Abstract
Fracture healing involves rapid stem and progenitor cell migration, homing, and differentiation. SDF-1 (CXCL12) is considered a master regulator of CXCR4-positive stem and progenitor cell trafficking to sites of ischemic (hypoxic) injury and regulates their subsequent differentiation into mature reparative cells. In this study, we investigated the role of SDF-1/CXCR4 signaling in fracture healing where vascular disruption results in hypoxia and SDF-1 expression. Mice were injected with AMD3100, a CXCR4 antagonist, or vehicle twice daily until euthanasia with the intent to impair stem cell homing to the fracture site and/or their differentiation. Fracture healing was evaluated using micro-computed tomography, histology, quantitative PCR, and mechanical testing. AMD3100 administration resulted in a significantly reduced hyaline cartilage volume (day 14), callus volume (day 42) and mineralized bone volume (day 42) and reduced expression of genes associated with endochondral ossification including collagen Type 1 alpha 1, collagen Type 2 alpha 1, vascular endothelial growth factor, Annexin A5, nitric oxide synthase 2, and mechanistic target of rapamycin. Our data suggest that the SDF-1/CXCR4 signaling plays a central role in bone healing possibly by regulating the recruitment and/or differentiation of stem and progenitor cells.
Keywords: fracture, SDF-1, CXCR4, AMD3100, adult-derived stem cells
Recruitment of stem and progenitor cells to areas of bone damage is important for remodeling and repair.1 Mesenchymal stem cell (MSC) populations may be a source of new chondrogenic and osteogenic cells, endothelial progenitor cells (EPCs) play a role in revascularization and osteoclasts are derived from hematopoietic stem cell (HSC) populations. However, the site of origin of MSCs in particular is controversial. In a normal fracture, it is likely that recruited progenitors originate from a combination of sources including bone marrow,2,3 periosteum,4 blood vessel walls,5 and peripheral blood.6–8 What remains unclear are the cellular mechanisms that drive their migration to the site of injury and regulate their differentiation.
Progenitors are recruited to the fracture site in the initial stages of healing (day 1) and proliferate around day 3.9 At this time, the fracture environment is complex: vascular disruption during fracture creates a localized hypoxic environment10 that acts as a powerful regulatory stimulus for many cells, including MSCs and bone cells.11,12 New bone formation is thought to take place under low oxygen tension.10 Hypoxic tissues express genes that increase cell survival under low oxygen conditions and re-establish vasculature for oxygen delivery.13 Additionally, hypoxia induces the production of chemotactic factors implicated in cell migration, differentiation, and bone formation.11,14
Hypoxia-induced tissue damage can increase the production of chemokines, including stromal cell-derived factor-1 (SDF-1, CXCL12), which is considered to be a master regulator of cell trafficking and to play a role in cell survival, growth, and development.15 SDF-1 belongs to the CXC family and binds specifically to chemokine (CXC motif) receptor type 4 (CXCR4)16 to promote the chemotactic recruitment of a number of cell types, including MSCs, EPCs, and HSCs. Tissue-committed CXCR4-positive stem cells follow SDF-1 gradients in vitro17 and SDF-1 plays a major role in their subsequent differentiation.18–20 SDF-1 stimulates chondrocyte hypertrophy,21 regulates BMP2-stimulated osteogenic differentiation,18 mediates EPC differentiation by enhancing cell adhesion,20 and promotes early osteoclast differentiation.19 In addition, CXCR4 regulates osteoblast development in post-natal bone.22 SDF-1 expression and release increases rapidly at sites of ischemic tissue damage23 and in cells exposed to hypoxia in vitro and in vivo.14 Together, these studies indicate that the SDF-1/CXCR4 axis may play a pivotal role in cell migration and differentiation at a fracture site.
In this study, we examined the role of SDF-1/ CXCR4 signaling in bone repair by using a CXCR4 antagonist, AMD3100,24 in a murine model of fracture healing. Our hypothesis was that AMD3100 treatment, by inhibiting the recruitment and/or differentiation of stem and progenitor cells, would impair fracture healing. We show that long-term antagonist treatment resulted in a significant reduction in expression of genes associated with endochondral ossification, and also significantly reduced cartilage volume and callus size. We conclude that SDF-1/ CXCR4 signaling plays a central role in bone healing possibly by controlling the recruitment and differentiation of osteo-chondrogenic, endothelial and hematopoietic progenitors.
MATERIALS AND METHODS
Animals
Transverse fractures were created in 115 13- to 14-week-old male C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). Half of the mice were injected with CXCR4 antagonist AMD3100 (Sigma, St. Louis, MO) at 1.25 mg/kg25 subcutaneously every 12 h until euthanasia, starting the morning before surgery and fracture. Control mice were injected with the same volume of saline. This dose and dosing regimen has been shown to significantly reduce the growth of murine breast cancer 4T1 cell metastases in the murine lung over 6 days,26 reduce the growth of glioblastoma xenografts in mice over 3 weeks by inhibiting proliferation and promoting apoptosis of tumor cells27 and reduce the recruitment of CXCR4+/CD45+cells to a murine glioblastoma and reduce neovascularization over a 2-week treatment period.25 Mice were euthanized via CO2 asphyxiation followed by cervical dislocation 3, 7, 14, and 42 days after surgery (Fig. 1A). All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
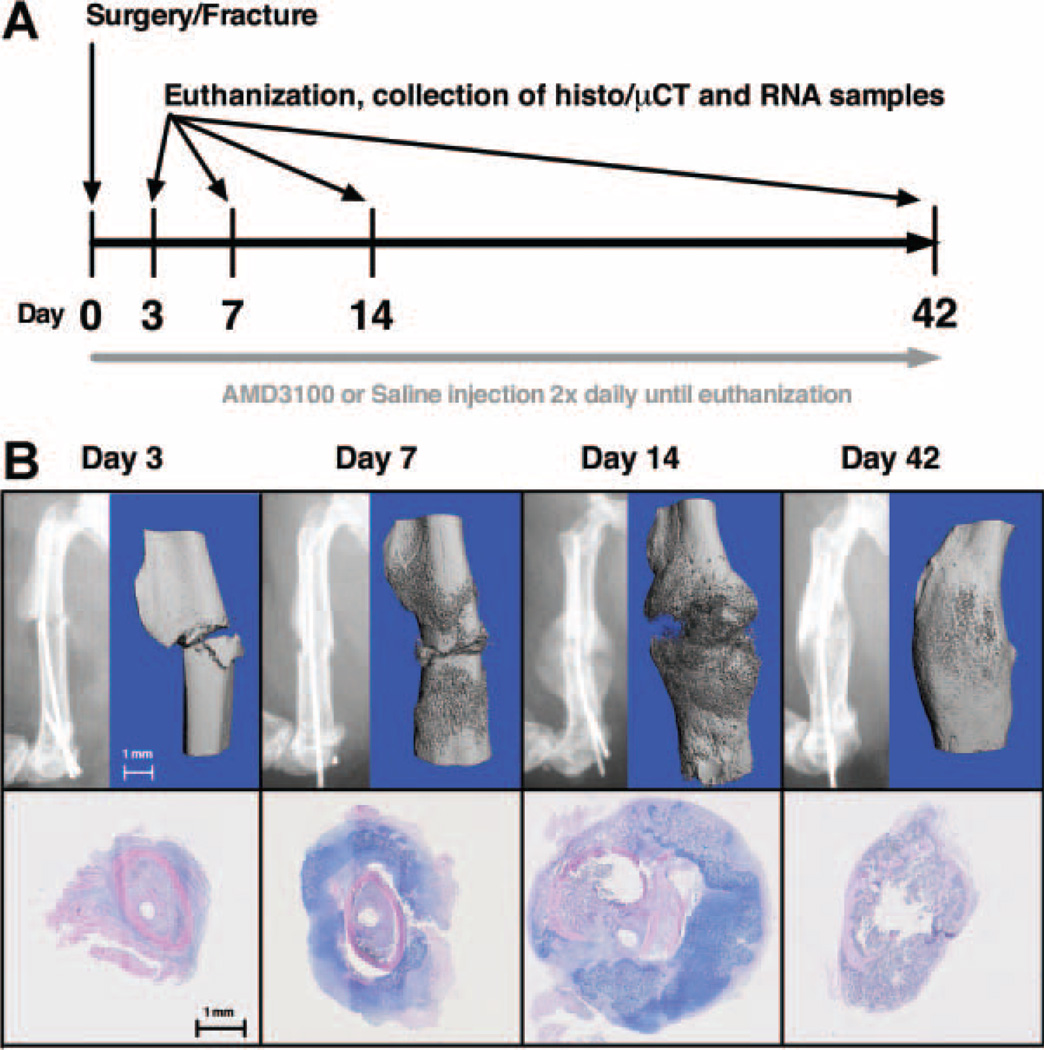
Figure 1.
(A) Experimental design: fractures were created on day 0 and AMD3100 or saline was administered every 12 h for the duration of the study. Animals were euthanized on days 3, 7, 14, and 42 and specimens collected for histology, μCT, and QTPCR evaluation. (B) Fracture model: radiographs, μCT-generated 3-D renditions, and H&E/alcian blue histological staining revealed changes to the structure and composition of the fracture callus that were consistent with a normal pattern of healing in our model.
Surgical Procedure
All mice were anesthetized using 1.5–2% isoflurane and injected with 0.05 mg/kg buprenorphine (Hospira Inc., Lake Forest, IL) for pain control. Eighteen mice were excluded from the study due to failed surgeries or misalignment of pin during healing. Consistent transverse fractures were created and stabilized by a 0.01 in. stainless steel wire pin (Small Parts, Seattle, WA) inserted in the right femur via the method developed by Bonnarens and Einhorn28,29 using a modified and improved fracture apparatus.30 Mice were radiographed to ascertain pin positioning and fracture configuration. The animals were fully weight-bearing and unrestricted activity was permitted postoperatively. Buprenorphine (0.05 mg/kg) was administered every 12 h for 48-h post-fracture. Saline-treated animals underwent normal fracture healing consistent with published reports (Fig. 1B).
Histology, Immunohistochemistry, and In Situ Hybridization
One hour before euthanasia, 37 mice were injected (IP) with 60 mg/kg Hypoxyprobe™ (pimonidazole hydrochloride; Hypoxyprobe, Inc., Burlington, MA). When diffused into hypoxic tissues, pimonidazole hydrochloride forms protein adducts in cells with a pO2 < 10 mmHg (1.4% O2). Mice were then euthanized, and both fractured and intact contralateral femurs were X-rayed (Faxitron, Lincolnshire, IL), extracted, any pins removed and placed into 4% phosphate-buffered formalin for 2–3 days at 4°C. Femurs were evaluated by micro- computed tomography (μCT) and then decalcified (2.3:1 solution of 10% EDTA and 4% phosphate-buffered formalin) for 10 days at 4°C. Femurs were embedded in paraffin and serial-sectioned transversely through the whole fracture callus at 500 mm intervals.31 Slides were then deparaffinized, rehydrated, and stained with one of the following: hematoxylin and eosin (H&E) and alcian blue, immunohistochemically for pimonidazole protein adducts (clone 4.3.11.3 mouse FITCMAb, Hypoxyprobe, Inc.), SDF-1 protein (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), or probed for SDF-1 RNA (F-GTCCTCTTGCTGTCCAGCTC; R-TAATTTCGGGTCAATGCACA) or CXCR4 RNA (F-TTCTCATCCTGGCCTTCATC; R-ATGGAGTTGAGTGCATGCTG with T7 sequence) via in situ hybridization. T7 sequence was generated using T7 Transcription Kit (Roche, Indianapolis, IN). After blocking with Fc Receptor Blocker (Innovex Biosciences, Richmond, CA) for 2 h and Background Buster (Innovex Biosciences) for another 2 h, visualization for immunohistochemistry was achieved using Stat-Q AEC kit (Innovex Biosciences). Colorimetric visualization of in situ hybridization was performed using AP substrate-chromogen NBT/ BCIP (Sigma). Immunohistochemistry and in situ hybridization slides were counterstained using hematoxylin (Sigma). The compartment volume of hyaline cartilage was estimated using an established stereological point counting technique using a point grid overlay.32
μCT Analysis
Femurs from 33 mice were scanned at 55 KVP, 145 mA, with a voxel size of 6 mm (Scanco Medical μCT 35, Brüttisellen, Switzerland) in 4% phosphate-buffered formalin. The volume of interest was 1,157 mm3, but only slices with callus were included in the analysis. The outer and inner edges of the calluses were manually contoured in order to exclude the original cortical bone from the measurements as previously described.33 A fixed, global threshold was used to distinguish mineralized from non-mineralized tissue with the lower limit corresponding to a mineral density of 421 mg HA/cm3 and the upper limit corresponding to 3,000 mg HA/cm3. Then, femurs were evaluated with a Gaussian filter (sigma = 0.8, support = 1.0) for noise reduction. Total callus volume (TV), mineralized callus volume (BV), fraction of mineralized callus (BV/TV), bone mineral density (BMD), and bone tissue mineral density (TMD) were calculated.
Mechanical Testing
Right and left femurs from six AMD3100- and eight saline-treated mice were harvested 42 days after fracture. Femurs were potted between two aluminum blocks with Wood’s metal (McMaster-Carr, Cleveland, OH). Femurs were secured in a materials testing machine (5800R; Instron, Norwood, MA) with an effective testing length of 6.5 mm. Preloading was performed with three cycles to a target rotation of ±0.5° at 1° per second. Torsional test to failure was performed such that the femoral head was externally rotated to 45° at 1° per second. Time, position, and torque data were recorded at 30 Hz, and torsional stiffness (Nm/degree), yield point (Nm), and maximum torque (Nm) were calculated. Peak force was identified as the maximum torque achieved at <20° rotation (there are interactions between fractured bone segments beyond 20° that can create large torques). All bones failed within 20° of rotation.
Quantitative PCR Analysis
The callus was extracted from 46 fractured femurs by cutting the bone 2 mm away from each edge of the callus. An equal length of the diaphysis was extracted from the contralateral limbs. The bone samples were then flash-frozen in liquid nitrogen and processed using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA was purified using Qiaquick RNeasy mini-kit (Qiagen, Valencia, CA) and cDNA was generated from total RNA using the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative reverse transcription PCR (qPCR) was performed using primer and TaqMan probe sets (Applied Biosystems, Foster City, CA) and QuantiFast Probe PCR kit (Qiagen) on a Mastercycler realplex2 (Eppendorf, Westbury, NY). Amplification conditions were 95°C for 3 min, followed by 40 cycles at 95°C for 3 s and 60°C for 30 s. Quantitative PCR results were first normalized to ActB (Mm00607939_s1) transcript to yield ΔCt, and then normalized to contralateral limb to yield ΔΔCt. Results are expressed as 2−ΔΔCt .34 Genes probed were collagen Type 1 alpha 1 (Col1a1, Mm00801666_g1), collagen Type 2 alpha 1 (Col2a1, Mm01309565_m1), collagen Type 10 alpha 1 (Col10a1, Mm00487041_m1), SDF-1 (Sdf1, Mm00445553_m1), bone sialoprotein (Ibsp, Mm00492555_m1), vascular endothelial growth factor (Vegf, Mm01281449_m1), Annexin A5 (AnxA5, Mm01293059_m1), early growth response 1 (Egr1, Mm00656724_m1), nitric oxide synthase 2 (Nos2, Mm00440502_m1), and mechanistic target of rapamycin (mTOR; Frap1, Mm00444968_m1).
Statistical Analysis
Comparisons between AMD3100 and control groups at each time point were evaluated using one- and two-tailed Student’s t-tests (GraphPad Software, Inc., La Jolla, CA). Differences were considered significant at p < 0.05.
RESULTS
Immunohistochemical and In Situ Hybridization Analysis
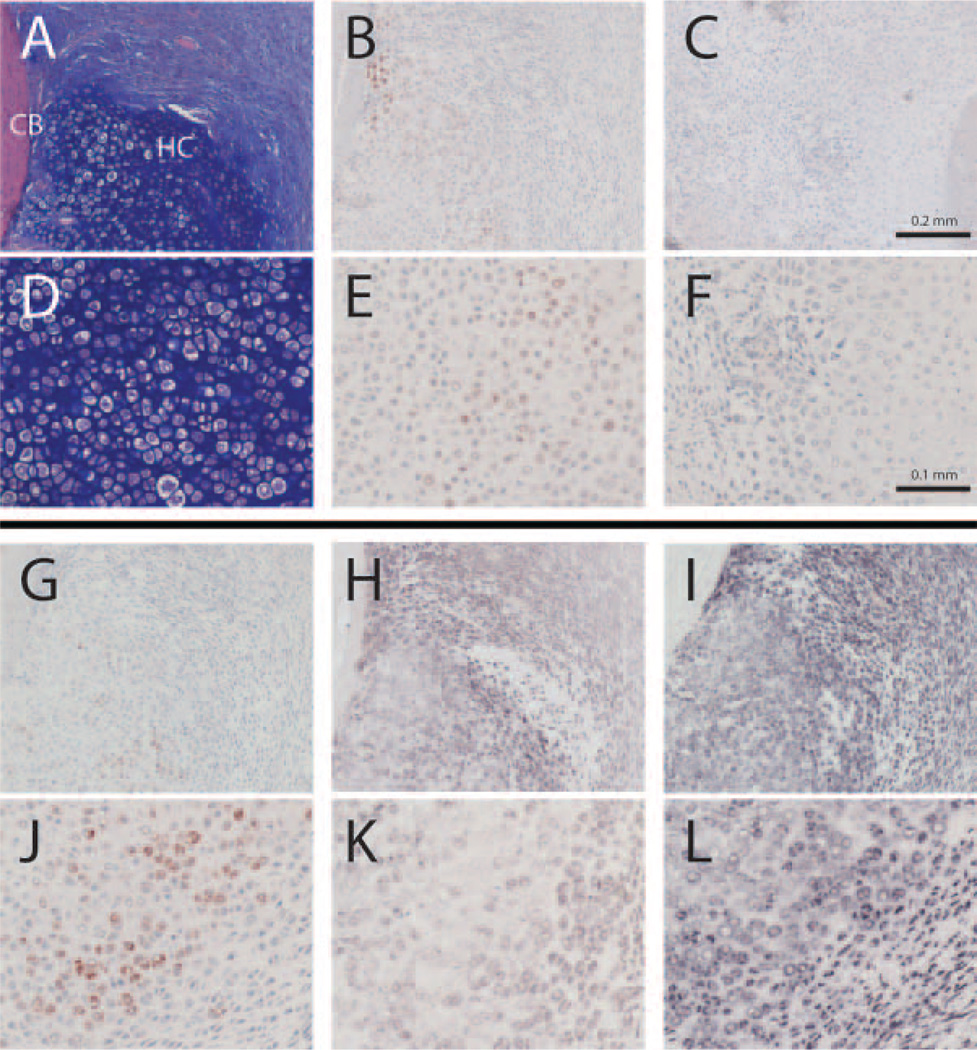
Regions of hypoxia were identified by Hypoxyprobe™ staining. Hypertrophic cartilage and regions of immature hyaline cartilage close to pre-existing cortical bone stained positively for Hypoxyprobe™ as well as SDF-1 protein (Fig. 2A/D, B/E, and G/J). We saw no differences in Hypoxyprobe™ staining between AMD3100 and saline-treated mice (data not shown). In order to verify the validity of our immunohistochemical stains, we confirmed that Hypoxyprobe™ staining with no Hypoxyprobe™ injection prior to euthanasia was negative (Fig. 2C/F) and that SDF-1 RNA staining via in situ hybridization co-localizes with SDF-1 protein staining (Fig. 2H/K). In addition, almost all cells stained positively for CXCR4 RNA via in situ hybridization, especially hypertrophic and prehypertrophic chondrocytes, osteoblasts, osteoclasts, and undifferentiated mesenchymal tissue cells (Fig. 2I/L).
Figure 2.
Detection of hypoxia, Sdf-1 protein, and Sdf-1 and CXCR4 RNA in sequential cross-sections through the midcallus on day 7 in the same control fracture. A–C and G–I are at 10× magnification, D–F, and J–L are at 20× magnification. (A/D) H&E/alcian blue staining showing a portion of hyaline cartilage (HC) adjacent to the cortical bone (CB). (B/E) Pimonidazole hydrochloride (Hypoxyprobe™) immunohistochemical staining. (C/ F) Negative Hypoxyprobe™ immunohistochemical control in a mouse where no Hypoxyprobe™ was injected prior to euthanization. (G/J) Fracture callus immunohistochemical staining of Sdf- 1 protein that colocalizes with Hypoxyprobe™. (H/K) in situ hybridization staining of Sdf-1 RNA co-localizes with immunohistochemical staining of SDF-1 protein, confirming the validity of the SDF-1 immunohistochemical stain. (I/L) in situ hybridization staining of CXCR4.
μCT and Histomorphometric Analysis
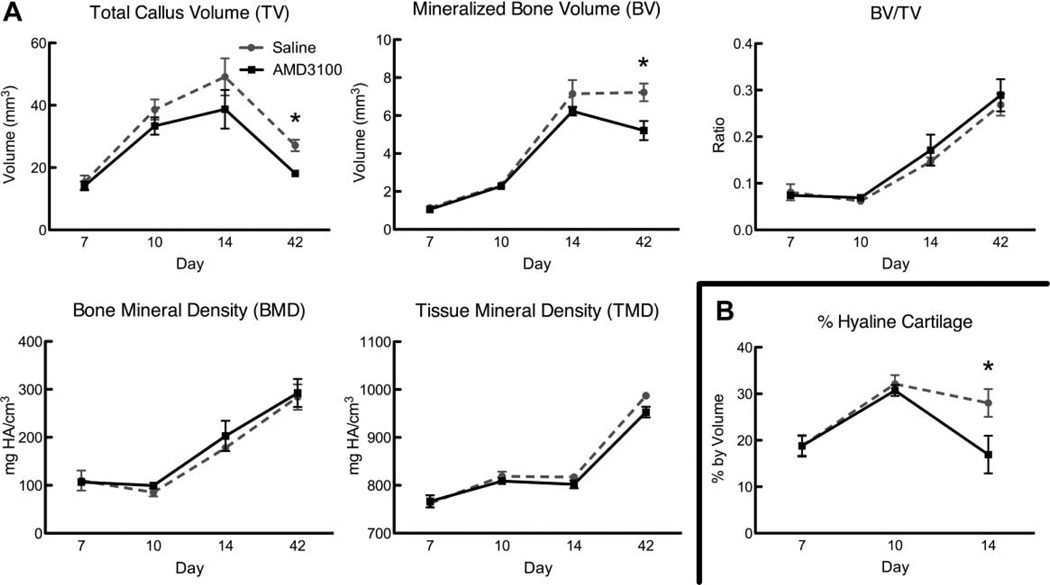
TV and BV were similar in AMD3100- and saline-treated control mice on days 7 and 10 (Fig. 3A). On day 14, there was a trend for both lower TV (p = 0.2927) and BV(p = 0.2929) in AMD3100-treated mice compared to controls, which reached statistical significance at day 42 (p = 0.0113 and 0.0422, respectively; Fig. 3A). BV/TV, BMD, and TMD were similar in AMD3100-treated and control mice. In addition AMD3100-treated mice had significantly less cartilage volume at day 14 (p = 0.0364) in the callus compared to controls (Fig. 3B).
Figure 3.
μCT analysis and histomorphometry. (A) μCT evaluation of TV, BV, BV/TV, BMD, and TMD. Each point represents the mean ± SEM, n = 3; *p < 0.05). (B) Percent by volume of hyaline cartilage present in the callus. Each point represents the mean ± SEM, n = 3–4; *p < 0.05). There is no cartilage evident in the callus at day 42 in any of the specimens.
Quantitative PCR Analysis
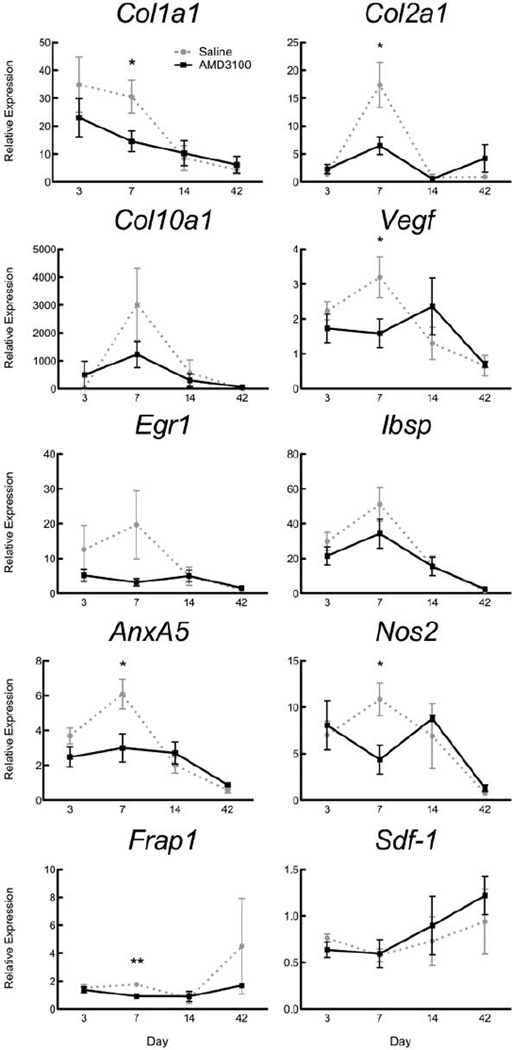
Seven days after fracture, the expression of key genes involved in endochondral ossification, Col1a1, Col2a1, Vegf, AnxA5, Nos2, and Frap1 was significantly lower in the callus of AMD3100-treated mice compared to saline controls (Fig. 4). A similar trend was observed for expression of Col10a1, Egr1, and Ibsp at day 7. In contrast there was a trend for increased Sdf1 expression in AMD3100-treated mice at the later time-points of 14 and 42 days, though this did not reach statistical significance (Fig. 4).
Figure 4.
Expression of key genes involved in fracture repair. Expression levels were measured at days 3–42 in mice treated with AMD3100 or saline. Results are expressed as mean 2−ΔΔCt ± SEM, with expression normalized to beta-actin transcript level and contralateral limb. Day 3, n = 8; day 7, n = 9; days 14 and 42, n = 3; *p < 0.05, **p < 0.01).
Mechanical Testing
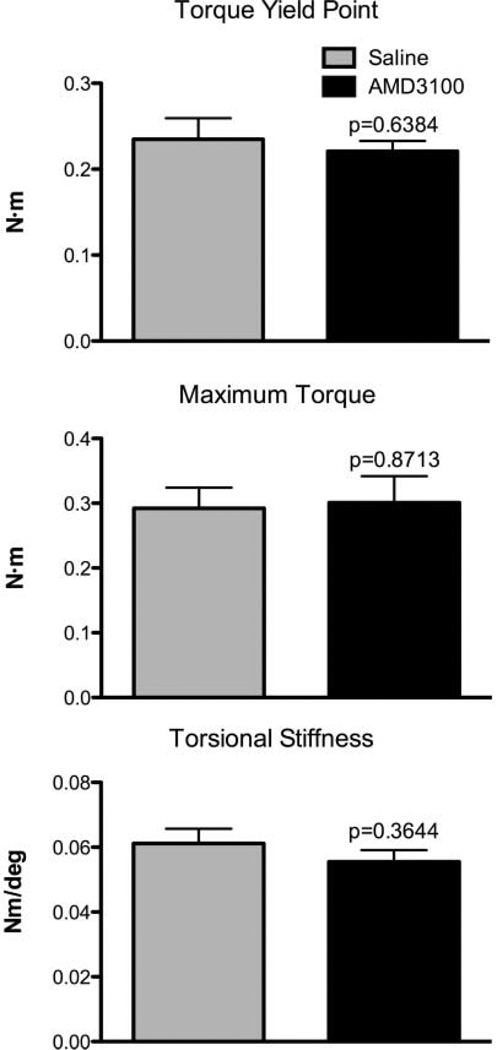
There were no significant differences between AMD3100 and saline-treated groups in any of the mechanical testing parameters examined, although there were non-significant trends suggesting that AMD3100- treated femurs had lower yield point (p = 0.64) and torsional stiffness (p = 0.36; Fig. 5).
Figure 5.
Torsional mechanical testing results of 42-day-old AMD3100 and saline-treated fracture calluses. Evaluation of torsional yield point, maximum torque, and torsional stiffness. Results are expressed as mean ± SEM, n = 6–7.
DISCUSSION
The fracture callus is hypoxic as early as 90 min after fracture and remains so for days and weeks afterwards.10,35–37 We observed strong Hypoxyprobe™ staining in both treated and untreated femurs, indicating tissue hypoxia, in immature and mature hyaline cartilage developing around the original cortical bone diaphysis. As early as the 1960s it has been reported that low oxygen promotes the formation of cartilage.38 Indeed, using a similar chemical marker of hypoxia, Schipani et al.39 demonstrated that the fetal growth plate is hypoxic. Taken together these data suggest that disruption of vasculature and rapid elaboration of the callus result in tissue hypoxia that promotes the formation of cartilage. Ceradini et al.14 showed that expression of SDF-1 is increased in hypoxic tissues. In this study, we show that markers of hypoxia (Hypoxyprobe™) and Sdf-1 mRNA and protein colocalized around regions of immature and mature hyaline cartilage suggesting that SDF-1 levels are increased in these hypoxic areas. The most strongly staining cells for CXCR4 mRNA include hypertrophic and prehypertrophic chondrocytes, osteoblasts, osteoclasts, and undifferentiated mesenchymal tissue cells. CXCR4 expression may be widespread throughout the callus as SDF-1 plays major roles in HSC, EPC, and MSC differentiation.
The effects of the CXCR4/SDF-1 blockade were observed in the structure and size of the callus. Histomorphometric analysis of the callus revealed significantly less cartilage volume after 2 weeks. Similarly, μCT data at 2 weeks showed a trend for reduced total callus volume and bone volume in AMD3100- treated animals, which reached statistical significance at 6 weeks. Interestingly, there was no difference in BMD and TMD suggesting that the quality of bone that was formed in AMD3100-treated mice was similar to that of control mice. In a related study, new bone formation was reduced around bone grafts in mice treated with an SDF-1 neutralizing antibody or with TF14016, a CXCR4 antagonist, as well as in Sdf-1+/− and Cxcr4+/− mice.40 In this same study, BrdU-labeled murine bone marrow-derived stromal cells were identified in the callus around a bone graft 1 week after intravenous injection.40 The number of BrdU-positive cells was inhibited by treatment with TF14016. In contrast, a significantly larger callus developed with enhanced biomechanical properties in a murine tibial fracture study where injection of MSCs increased the number of cells in circulation.41 In this same study, only MSCs expressing CXCR4 were able to home to the fracture site.41
Disruption of the SDF-1/CXCR4 axis also has effects on progenitor cell differentiation. Hosogane et al.18 found that inhibition of the SDF-1/CXCR4 axis during BMP-2-induced osteogenic differentiation of human and murine mesenchymal stem cells with AMD3100,- decreased ALP activity and osteocalcin production, inhibited bone nodule mineralization, and reduced the expression of Runt-related factor-2 (Runx2) and Osterix. In addition, AMD3100 partially inhibited Runx2 expression during murine chondrogenesis.21 The SDF-1/CXCR4 axis also plays an important role in EPC differentiation by enhancing cell adhesion20 and promotes early osteoclast differentiation.19 Taken together these data suggest that the CXCR4/SDF-1 signaling pathway plays a critical role in bone healing. CXCR4/SDF-1 signaling may affect the migration and differentiation of progenitor cells at sites of bone injury. Such cells may contribute directly to tissue healing by engraftment, cell differentiation and tissue elaboration or indirectly through release of paracrine factors to regulate bone healing. Surprisingly, given the significant negative effects of AMD3100 in the fracture callus assessed by μCT, histology and PCR, there was no significant difference in mechanical strength parameters at day 42. It is possible that our mechanical testing conditions were not sensitive enough to detect small differences (3.1–8.2%) in mechanical properties in this model. For example, based on the means and standard deviations for these tests, it would require n = 71 femurs/group in order to observe a significant difference in torsional stiffness between treated and untreated femurs.
The most significant changes in gene expression were observed at day 7, when a large cartilaginous callus is present, as well as de novo bone formation. In AMD3100-treated mice, we saw significant reductions in mRNA expression of Col1a1, Col2a1, Col10a1, Ibsp, and AnxA5, which are involved in the elaboration and subsequent mineralization of cartilage and bone.42 There was also decreased expression of mRNA for Vegf and Egr1, which play critical roles in revascularization.43 Nitric oxide influences bone turnover and increase osteogenic activity.44 Nos2 expression increases after fracture,45 and deletion of the Nos2 gene impairs fracture healing46. In our study, we also saw a significant reduction in mRNA for Nos2 in the fracture callus of animals treated with AMD3100. We also observed significant decreases in mRNA for Frap1, which plays a positive role in MSC proliferation and early osteoblast differentiation,47 chondrocyte differentiation,48 and osteoclast survival.49
We conclude that SDF-1 plays a significant role in bone healing. The mechanism by which SDF-1 exerts its effects remains to be elucidated but likely include effects on cell migration and differentiation. Furthermore SDF-1 exerts its effects on a wide variety of cell types including those from the hematopoietic and mesenchymal lineages and endothelial cells. Thus, SDF-1 has the potential to affect many of the cellular processes involved in bone healing including chondrogenesis and osteogenesis, bone remodeling and vascularization. Further studies will determine how SDF-1 might regulate the trafficking and differentiation of these distinct cell populations and how they each contribute to tissue healing. Such insight may provide us with therapeutic tools to manipulate progenitor cell homing and differentiation for enhanced healing.
ACKNOWLEDGMENTS
Funding was provided by a private grant from Dick and Carolyn Randall. D.C. Genetos was supported by NIH NIAMS R03 AR057547. We acknowledge Tanya Garcia-Nolen for her expertise with μCT analysis and Diane Naydan for developing the IHC protocols. DCG was supported by NIH NIAMS grant AR057547, and DM and GGL were supported by NIH grant NIDDK DK075730; work by DM and GGL was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Footnotes
Conflicts of interest: none to declare.
REFERENCES
- 1.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 2.Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350:557–561. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi K, Ogawa R, Migita M, et al. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 4.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 6.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–497. doi: 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai K, Vasanji A, Drazba JA, et al. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 9.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Brighton CT, Krebs AG. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–332. [PubMed] [Google Scholar]
- 11.Raheja LF, Genetos DC, Yellowley CE. The effect ofoxygen tension on the long-term osteogenic differentiation and MMP/TIMP expression of human mesenchymal stem cells. Cells Tissues Organs. 2010;191:175–184. doi: 10.1159/000235679. [DOI] [PubMed] [Google Scholar]
- 12.Genetos DC, Toupadakis CA, Raheja LF, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J Cell Biochem. 2010;110:457–467. doi: 10.1002/jcb.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 14.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 15.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 16.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 17.Kucia M, Wojakowski W, Reca R, et al. The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF-dependent manner. Arch Immunol Ther Exp (Warsz) 2006;54:121–135. doi: 10.1007/s00005-006-0015-1. [DOI] [PubMed] [Google Scholar]
- 18.Hosogane N, Huang Z, Rawlins BA, et al. Stromal derived factor-1 regulates bone morphogenetic protein 2- induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol. 2010;42:1132–1141. doi: 10.1016/j.biocel.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright LM, Maloney W, Yu X, et al. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36:840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 20.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Kanbe K, Lee M, et al. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341:236–245. doi: 10.1016/j.ydbio.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Liang G, Huang Z, et al. Conditional inactivation of the CXCR4 receptor in osteoprecursors reduces postnatal bone formation due to impaired osteoblast development. J Biol Chem. 2011;286:26794–26805. doi: 10.1074/jbc.M111.250985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 24.Hatse S, Princen K, Bridger G, et al. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 25.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 27.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 29.Manigrasso MB, O’Connor JP. Characterization of a closed femur fracture model in mice. J Orthop Trauma. 2004;18:687–695. doi: 10.1097/00005131-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Marturano JE, Cleveland BC, Byrne MA, et al. An improved murine femur fracture device for bone healing studies. J Biomech. 2008;41:1222–1228. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Gerstenfeld LC, Wronski TJ, Hollinger JO, et al. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20:1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 32.Howard C, Reed MG. Unbiased stereology. New York: Garland Science/BIOS Scientific Publishers; 2005. [Google Scholar]
- 33.Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Aro H, Eerola E, Aho AJ, et al. Tissue oxygen tension in externally stabilized tibial fractures in rabbits during normal healing and infection. J Surg Res. 1984;37:202–207. doi: 10.1016/0022-4804(84)90181-1. [DOI] [PubMed] [Google Scholar]
- 36.Epari DR, Lienau J, Schell H, et al. Pressure, oxygen tension and temperature in the periosteal callus during bone healing—an in vivo study in sheep. Bone. 2008;43:734–739. doi: 10.1016/j.bone.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, Rollins M, Hou H, et al. Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J. 2008;28:14–21. [PMC free article] [PubMed] [Google Scholar]
- 38.Bassett CA, Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. 1961;190:460–461. doi: 10.1038/190460a0. [DOI] [PubMed] [Google Scholar]
- 39.Schipani E, Ryan HE, Didrickson S, et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 41.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HJ, Kirsch T. Collagen/annexin V interactions regulate chondrocyte mineralization. J Biol Chem. 2008;283:10310–10317. doi: 10.1074/jbc.M708456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Li X, Tomin E, et al. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23:671–679. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Watanuki M, Sakai A, Sakata T, et al. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J Bone Miner Res. 2002;17:1015–1025. doi: 10.1359/jbmr.2002.17.6.1015. [DOI] [PubMed] [Google Scholar]
- 45.Diwan AD, Wang MX, Jang D, et al. Nitric oxide modulates fracture healing. J Bone Miner Res. 2000;15:342–351. doi: 10.1359/jbmr.2000.15.2.342. [DOI] [PubMed] [Google Scholar]
- 46.Baldik Y, Diwan AD, Appleyard RC, et al. Deletion of iNOS gene impairs mouse fracture healing. Bone. 2005;37:32–36. doi: 10.1016/j.bone.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Singha UK, Jiang Y, Yu S, et al. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103:434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- 48.Phornphutkul C, Wu KY, Auyeung V, et al. mTOR signaling contributes to chondrocyte differentiation. Dev Dyn. 2008;237:702–712. doi: 10.1002/dvdy.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glantschnig H, Fisher JE, Wesolowski G, et al. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]