7 inhibited fibrosis of the wounded skin
During the course of our experiments, we consistently noticed that rC7-treated wounds appeared less scarred than vehicle-treated wounds. To determine if rC7 has anti-contraction activity, we performed a standard fibroblast-populated collagen I lattice contraction assay.24 Previously, we have shown that collagen lattices are contracted by human dermal fibroblasts pulling on a specific domain of type I collagen, the slightly unraveled telopeptides.25 In this experiment, we performed this assay with and without the presence of purified rC7 or its NC1 domain. As expected, maximum contraction occurred in the presence of serum GFs while there was no contraction without them (Figure 4a). Interestingly, full-length rC7 inhibited contraction of the collagen lattices, while the NC1 domain did not. As we showed previously that the NC1 domain of C7 was unable to promote skin cell migration in vitro or accelerate wound closure in vivo, our data suggest that the domain of C7 that promotes cell migration resides within the same domain of C7 that is required for the inhibition of collagen lattice contraction. Furthermore, rC7 inhibited collagen lattice contraction in a dose-dependent manner with maximum levels of inhibition at a concentration of 25 µg/ml (Figure 4b).
Figure 4.
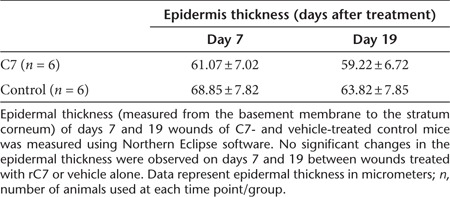
C7 inhibited the contraction of collagen lattices in vitro and reduces the presence of myofibroblasts, connective tissue growth factor (CTGF) expression, and collagen deposition in vivo. (a) Early passage, human dermal fibroblasts were isolated and mixed with serum and type I collagen. Collagen lattices, with size of 0.6 cm3 and cell density at 100,000 cells/cm3, were released and floated in 1 ml DMEM. rC7 (C7) or NC1 of 30 µg were added to the serum containing medium and incubated at 37 °C for 24 hours. Contraction assays were also carried out without serum added (GF−) or with serum added (GF+). Contraction of the lattices was determined by measuring the gel weight. These data represent the mean ± SD of triplicate determinations in one representative experiment. Similar results were obtained in two other independent experiments. (b) Dose-dependent inhibition of contraction of collagen lattices by rC7. rC7 at the indicated concentrations were added to the contraction assays. These data represent the mean ± SD of triplicate determinations in one representative experiment. Similar results were obtained in two other independent experiments. (c) Immunohistochemistry staining of healed mouse skin was performed with the antibodies specific for α smooth muscle actin (α-SMA) and connective tissue growth factor (CTGF) at 2 weeks after topical application of vehicle (VE) and rC7 (C7). Ten randomly selected images per condition from three independent experiments were analyzed. The representative images are shown. Scale bars: 0.2 mm. Note that the wounds treated with topical rC7 showed significantly diminished α-SMA–positive fibroblasts (myofibroblasts) as well as decreased CTGF expression. (d) Collagen deposition was assessed by Masson's trichrome staining and Picrosirius red staining of healed wounds at 14 days after topical application of rC7 (C7) or vehicle (VE). Ten randomly selected images per condition from three independent experiments were analyzed. The representative images are shown. Scale bars: 0.2 mm. Note that rC7-treated wounds have less collagen deposition in the dermis by both staining methods compared with vehicle-treated wounds which show perturbed fiber architecture. (e) Immunofluorescence staining of healed mouse skin was performed with the polyclonal antibodies specific for TGF-β2 and TGF-β3 at 2 weeks after topical application of vehicle (VE) and rC7 (C7). Ten totally randomly selected images per condition from three independent experiments were analyzed. The representative images are shown. Scale bars: 0.2 mm. Note that the wounds treated with topical rC7 display reduced expression of TGF-β2 and increased expression of TGF-β3.
The transformation of fibroblasts to α smooth muscle actin (α-SMA)–positive myofibroblasts is responsible for converting granulation tissue into a permanent scar. Therefore, the upregulation of SMA is associated with fibrosis.26 To determine if rC7 also inhibits wound fibrosis in vivo, we performed immunohistochemistry on healed skin wounds with antibodies specific for α-SMA at 2 weeks after the topical application of rC7 or vehicle. As shown in Figure 4c, there was a significant decrease in the quantity of myofibroblasts in wounds treated with topical rC7- versus vehicle-treated wounds. Connective tissue GF (CTGF/CCN2) is required for TGF-β–induced transdifferentiation of fibroblasts to myofibroblasts.27 As shown in Figure 4c, rC7 also reduced the expression of CTGF/CCN2 protein at 2 weeks in comparison with vehicle control. Histological evaluation of healed skin tissues stained with Masson's trichrome and Picrosirius red stain revealed less dermal collagen deposition in rC7-treated wounds compared with vehicle-treated wounds (Figure 4d). In addition, rC7-treated wounds revealed collagen fibers organized in a parallel fashion compared with vehicle-treated wounds which showed a haphazard, disarrayed arrangement of collagen fibers. Collectively, the in vivo data showed that the topical application of rC7 to skin wounds resulted in a significant reduction in fibroblast-to-myofibroblast differentiation, CTGF expression and collagen deposition, suggesting that C7 may inhibit excessive fibrosis that leads to scar formation.
Transforming GF-β (TGF-β) is a multifunctional GF involved in many aspects of wound healing.2 However, an excess of TGF-β can lead to pathological scar formation. There are three mammalian TGF-β isoforms: TGF-β1, TGF-β2, and TGF-β3. TGF-β1 and TGF-β2 isoforms exhibit pro-scarring properties, whereas TGF-β3 displays anti-scarring properties.28 To determine the effects of topical application of rC7 on the expression of TGF isoforms, we performed immunostaining on healed skin wounds with antibodies specific for TGF-β1, TGF-β2, and TGF-β3 at 2 weeks after the topical application of rC7 or vehicle. As shown in Figure 4e, the expression of pro-fibrogenic TGF-β2 was significantly reduced while the expression of anti-fibrogenic TGF-β3 was significantly increased in healed wounds treated with topical rC7- versus vehicle-treated wounds. We did not detect any significant difference in the expression of TGF-β1 (data not shown). These data indicate that one mechanism by which C7 affects the fibrotic pathway is by regulating the expression of TGF-β isoforms.
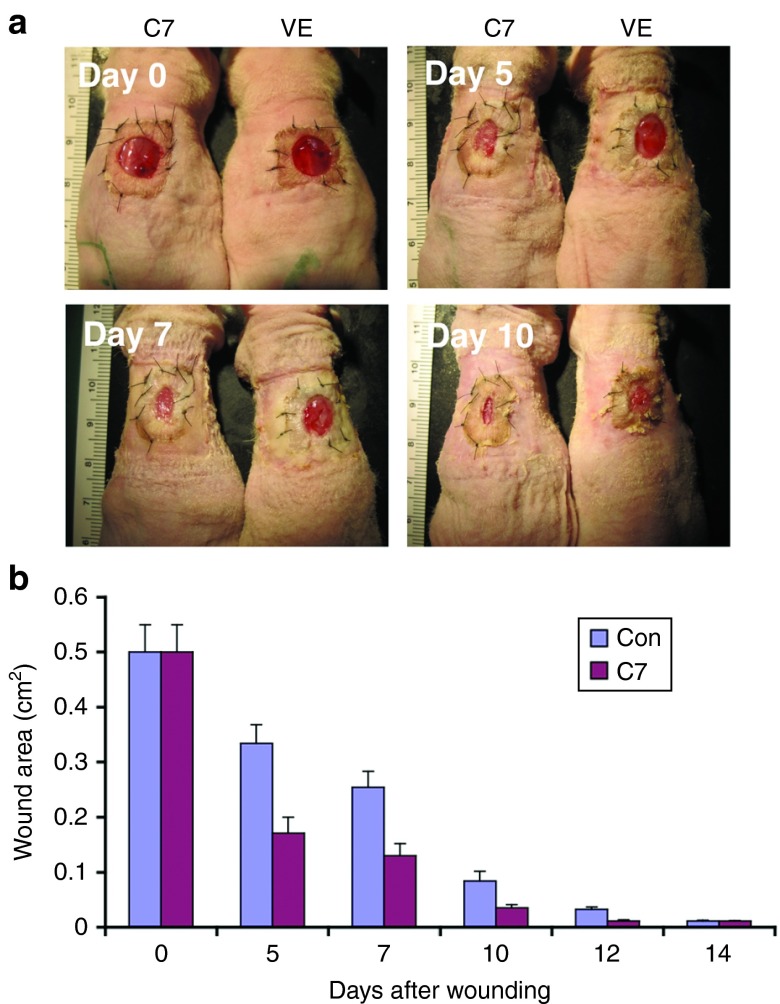
We also assessed the effect of topical rC7 on the healing wounds in human skin grafts. We grafted 1.5 × 1.5 cm pieces of human skin onto the backs of athymic nude mice as previously described.29 Eight weeks after grafting, the human skin grafts were wounded with an 8 mm punch biopsy tool. We then topically applied rC7 or vehicle onto the wounds. As shown in Figure 5, human skin wounds healed significantly faster when treated with topical rC7 compared with vehicle controls. Taken together, these data indicate that topically applied rC7 to healing wounds accelerates the closure of skin wounds in both murine and human skin graft model systems.
Figure 5.
Topically applied rC7 promoted wound closure of human skin. A 0.5 cm2 (8 mm diameter punch biopsy) full-thickness excision wound was made in human skin grafted onto the mid-back of 8- to 10-week-old athymic nude mice. The wounds were then treated with topically applied 30 µg rC7 (C7) or vehicle (VE) (n = 5 mice per group). (a) Representative days 0, 5, 7, and 10 wounds are shown. Wound sizes were reduced in wounds treated with rC7 compared with vehicle control at 5, 7, and 10 days after wounding. (b) Mean + SD wound size measurements at days 0, 5, 7, 10, 12, and 14 after wounding (n = 5 mice for each group).
Topical rC7 restored C7 expression in engrafted RDEB mouse skin in vivo
Patients with RDEB manifest with widespread open wounds and chronic erosions. To evaluate the efficacy of topical rC7 for a murine model of RDEB wounds, we transplanted skin from C7-knock out mice onto the backs of the athymic nude mice. These COL7A1-null mice have no C7 at the DEJ of their skin, and they entirely lack AFs.30 Clinically, these newborn mice exhibit extensive blisters and die within the first week of life. Thus, these C7-null mice recapitulate many of the clinical, genetic, and ultrastructural features of severe patients with RDEB. Because these RDEB mice are very fragile and small and die within a few days after birth, it was not technically feasible to apply rC7 topically onto these mice. As an alternative, we killed these mice and transplanted their skin onto the backs of adult athymic nude mice. After engraftment of the transplanted RDEB mouse skin, we made a 6 mm punch biopsy wound in the transplanted RDEB graft and topically applied rC7. As shown in Figure 6a (panels A and D), RDEB skin grafts before treatment showed histological evidence of dermal–epidermal separation and entirely lacked C7 at the DEJ, characteristic of RDEB-diseased donor skin. Similarly, vehicle-treated wounds displayed dermal–epidermal separation and exhibited no C7 at the DEJ (Figure 6a, panels B and E). In contrast, RDEB skin grafts that received topical rC7 exhibited improved dermal–epidermal adherence and had C7 restored at the DEJ in a linear distribution (Figure 6a, panels C and F).
Figure 6.
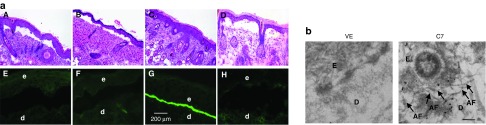
Topical application of rC7 incorporated into the DEJ of RDEB mouse skin grafts and forms AFs in vivo. (a) Histological appearance (A–D) and immunofluorescence staining (E–H) of engrafted RDEB mouse skin using a polyclonal antibody to the NC1 domain of C7. Panels A and E, 2 weeks after grafting of RDEB mouse skin and before treatment (n = 30 mice); panels B and F, wounded and engrafted RDEB skin 2 weeks after topically applied vehicle (n = 4 mice); panels C and G, wounded and engrafted RDEB skin 2 weeks after 30 µg of rC7 was applied topically (n = 15 mice); panels D and H, unwounded engrafted RDEB skin 2 weeks after 30 µg of rC7 was applied topically (n = 4 mice). (b) Immunogold labeling of engrafted murine RDEB skin topically applied with 30 µg rC7 (C7) or vehicle (VE) was performed using an anti-NC1 polyclonal antibody and revealed that the topical rC7 incorporated into the RDEB skin grafts and formed AFs. Note restoration of numerous arching AFs depicted with arrows and labeled with gold particles decorating the DEJ in rC7-treated RDEB skin grafts. AF, anchoring fibrils; d, dermis; e, epidermis; Scale bar: 100 nm.
Full-thickness, skin wounds are much different from the superficial bullous wounds of RDEB skin, which occur at the dermal–epidermal interface. Having shown that topical rC7 incorporated into the DEJ of full-thickness, skin wounds, we wished to evaluate if topical rC7 would be able to incorporate into the engrafted RDEB skin wounds by performing experiments without wounding the RDEB skin grafts. As shown in Figure 6a, the rC7-treated RDEB skin grafts displayed dermal–epidermal separation and lacked C7 expression at the DEJ (Figure 6a, panels D and H). These results indicate that wounding the RDEB skin is a prerequisite for topical rC7 to incorporate into the DEJ.
Immunoelectron microscopy showed that topically applied rC7 restored the formation of AFs in the grafted RDEB skin (Figure 6b), suggesting correction of the major ultrastructural disease abnormality. Taken together, these data suggest that protein-based therapy by topical application of rC7 may correct the defects in RDEB skin, namely lack of C7, lack of AFs and compromised dermal–epidermal adherence.
Discussion
In this study, we used a murine wound healing model and a RDEB skin transplant model to evaluate the feasibility of topically applied rC7 to treat RDEB and normal skin wounds. Our studies showed that topical rC7 stably incorporated into the DEJ of healing wounds and accelerated wound closure by increasing re-epithelialization. Furthermore, we observed that C7-mediated acceleration of wound closure was accompanied by decreases in the expression of CTGF and the presence of α-SMA myofibroblasts, markers associated with scar formation. In addition, our results indicated that there is a correlation between the domain of C7 that promotes keratinocyte migration and the domain of C7 that accelerates wound closure. With the RDEB skin transplantation model, we demonstrated that topical rC7 incorporated correctly into the DEJ of RDEB skin, formed AFs and corrected the RDEB clinical phenotype.
Numerous GFs and other agents that could potentially enhance tissue regeneration have been identified, but their therapeutic application has been limited in clinical medicine for several reasons. These include difficulty in maintaining bioactivity of locally applied therapeutic agents in regenerating tissue because of a lack of retention of the agent, poor tissue penetration, and instability of therapeutic proteins in the protease-rich environment of a healing skin wound. It is thought that for these reasons GFs, such as PDGF-BB (Regranex, Healthpoint Biotherapeutics, Fort Worth, TX), have only a modest effect on wound healing and need to be administered daily. C7 is a large, stable collagen molecule with a molecular mass of 900 kDa. It is a stable molecule that is relatively resistant to protease degradation. In fact, we have left purified C7 in neutral buffer at room temperature for up to 3 months or at 4 °C for up to 6 months and found no degradation or loss of biological activity (data not shown). The stability of C7, and its large size may offer advantages over GFs for use as a wound healing agent.
As for treatment of RDEB, our data demonstrate that topical rC7 stably incorporated into the mouse DEJ for up to 2 months. The actual turnover time of C7 in human skin is not known. Our study with human RDEB skin equivalents transplanted onto immunodeficient mice shows that human rC7 administered to skin equivalents endures for at least 3 months.20 We also know that one does not have to have 100% of the normal complement of AFs to have physiological normal dermal–epidermal adherence, and in fact, only about 35% of the normal AF complement is needed to prevent clinical skin fragility and blistering.31 With regards to patients with RDEB, it may be possible to apply topically rC7 and improve the patient's skin fragility and bullae/erosion formation with periodic applications. This will be determined by future clinical trial.
We have not done parallel systematic studies to compare experiments comparing the delivering of rC7 by the three different potential administration routes; topical application, intravenous infusion and intradermal injection. We showed previously that the intradermal injection of 20 µg of rC7 was sufficient to restore C7 and reverse the RDEB phenotype in a square centimeter of RDEB skin equivalent.19 In a recently published paper using an intravenous injection approach, we showed that functional correction and clinical benefit was achieved using 60 µg of rC7 in ~1 square centimeter of grafted RDEB mouse skin.32 In the topical rC7 study herein, 30 µg of topical rC7 was used to achieve reversal of the RDEB phenotype. It appears that topical and intradermal routes of delivery may require less rC7 in comparison with intravenously administration of rC7. As we have not compared systematically all three approaches at the same time, we cannot extrapolate and say which route will be the most efficient and efficacious in actual patients with RDEB.
We previously showed that the wounds of mice intravenously injected with gene-corrected RDEB fibroblasts over-expressing C7 demonstrated accelerated wound healing compared with control mice injected with uncorrected RDEB cells.22 In the present study, we demonstrated that rC7 topically delivered to skin wounds accelerated their wound closure. How exogenously delivered rC7 promotes healing is unknown. It is known that the process of wound healing involves a series of synchronized events, namely clot formation, inflammation, re-epithelialization, production of granulation tissue, fibroplasia, angiogenesis, and connective tissue remodeling.2 The migration of keratinocytes is the first essential step for wound re-epithelialization. When the skin is wounded, the keratinocytes come into contact with collagens and dermal glycoproteins, such as fibronectin, and start to migrate across the wound bed.33,34 Our previous studies demonstrated that C7 is the most potent pro-motility matrix among other extracellular cellular matrices (including collagens type I and IV, and fibronectin) for driving human keratinocyte migration. However, during the normal wound healing process, C7 was detected very late and appeared at the DEJ on day 7 after wounding.35 It is possible that exogenously delivered rC7 by topical application during early wound healing will provide a matrix that promotes cell motility and allows keratinocytes to migrate and close the wounds. Consistent with this notion, we showed in this study that rC7-treated wounds demonstrated increased re-epithelialization. This notion is further supported by our data showing that the NC1 domain of C7 that was unable to promote keratinocyte migration in vitro also failed to accelerate skin wound closure in vivo.
Our data in this study showed that rC7 inhibits collagen lattice contraction in vitro. In addition, topical rC7 reduces the number of myofibroblasts, the expression of CTGF and disorganized collagen deposition in the neodermis of healed skin wounds. Myofibroblasts and CTGF are key players in fibrotic processes. These observations raise the question of whether exogenous rC7 topically applied to skin wounds could result in wounds with less scarring and fibrosis. There are several lines of evidence to support this notion. First, patients with RDEB who lack functional C7 heal wounds with significant scarring. Second, hypomorphic RDEB mice that only express C7 at 10% of the normal level exhibit enhanced fibrotic processes with excessive contraction and deposition of extracellular matrix.36 This fibrosis is associated with an upregulation of TGF-β and CTGF, neoexpression of α-SMA–positive cells, and induction of tenascin C. Third, when fetuses in utero are subjected to skin wounds within the first trimester, the skin wounds heal without scars, so-called “scarless healing”.37 It is interesting to note that during fetal life, the fetus is bathed in amniotic fluid and surrounded with an amniotic membrane that is very rich in C7 and laminin 332, two large matrix molecules that improve wound healing.38 In concordance with these observations, amniotic membranes have been used topically on burn wounds, corneal abrasions, and skin wounds to promote re-epithelialization and healing.39 One could speculate that the molecular basis for these clinical observations is that the C7 within amniotic membranes promotes scarless healing in fetal skin wounds.
Many soluble factors participate in wound repair. One factor that has been implicated in scar formation is TGF-β. The TGF-β family of proteins consists of several structurally related but functionally distinct isoforms. In mammals, three isoforms, TGF-β1, -β2, and -β3, have been identified. These isoforms play distinct roles in the modulation of wound repair.2 TGF-β1 and TGF-β2 possess pro-fibrogenic activities, while TGF-β3 exhibits anti-fibrogenic activity.28 In this study, we showed that topical rC7 induced the upregulation of TGF-β3 (anti-fibrogenic isoform) and downregulation of TGF-β2 (the pro-fibrogenic isoform). These data, taken together with the reduction of several scarring parameters (α-SMA expression, collagen deposition and CTGF expression), suggest that rC7 not only accelerates wound closure but also contributes to improve wound healing quality with less scarring by modulating the expression of the TGF-β family.
The mice we used for the wound healing studies are athymic nude mice that are unable to produce T cells, and are therefore immunodeficient. We chose these mice simply because they cannot mount an immune response and produce anti-C7 antibodies after topical application of human C7. In addition, they do not reject grafted human skin or RDEB mouse skin. However, the rC7-mediated accelerated wound closure and reduced expression of α-SMA and CTGF were also observed when wound healing experiments were conducted with immunocompetent mice (data not shown).
Various therapeutic strategies for RDEB are under investigation, including gene, cell and protein therapies, but to date, no consistently low risk, effective treatment is available.40 Currently, there are several “proof-of-principal” clinical studies that have been planned or initiated to study therapeutic approaches for RDEB. Bone marrow/stem cells have been administered to a limited number of patients with RDEB with some success, but also with considerable mortality.15 The intradermal injection of C7-producing, allogeneic, human dermal fibroblasts into patients with RDEB has increased the expression of patients' own mutated C7 at their DEJ and improved their dermal–epidermal adherence.14 However, the injected cells did not persist longer than 2 weeks in the skin of the patients with RDEB'. Using two preclinical animal models, we showed that intradermal injection of C7 expressing fibroblasts, C7 protein and lentivectors expressing C7 into RDEB mice all restored C7 and AFs at the DEJ and significantly prolonged the survival of the RDEB mice.17–21 In a more recently study, we showed that rC7 administered intravenously to mice homed to engrafted RDEB mouse skin, incorporated into the DEJ of the grafts, and restored C7, AFs and dermal–epidermal adherence.32
If topically administered rC7 improves the health of patients with RDEB, it would have many advantages over other treatments such as lack of exposure to live cells, exogenous DNA or RNA or viral vectors and local application. Compared with bone marrow/stem cell transplantation and intravenous or intradermal injection of C7, topically applied rC7 would likely be painless and logistically simple. It would not require sophisticated medical expertise or equipment. Lastly, the topical application of rC7 could promote the closure of the existing wounds of the patient with RDEB. Nevertheless, there are several limitations associated with the topical approach. This includes the inability to treat esophageal lesions that are common in the most severely affected patients with RDEB. In addition, as rC7 does not penetrate intact, unwounded skin and only works locally in existing open wounds, another limitation is that topical rC7 will not prevent the onset of new skin blisters when applied to non-lesional RDEB skin. Lastly, protein replacement therapy requires lifelong repeated administration of rC7 regardless of the mode of administration: intradermal injection, topical application, or intravenous injection.
In summary, our studies demonstrate that rC7 applied topically onto skin wounds stably incorporated into the newly regenerated DEJ of healed wounds and accelerated wound closure. In addition to being a therapy for chronic skin wounds, this strategy may be particularly useful for patients with RDEB who have multiple open wounds and often die from metastatic squamous cell carcinomas arising in chronic skin wounds. The topical application of rC7 may provide a feasible treatment option for not only accelerating wound healing, but also normalizing the devastating cutaneous phenotype of RDEB. These preclinical results are very encouraging though additional studies are needed to determine the safety and efficacy in humans.
Materials and Methods
Purification of rC7. Recombinant C7 was purified from serum-free media from RDEB dermal fibroblasts stably transduced with a lentiviral vector coding for full-length C7 as described.20 Briefly, serum-free media were equilibrated to 5 mmol/l EDTA, 50 µmol/l PMSF, and 50 µmol/l NEM and precipitated with 300 mg/ml ammonium sulfate at 4 °C overnight with constant stirring. Precipitated proteins were collected by centrifuging at 1.2 × 106 g/minute for 1 hour, resuspended, and dialyzed in Buffer A (65 mmol/l NaCl, 25 mmol/l Tris–HCl, pH 7.8). Following dialysis, insoluble material was collected by centrifugation at 8,600g for 20 minute, and the pellet redissolved in Buffer B (50 mmol/l Tris–HCl pH 7.5, 150 mmol/l NaCl, 5 mmol/l EDTA, 2 mmol/l NEM, 2 mmol/l PMSF). The solution was clarified as above, and the supernatant, S1', was passed over a Q-sepharose column (Pharmacia, Piscataway, NJ) equilibrated in the same buffer. Elution was then carried out with a linear gradient from 0.2 to 1.0 mol/l NaCl of appropriate volume size. The rC7 eluted at 0.7–1 mol/l NaCl. The NC1 domain of C7 was purified from 293 cells stably transfected with cDNA encoding for NC1 as described.41
Topical application of rC7 onto mice. We first made a 1.0 × 1.0 cm full-thickness excisional wound by lifting the skin with forceps and removing full thickness skin with a scissors on the mid-back of 8- to 10-week-old athymic nude mice (Simonsen Laboratory, Gilroy, CA). Immediately after wounding, 8–32 µg of rC7 or 30 µg NC1 in a 10% carboxymethylcellulose gel were applied to the wound surface. We then covered the wound with a band-aid and a Coban self-adherent wrap, to prevent desiccation. We treated 30 mice with topical rC7, 10 mice with NC1, and 20 mice with the vehicle as a negative control. Two to 8 weeks after topical application, biopsies from the wounds were obtained and subjected to immunostaining using an antibody specific for human C7 (clone LH 7.2; Sigma, St Louis, MO) or a rabbit polyclonal antibody that recognizes both human and mouse C7.42 To measure the wound size, standardized digital photographs were taken of the wounds at various days after wounding and open wound areas determined with an image analyzer (AlphaEase FC version 4.1.0; Alpha Innotech, Johannesburg, South Africa) on a personal Macintosh computer. The total pixels that covered the unhealed areas were drawn onto the digital photographs using a pattern overlay in ImageJ (http://rsbweb.nih.gov/ij/). The number of pixels covering an open wound area on a given day was divided by the number of pixels spreading over the initial wound on day 0 to obtain the percentage of closure.
For RDEB mouse skin transplantation studies, 1.2 by 1.2 cm of skin from 2 to 5 days old, newborn RDEB mice was transplanted onto the back of athymic nude mice. At 12–14 days after skin transplantation and engraftment, the RDEB skin grafts were either unwounded or wounded with a 6 mm punch biopsy instrument. We then topically applied 30 µg of rC7 (n = 15 mice) or vehicle (n = 4) to the wounded RDEB skin or unwounded RDEB skin (n = 4) as described above.
For evaluating wound healing of human skin, a 1.5 × 1.5 cm square of full-thickness human skin was grafted onto athymic nude mice as previously described.29 Eight weeks after grafting the engrafted human skin was wounded using an 8 mm punch biopsy tool. We then topically applied rC7 (30 µg) or vehicle to the wounds and bandaged as described above. The assessment of wound healing was then performed using area planimetry, as described above for the murine wounds. All animal studies were conducted using protocols approved by the University of Southern California Institutional Animal Use Committee.
Immunofluorescence staining and ultrastructural analysis of tissue. Five-micrometer thick sections of OCT-embedded frozen tissues were cut on a cryostat, fixed for 5 minutes in cold acetone, and air-dried. Immunolabeling of the tissue was performed using standard immunofluorescence methods as described previously.17–20 Briefly, for single- and double-immunofluorescence staining, sections were blocked with M.O.M. Mouse IgG Blocking Reagent (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Primary antibodies were diluted in phosphate buffered saline with 1% bovine serum albumin. For C7 staining, we used monoclonal antibodies against human C7, clone LH 7.2 (Sigma), or a rabbit polyclonal antibody that recognizes both mouse and human C7.42 For double-immunofluorescence staining, we incubated the mouse monoclonal anti-human C7 antibody together with a rabbit polyclonal antibody to both human and mouse C7. For TGF-β staining, we used polyclonal antibodies against TGF-β1 (sc-146, Santa Cruz Biotechnology, Santa Cruz, CA), TGF-β2 (sc-90, Santa Cruz Biotechnology), or TGF-β3 (sc-82, Santa Cruz Biotechnology). All primary antibody dilutions were 1:200. After incubation for 1 hour at room temperature, sections were washed in phosphate buffered saline three times and stained for 1 hour with FITC-conjugated goat anti-mouse IgG1 with or without Cy3-conjugated goat anti-rabbit IgG (Sigma) diluted 1:300 in phosphate buffered saline with 1% bovine serum albumin. Slides were mounted with 40% glycerol. Photographs of stained sections were taken using a Zeiss Axioplan fluorescence microscope equipped with a Zeiss Axiocam MRM digital camera system (Carl Zeiss International, Göttingen, Germany).
Immunogold electron microscopy was performed on the engrafted RDEB mouse skin using a standardized method as described previously.43,44 To assess human AF formation and ultrastructure, 40 micron sections were fixed in 0.1% glutaraldehyde, rinsed in 0.15 mol/l Tris pH 7.5, then incubated in our polyclonal anti-NC1 antibody followed by 5 nm gold secondary antibody and enhancement as described.43,44
Histological analysis of tissues. The mice whose wounds were treated with topical rC7 or vehicle were euthanized at 7 or 14 days after treatment. The wounds, together with unwounded skin margins, were excised and put into 10% formaldehyde. H&E staining was carried out as previously described.45 To show the entire wound, multiple overlapping photographs were taken under a microscope (Nikon, Eclipse TE2000-U, ×4; Nikon, Tokyo, Japan) and used to reconstitute the entire wound. A standard immunohistochemistry staining procedure was carried out as described.46 We used a mouse monoclonal antibody to pan keratin (Clone 80; Abcam, Cambridge, MA), a rabbit polyclonal antibody to PECAM-1 (Clone M-20; Santa Cruz Biotechnology), a mouse monoclonal antibody to α-SMA (Clone 1A4; Dako Denmark A/S, Glostrup, Denmark), a mouse monoclonal antibody to PCNA (Clone PC10; EMD Millipore, Billerica, MA), and a rabbit polyclonal antibody to CTGF (Abcam). All antibodies were used in 1:100 dilutions.
Collagen lattice contraction assay (FPCL). Type I collagen (Sigma) lattices were prepared as previously described.24 Human dermal fibroblasts were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum with antibiotics. The lattices were prepared with a final fibroblast density of 100,000 cells/ml in a 0.6 ml volume per well in 24-well, non-tissue culture plates (Becton Dickinson, Le Pont De Claix, France). After polymerization of the collagen (VitrogenTM, Cohesion Technologies, Palo Alto, CA), the lattices were incubated in 1.0 ml Dulbecco's modified Eagle's medium with or without fetal bovine serum. Either NC1 or rC7 was added to the individual dishes at concentrations ranging from 6.25–30 µg. The collagen lattices were incubated at 37 °C with 5% CO2 and the contraction of the lattices was measured by weight.24 All experiments were carried out in triplicate and repeated three times, and data points and error bars in the figures represent averages and SDs.
Acknowledgments
This work was supported by grants (NIH RO1 AR47981 to M.C, RO1 AR33625 to M.C. and D.T.W., Sponsored Research Project from Lotus Tissue Repair to M.C. and D.T.W.). However, the content is the sole responsibility of the authors and does not necessarily represent the views of Lotus Tissue Repair or its affiliates. We thank Sara Tufa for technical support of immuno-EM. Microscopy services were provided by the Cell and Tissue Imaging Core of USC Research Center for Liver Diseases (NIH grants No. P30 DK048522 and S10 RR022508). M.C. and D.T.W. are consultants for Lotus Tissue Repair, and hold stock in the company. M.C., D.T.W., and the University of Southern California hold patents for recombinant type VII collagen and have filed a Conflict of Interest Declaration with Randoph W Hall, Vice Provost for Research Advancement at the University of Southern California.
References
- Gordois A, Posnett J, Borris L, Bossuyt P, Jönsson B, Levy E, et al. The cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against thromboembolism following major orthopedic surgery. J Thromb Haemost. 2003;1:2167–2174. doi: 10.1046/j.1538-7836.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am J Surg. 1998;176 2A Suppl:48S–54S. doi: 10.1016/s0002-9610(98)00177-9. [DOI] [PubMed] [Google Scholar]
- Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335–346. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- Lin AN, Carter DM.eds) (1992Epidermolysis Bullosa: Basic and Clinical Aspects. Springer-Verlag: New York [Google Scholar]
- Uitto J, Christiano AM. Molecular basis for the dystrophic forms of epidermolysis bullosa: mutations in the type VII collagen gene. Arch Dermatol Res. 1994;287:16–22. doi: 10.1007/BF00370713. [DOI] [PubMed] [Google Scholar]
- Uitto J, Christiano AM. Molecular genetics of the cutaneous basement membrane zone. Perspectives on epidermolysis bullosa and other blistering skin diseases. J Clin Invest. 1992;90:687–692. doi: 10.1172/JCI115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente MG, Chung LC, Ryynänen J, Woodley DT, Wynn KC, Bauer EA, et al. Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proc Natl Acad Sci USA. 1991;88:6931–6935. doi: 10.1073/pnas.88.16.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101:252–255. doi: 10.1111/1523-1747.ep12365129. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986;103:1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NP, Keene DR, Glanville RW, Bentz H, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem. 1986;261:5638–5644. [PubMed] [Google Scholar]
- Bruckner-Tuderman L, Nilssen O, Zimmermann DR, Dours-Zimmermann MT, Kalinke DU, Gedde-Dahl T, Jr, et al. Immunohistochemical and mutation analyses demonstrate that procollagen VII is processed to collagen VII through removal of the NC-2 domain. J Cell Biol. 1995;131:551–559. doi: 10.1083/jcb.131.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T, Gammon L, Liu L, Mellerio JE, Dopping-Hepenstal PJ, Pacy J, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2008;128:2179–2189. doi: 10.1038/jid.2008.78. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–639. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urda S, Thyagarajan B, Keene DR, Lin Q, Fang M, Calos MP, et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med. 2002;8:1166–1170. doi: 10.1038/nm766. [DOI] [PubMed] [Google Scholar]
- Chen M, Kasahara N, Keene DR, Chan L, Hoeffler WK, Finlay D, et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat Genet. 2002;32:670–675. doi: 10.1038/ng1041. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Krueger GG, Jorgensen CM, Fairley JA, Atha T, Huang Y, et al. Normal and gene-corrected dystrophic epidermolysis bullosa fibroblasts alone can produce type VII collagen at the basement membrane zone. J Invest Dermatol. 2003;121:1021–1028. doi: 10.1046/j.1523-1747.2003.12571.x. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Keene DR, Atha T, Huang Y, Ram R, Kasahara N, et al. Intradermal injection of lentiviral vectors corrects regenerated human dystrophic epidermolysis bullosa skin tissue in vivo. Mol Ther. 2004;10:318–326. doi: 10.1016/j.ymthe.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Keene DR, Atha T, Huang Y, Lipman K, Li W, et al. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med. 2004;10:693–695. doi: 10.1038/nm1063. [DOI] [PubMed] [Google Scholar]
- Remington J, Wang X, Hou Y, Zhou H, Burnett J, Muirhead T, et al. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther. 2009;17:26–33. doi: 10.1038/mt.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley DT, Remington J, Huang Y, Hou Y, Li W, Keene DR, et al. Intravenously injected human fibroblasts home to skin wounds, deliver type VII collagen, and promote wound healing. Mol Ther. 2007;15:628–635. doi: 10.1038/sj.mt.6300041. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Hou Y, Martin S, Li W, Chen M. Characterization of molecular mechanisms underlying mutations in dystrophic epidermolysis bullosa using site-directed mutagenesis. J Biol Chem. 2008;283:17838–17845. doi: 10.1074/jbc.M709452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YP, Nien YD, Garner WL. Recombinant human platelet-derived growth factor and transforming growth factor-beta mediated contraction of human dermal fibroblast populated lattices is inhibited by Rho/GTPase inhibitor but does not require phosphatidylinositol-3' kinase. Wound Repair Regen. 2002;10:169–176. doi: 10.1046/j.1524-475x.2002.10801.x. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Yamauchi M, Wynn KC, Mechanic G, Briggaman RA. Collagen telopeptides (cross-linking sites) play a role in collagen gel lattice contraction. J Invest Dermatol. 1991;97:580–585. doi: 10.1111/1523-1747.ep12481920. [DOI] [PubMed] [Google Scholar]
- Akasaka Y, Ono I, Tominaga A, Ishikawa Y, Ito K, Suzuki T, et al. Basic fibroblast growth factor in an artificial dermis promotes apoptosis and inhibits expression of alpha-smooth muscle actin, leading to reduction of wound contraction. Wound Repair Regen. 2007;15:378–389. doi: 10.1111/j.1524-475X.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108 (Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Kim YH, Woodley DT, Wynn KC, Giomi W, Bauer EA. Recessive dystrophic epidermolysis bullosa phenotype is preserved in xenografts using SCID mice: development of an experimental in vivo model. J Invest Dermatol. 1992;98:191–197. doi: 10.1111/1523-1747.ep12555849. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Männikkö M, Klement JF, Whitaker-Menezes D, Murphy GF, Uitto J. Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: a model for recessive dystrophic epidermolysis bullosa. J Cell Sci. 1999;112 (Pt 21):3641–3648. doi: 10.1242/jcs.112.21.3641. [DOI] [PubMed] [Google Scholar]
- Kern JS, Loeckermann S, Fritsch A, Hausser I, Roth W, Magin TM, et al. Mechanisms of fibroblast cell therapy for dystrophic epidermolysis bullosa: high stability of collagen VII favors long-term skin integrity. Mol Ther. 2009;17:1605–1615. doi: 10.1038/mt.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley DT, Wang X, Amir M, Hwang B, Remington J, Hou Y, et al. 2013Intravenously Injected Recombinant Human Type VII Collagen Homes to Skin Wounds and Restores Skin Integrity of Dystrophic Epidermolysis Bullosa. J Invest Dermatole-pub ahead of print) [DOI] [PMC free article] [PubMed]
- Kirfel G, Herzog V. Migration of epidermal keratinocytes: mechanisms, regulation, and biological significance. Protoplasma. 2004;223:67–78. doi: 10.1007/s00709-003-0031-5. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, Woodley DT. Mechanisms of human skin cell motility. Histol Histopathol. 2004;19:1311–1324. doi: 10.14670/HH-19.1311. [DOI] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch A, Loeckermann S, Kern JS, Braun A, Bösl MR, Bley TA, et al. A hypomorphic mouse model of dystrophic epidermolysis bullosa reveals mechanisms of disease and response to fibroblast therapy. J Clin Invest. 2008;118:1669–1679. doi: 10.1172/JCI34292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin. Int J Dermatol. 2011;50:85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD, 2nd, Guo Z, Hopkinson S, Lazar AJ, Brenn T, et al. A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4A activated in wound healing and senescence. Am J Pathol. 2006;168:1821–1837. doi: 10.2353/ajpath.2006.051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelford JD, Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979;134:833–845. doi: 10.1016/0002-9378(79)90957-8. [DOI] [PubMed] [Google Scholar]
- Uitto J. Molecular therapeutics for heritable skin diseases. J Invest Dermatol. 2012;132 E1:E29–E34. doi: 10.1038/skinbio.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Marinkovich MP, Veis A, Cai X, Rao CN, O'Toole EA, et al. Interactions of the amino-terminal noncollagenous (NC1) domain of type VII collagen with extracellular matrix components. A potential role in epidermal-dermal adherence in human skin. J Biol Chem. 1997;272:14516–14522. doi: 10.1074/jbc.272.23.14516. [DOI] [PubMed] [Google Scholar]
- Chen M, Petersen MJ, Li HL, Cai XY, O'Toole EA, Woodley DT. Ultraviolet A irradiation upregulates type VII collagen expression in human dermal fibroblasts. J Invest Dermatol. 1997;108:125–128. doi: 10.1111/1523-1747.ep12332300. [DOI] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Morris NP, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986;103:1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR.1994Fibrillin: monomers and microfibrils. Ruoslahti E.andEngvall E.eds). Methods in Enzymology vol. 245Academic Press: New York; pp. 47–50. [DOI] [PubMed] [Google Scholar]
- van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J, Kim R, et al. A fragment of secreted Hsp90a carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J Clin Invest. 2011;121:4348–4361. doi: 10.1172/JCI46475. [DOI] [PMC free article] [PubMed] [Google Scholar]