Abstract
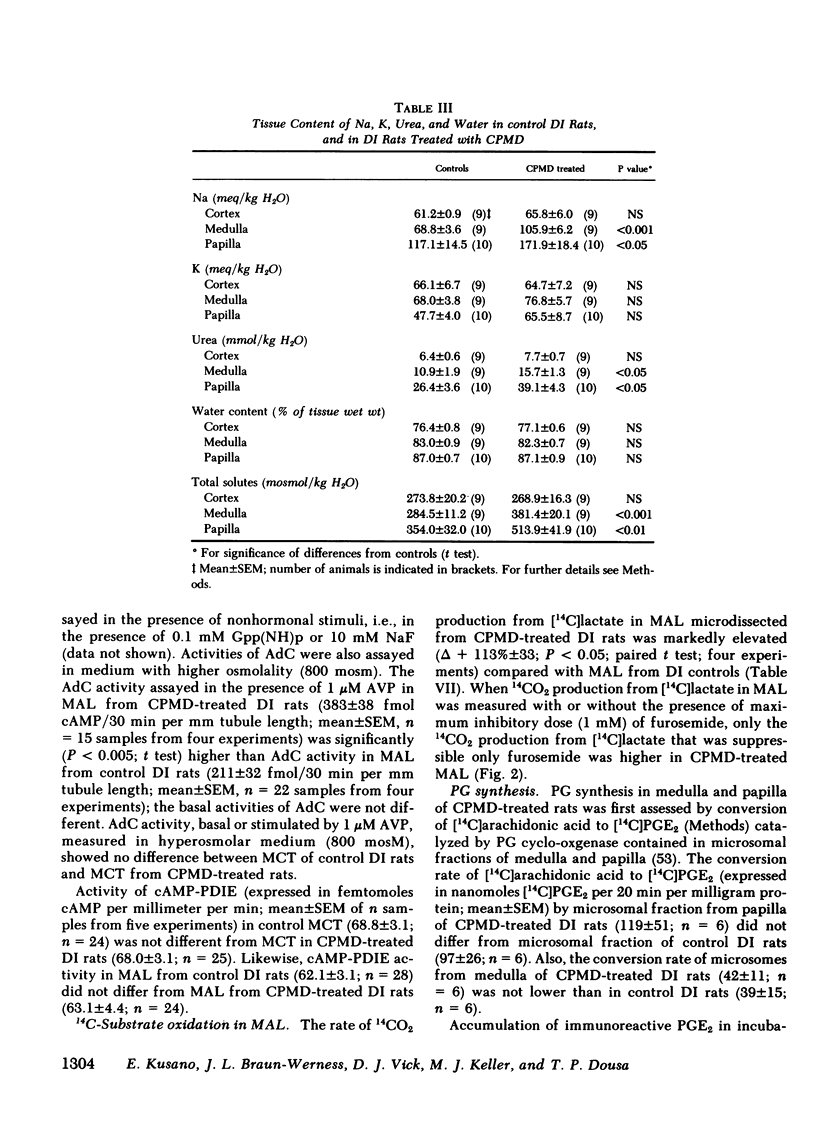
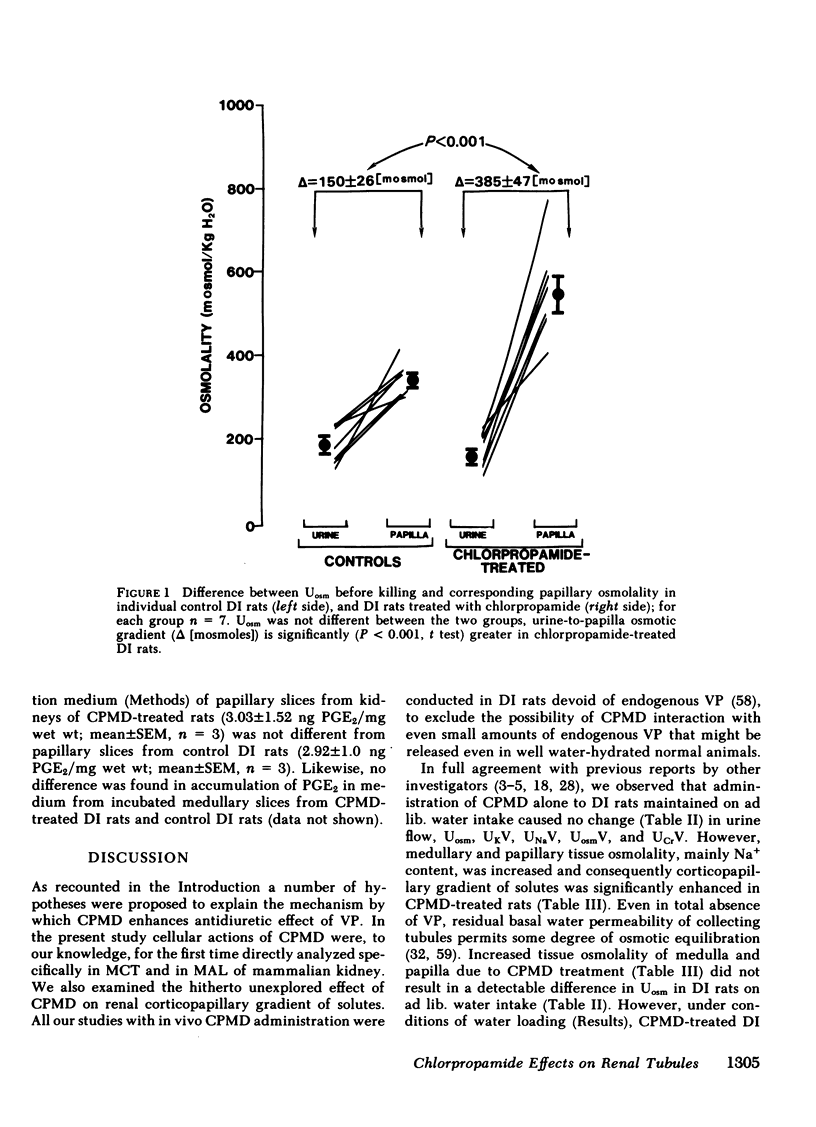
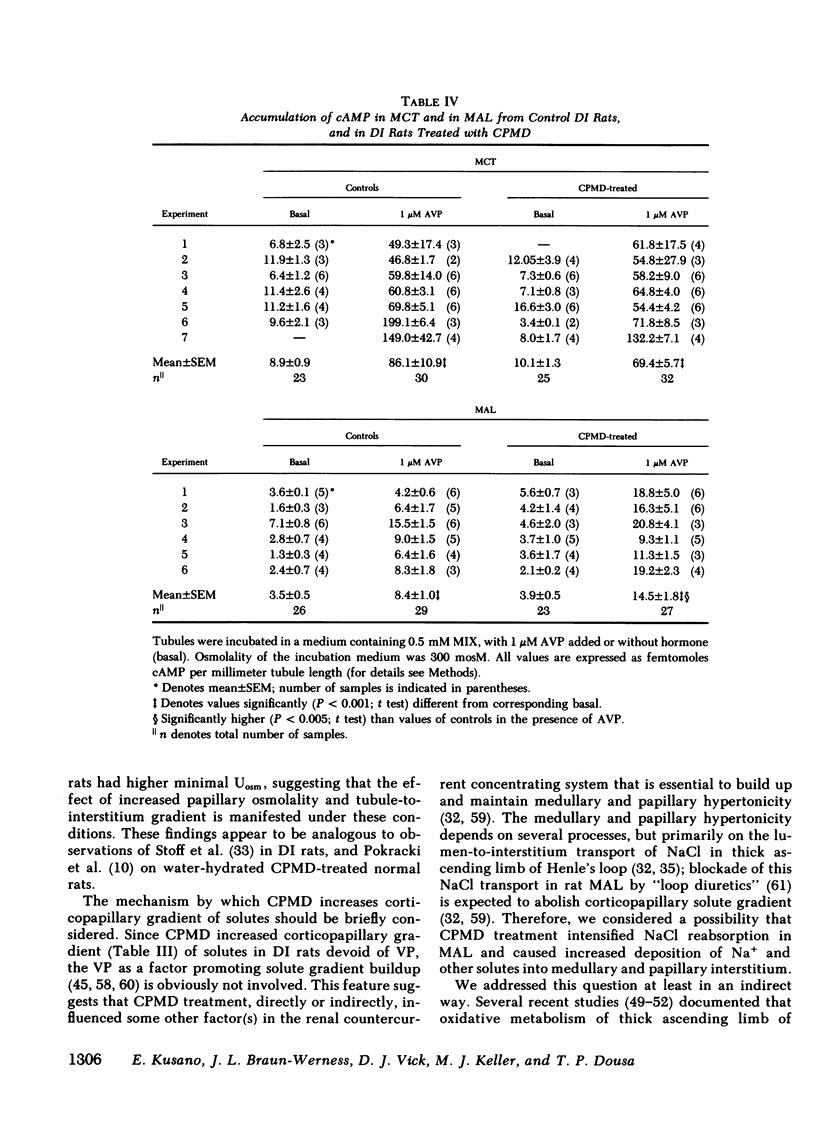
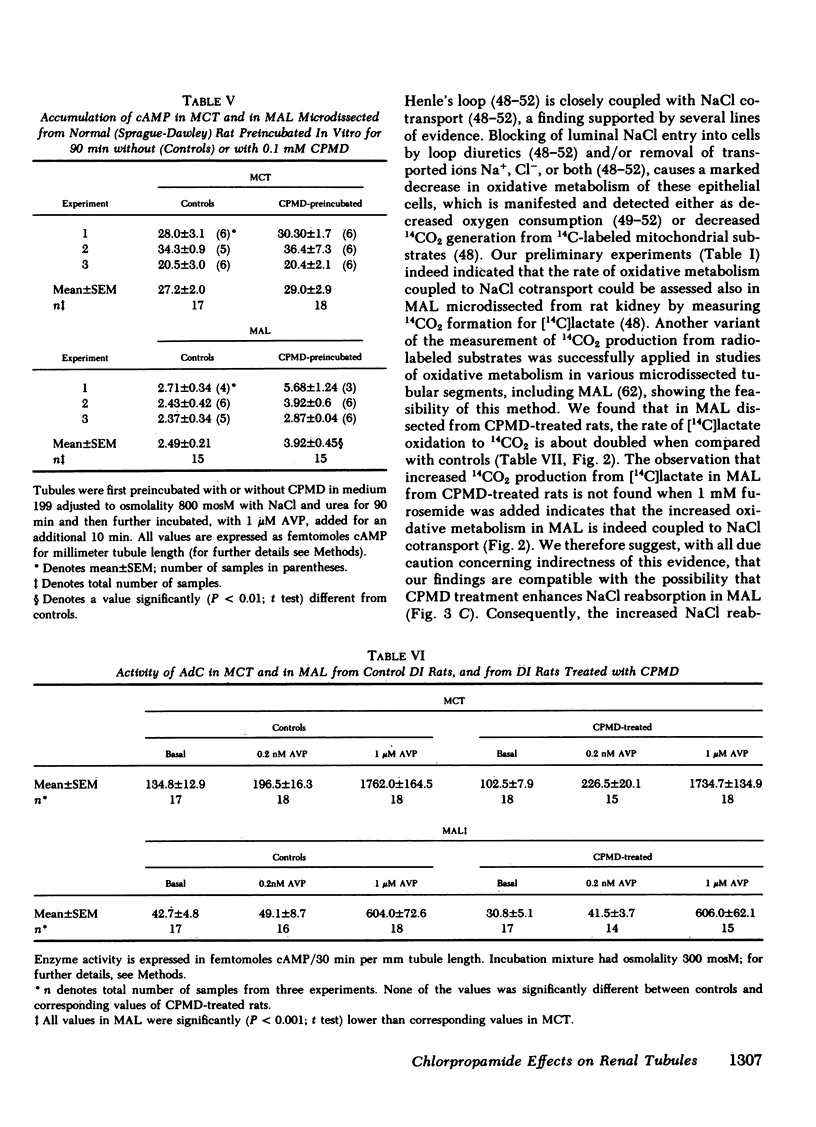
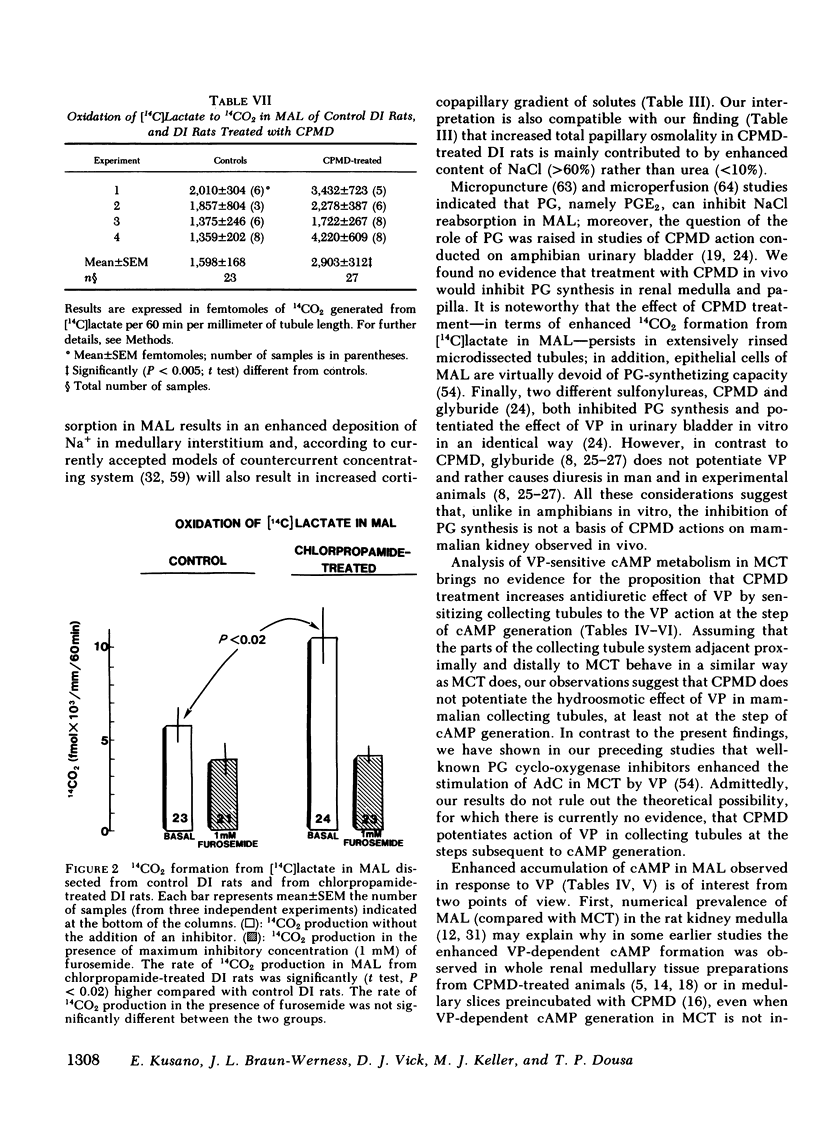
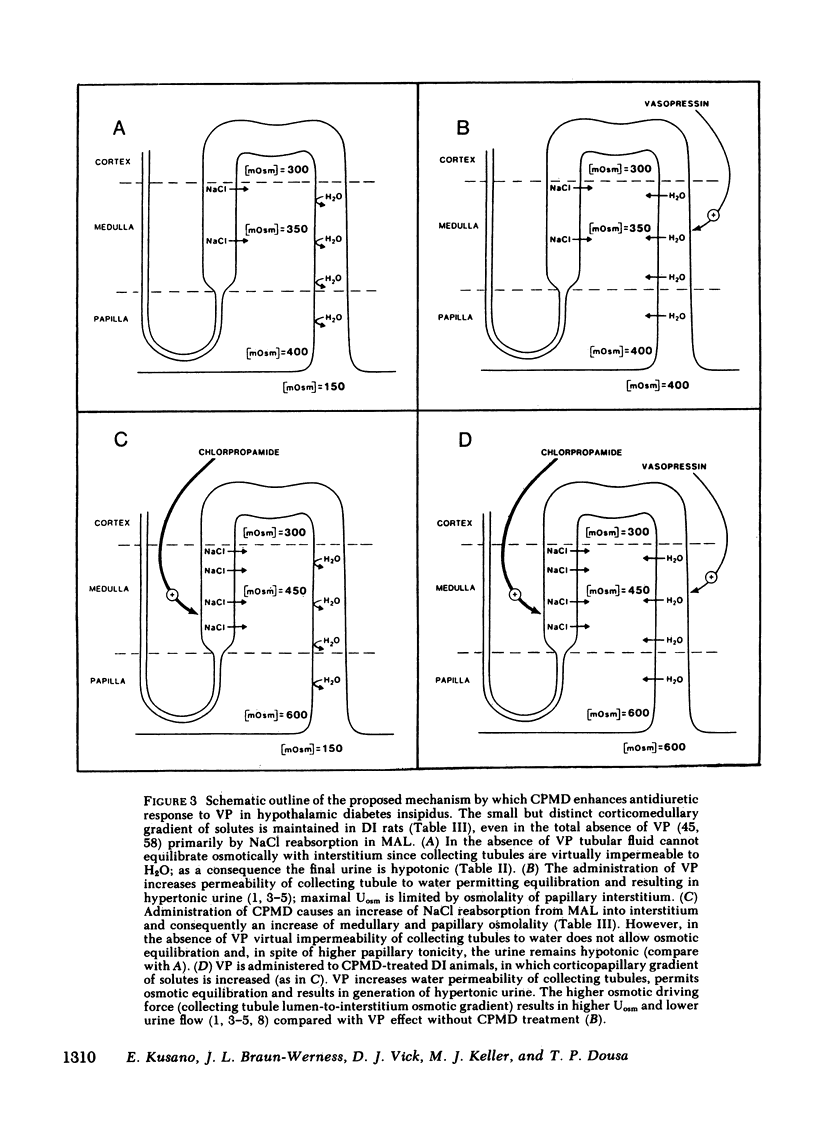
To determine vasopressin (VP)-potentiating effect of chlorpropamide (CPMD), we studied the effect of CPMD in vivo and in vitro in kidneys and in specific tubule segments of rats with hypothalamic diabetes insipidus, homozygotes of the Brattleboro strain (DI rats). Rats on ad lib. water intake were treated with CPMD (20 mg/100 g body wt s.c. daily) for 7 d. While on ad lib. water intake, the urine flow, urine osmolality, urinary excretion of Na +, K +, creatinine, or total solute excretion did not change. However, corticopapillary gradient of solutes was significantly increased in CPMD-treated rats. Higher tissue osmolality was due to significantly increased concentration of Na +, and to a lesser degree urea, in the medulla and papilla of CPMD-treated rats. Consequently, the osmotic gradient between urine and papillary tissue of CPMD-treated rats (delta = 385 +/- 47 mosM) was significantly (P less than 0.001) higher compared with controls (delta = 150 +/- 26 mosM). Minimum urine osmolality after water loading was higher in CPMD-treated DI rats than in controls. Oxidation of [14C]lactate to 14CO2 coupled to NaCl cotransport was measured in thick medullary ascending limb of Henle's loop (MAL) microdissected from control and CPMD-treated rats. The rate of 14CO2 production was higher (delta + 113% +/- 20; P less than 0.01) in CPMD-treated MAL compared with controls, but 14CO2 production in the presence of 10(-3) M furosemide did not differ between MAL from control and from CPMD-treated rats. These observations suggest that CPMD treatment enhances NaCl transport in MAL. Cyclic AMP metabolism was analyzed in microdissected MAL and in medullary collecting tubule (MCT). MCT from control and from CPMD-treated rats did not differ in the basal or VP-stimulated accumulated of cAMP. The increase in cAMP content elicited by 10(-6) M VP in MAL from CPMD-treated rats (delta + 12.0 +/- 1.8 fmol cAMP/mm) was significantly (P less than 0.02) higher compared with MAL from control rats (delta + 5.1 +/- 1.0 fmol cAMP/mm). Preincubation of MAL dissected from Sprague-Dawley rats with 10(-4) M CPMD in vitro increased cAMP accumulation in the presence of VP, but no such enhancement was found in preincubated MCT. Adenylate cyclase activity, basal or stimulated by VP, 5'guanylimidodiphosphate, or by NaF, assayed in isotonic medium did not differ between MAL or MCT from control rats and MAL or MCT from CPMD-treated rats. When assayed in hypertonic medium (800 mosM), the adenylate cyclase activity in the presence of 10(-6) M VP was significantly higher in MAL of CPMD-treated rats. MAL and MCT from control and CPMD-treated rats did not differ in the activities of cAMP phosphodiesterase. The rate of [(14)C]prostaglandin E2 by medullary and papillary microsomes was not different between the control and CPMD-treated rats; likewise, there was no difference in accumulation of immunoreactive prostaglandin E2 in the medium of in vitro incubated medullary or papillary slices prepared from control and CPMD-treated rats. Based on the findings recounted above, we propose a hypothesis that CPMD administration enhances the antidiuretic effect of VP, primarily by increasing medullary and papillary tonicity dye to increased NaCl reabsorption in MAL. There is no evidence that CPMD sensitizes collecting tubules to the action of VP, at least at the camp-generation step. Therefore, increased antidiuretic response to VP in the kidneys of CPMD-treated DI rats is due to enhanced osmotic driving force for water reabsorption (lumen-to-interstitium osmotic gradient) in collecting tubules, rather than due to increased VP-dependent water permeability of tubular epithelium.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELBOOM J. W., BRODSKY W. A., TUTTLE W. S., DIAMOND I. The freezing point depression of mammalian tissues after sudden heating in boiling distilled water. J Gen Physiol. 1958 Jul 20;41(6):1153–1169. doi: 10.1085/jgp.41.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck N., Kim K. S., Davis B. B. Effect of chlorpropamide on cyclic AMP in rat renal medulla. Endocrinology. 1974 Sep;95(3):771–775. doi: 10.1210/endo-95-3-771. [DOI] [PubMed] [Google Scholar]
- Beierwaltes W. H., Schryver S., Olson P. S., Romero J. C. Interaction of the prostaglandin and renin-angiotensin systems in isolated rat glomeruli. Am J Physiol. 1980 Dec;239(6):F602–F608. doi: 10.1152/ajprenal.1980.239.6.F602. [DOI] [PubMed] [Google Scholar]
- Berndt W. O., Miller M., Kettyle W. M., Valtin H. Potentiation of the antidiuretic effect of vasopressin by chlorpropamide. Endocrinology. 1970 May;86(5):1028–1032. doi: 10.1210/endo-86-5-1028. [DOI] [PubMed] [Google Scholar]
- Brooker G., Fichman M. Chlorpropamide and tolbutamide inhibition of adenosine 3'5' cyclic monophosphate phosphodiesterase. Biochem Biophys Res Commun. 1971 Mar 5;42(5):824–828. doi: 10.1016/0006-291x(71)90504-3. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Gagnan-Brunette M., Imbert-Teboul M., Gontcharevskaia O., Montégut M., Clique A., Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest. 1980 Feb;65(2):439–448. doi: 10.1172/JCI109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Effects of colchicine and vinblastine on the cellular action of vasopressin in mammalian kidney. A possible role of microtubules. J Clin Invest. 1974 Aug;54(2):252–262. doi: 10.1172/JCI107760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D. Lithium-induced diuretic effect of antidiuretic hormone in rats. Am J Physiol. 1976 Dec;231(6):1754–1759. doi: 10.1152/ajplegacy.1976.231.6.1754. [DOI] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Dunn M. J., Kinter L. B., Beeuwkes R., 3rd, Shier D., Greeley H. P., Valtin H. Interaction of vasopressin and renal prostaglandins in the homozygous diabetes insipidus rat. Adv Prostaglandin Thromboxane Res. 1980;7:1009–1013. [PubMed] [Google Scholar]
- Edwards R. M., Jackson B. A., Dousa T. P. ADH-sensitive cAMP system in papillary collecting duct: effect of osmolality and PGE2. Am J Physiol. 1981 Apr;240(4):F311–F318. doi: 10.1152/ajprenal.1981.240.4.F311. [DOI] [PubMed] [Google Scholar]
- Edwards R. M., Jackson B. A., Dousa T. P. Protein kinase activity in isolated tubules of rat renal medulla. Am J Physiol. 1980 Apr;238(4):F269–F278. doi: 10.1152/ajprenal.1980.238.4.F269. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Haase W., Kinne R. Biochemical and physiological studies on cells isolated from the medullary thick ascending limb of Henle's loop. Int J Biochem. 1980;12(1-2):55–59. doi: 10.1016/0020-711x(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Silva P., Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflugers Arch. 1981 Mar;389(3):263–270. doi: 10.1007/BF00584788. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Haase W., Kinne R. Separation of renal medullary cells: isolation of cells from the thick ascending limb of Henle's loop. J Cell Biol. 1980 Dec;87(3 Pt 1):672–681. doi: 10.1083/jcb.87.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Varney D. M. Effect of vasopressin on electrical potential difference and chloride transport in mouse medullary thick ascending limb of Henle's loop. J Clin Invest. 1980 Oct;66(4):792–802. doi: 10.1172/JCI109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S. C., Culpepper R. M., Andreoli T. E. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol. 1981 Oct;241(4):F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- Higashihara E., Stokes J. B., Kokko J. P., Campbell W. B., DuBose T. D., Jr Cortical and papillary micropuncture examination of chloride transport in segments of the rat kidney during inhibition of prostaglandin production. Possible role for prostaglandins in the chloruresis of acute volume expansion. J Clin Invest. 1979 Nov;64(5):1277–1287. doi: 10.1172/JCI109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M. Effect of bumetanide and furosemide on the thick ascending limb of Henle's loop of rabbits and rats perfused in vitro. Eur J Pharmacol. 1977 Feb 21;41(4):409–416. doi: 10.1016/0014-2999(77)90261-8. [DOI] [PubMed] [Google Scholar]
- Imbert-Teboul M., Chabardès D., Montégut M., Clique A., Morel F. Vasopressin-dependent adenylate cyclase activities in the rat kidney medulla: evidence for two separate sites of action. Endocrinology. 1978 Apr;102(4):1254–1261. doi: 10.1210/endo-102-4-1254. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J. R., Hays R. M. Evidence that chlorpropamide and vasopressin share a common site of action. J Clin Endocrinol Metab. 1969 May;29(5):738–740. doi: 10.1210/jcem-29-5-738. [DOI] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Lithium-induced polyuria: effect of lithium on adenylate cyclase and adenosine 3',5'-monophosphate phosphodiesterase in medullary ascending limb of Henle's loop and in medullary collecting tubules. Endocrinology. 1980 Dec;107(6):1693–1698. doi: 10.1210/endo-107-6-1693. [DOI] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Measurements of cyclic AMP and cyclic GMP phosphodiesterase activity in isolated tubular segments. Kidney Int. 1980 Oct;18(4):512–518. doi: 10.1038/ki.1980.166. [DOI] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Vasopressin-prostaglandin interactions in isolated tubules from rat outer medulla. J Lab Clin Med. 1980 Jul;96(1):119–128. [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Valtin H., Dousa T. P. Cellular action of vasopressin in medullary tubules of mice with hereditary nephrogenic diabetes insipidus. J Clin Invest. 1980 Jul;66(1):110–122. doi: 10.1172/JCI109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L., Oliver R. E. Disorders of urinary concentration and dilution. Am J Med. 1982 Feb;72(2):308–322. doi: 10.1016/0002-9343(82)90823-3. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Colon-Otero G., Ou S. Y., Turner S. T., Dousa T. P. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest. 1981 May;67(5):1347–1360. doi: 10.1172/JCI110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. K., Jackson B. A., Edwards R. M., Dousa T. P. Effect of potassium depletion on the vasopressin-sensitive cyclic AMP system in rat outer medullary tubules. J Lab Clin Med. 1982 Jan;99(1):29–38. [PubMed] [Google Scholar]
- Klein K. L., Wang M. S., Torikai S., Davidson W. D., Kurokawa K. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 1981 Jul;20(1):29–35. doi: 10.1038/ki.1981.100. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leichter S. B., Chase L. R. Differential effects of chlorpropamide on the control of adenosine 3',5'-monophosphate metabolism in rat renal cortex and medulla. Endocrinology. 1978 Mar;102(3):785–790. doi: 10.1210/endo-102-3-785. [DOI] [PubMed] [Google Scholar]
- Liberman B., Borges R., Wajchenberg B. L. Evidence for a role of antidiuretic hormone (ADH) in the antidiuretic action of chlorpropamide. J Clin Endocrinol Metab. 1973 May;36(5):894–900. doi: 10.1210/jcem-36-5-894. [DOI] [PubMed] [Google Scholar]
- Meinders A. E., van Leeuwen A. M., Borst J. G., Cejka V. Paradoxical diuresis after vasopressin administration to patients with neurohypophyseal diabetes insipidus treated with chlorpropamide, carbamazepine or clofibrate. Clin Sci Mol Med. 1975 Oct;49(4):283–290. doi: 10.1042/cs0490283. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Brown C. F., Jr Effect of chlorpropamide on osmotic water flow across toad bladder and the response to vasopressin, theophylline and cyclic AMP. J Clin Endocrinol Metab. 1974 May;38(5):883–889. doi: 10.1210/jcem-38-5-883. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A. Effect of chlorpropamide on the permeability of the urinary bladder of the toad and the response to vasopressin, adenosine-3',5'-monophosphate and theophylline. Endocrinology. 1969 Feb;84(2):411–414. doi: 10.1210/endo-84-2-411. [DOI] [PubMed] [Google Scholar]
- Miller M., Moses A. M. Potentiation of vasopressin action by chlorpropamide in vivo. Endocrinology. 1970 May;86(5):1024–1027. doi: 10.1210/endo-86-5-1024. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Coulson R. Augmentation by chlorpropamide of 1-deamino-8-D-arginine vasopressin-induced antidiuresis and stimulation of renal medullary adenylate cyclase and accumulation of adenosine 3',5'-monophosphate. Endocrinology. 1980 Mar;106(3):967–972. doi: 10.1210/endo-106-3-967. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Fenner R., Schroeder E. T., Coulson R. Further studies on the mechanism by which chlorpropamide alters the action of vasopressin. Endocrinology. 1982 Dec;111(6):2025–2030. doi: 10.1210/endo-111-6-2025. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Howanitz J., Miller M. Diuretic action of three sulfonylurea drugs. Ann Intern Med. 1973 Apr;78(4):541–544. doi: 10.7326/0003-4819-78-4-541. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Numann P., Miller M. Mechanism of chlorpropamide-induced antidiuresis in man: evidence for release of ADH and enhancement of peripheral action. Metabolism. 1973 Jan;22(1):59–66. doi: 10.1016/0026-0495(73)90029-2. [DOI] [PubMed] [Google Scholar]
- Moses A. M., van Gemert M., Miller M. Evidence that glyburide-induced diuresis is not mediated by inhibition of ADH. Horm Res. 1974;5(6):359–366. doi: 10.1159/000178651. [DOI] [PubMed] [Google Scholar]
- Omachi R. S., Robbie D. E., Handler J. S., Orloff J. Effects of ADH and other agents on cyclic AMP accumulation in toad bladder epithelium. Am J Physiol. 1974 May;226(5):1152–1157. doi: 10.1152/ajplegacy.1974.226.5.1152. [DOI] [PubMed] [Google Scholar]
- Ozer A., Sharp G. W. Modulation of adenyl cyclase action in toad bladder by chlorpropamide: antagonism to prostaglandin E. Eur J Pharmacol. 1973 Jun;22(3):227–232. doi: 10.1016/0014-2999(73)90020-4. [DOI] [PubMed] [Google Scholar]
- Pokracki F. J., Robinson A. G., Seif S. M. Chlorpropamide effect: measurement of neurophysin and vasopressin in humans and rats. Metabolism. 1981 Jan;30(1):72–78. doi: 10.1016/0026-0495(81)90222-5. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Briggs J., Schubert G. In situ studies of the distal convoluted tubule in the rat. I. Evidence for NaCl secretion. Am J Physiol. 1982 Aug;243(2):F160–F166. doi: 10.1152/ajprenal.1982.243.2.F160. [DOI] [PubMed] [Google Scholar]
- Stoff J. S., Rosa R. M., Silva P., Epstein F. H. Indomethacin impairs water diuresis in the DI rat: role of prostaglandins independent of ADH. Am J Physiol. 1981 Sep;241(3):F231–F237. doi: 10.1152/ajprenal.1981.241.3.F231. [DOI] [PubMed] [Google Scholar]
- Stokes J. B. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979 Aug;64(2):495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. N., White H. J., Towbin E. J. Histochemistry and electron microscopy of the renal papilla in a genetic strain of rats with diabetes insipidus. Nephron. 1972;9(5):308–317. doi: 10.1159/000180162. [DOI] [PubMed] [Google Scholar]
- Tai H. H., Tai C. L., Hollander C. S. Biosynthesis of prostaglandins in rabbit kidney medulla. Properties of prostaglandin synthase. Biochem J. 1976 Feb 15;154(2):257–264. doi: 10.1042/bj1540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtin H. Sequestration of urea and nonurea solutes in renal tissues of rats with hereditary hypothalamic diabetes insipidus: effect of vasopressin and dehydration on the countercurrent mechanism. J Clin Invest. 1966 Mar;45(3):337–345. doi: 10.1172/JCI105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D. G., Eveloff J. NaCl entry mechanisms in the luminal membrane of the renal tubule. Am J Physiol. 1982 Jun;242(6):F561–F574. doi: 10.1152/ajprenal.1982.242.6.F561. [DOI] [PubMed] [Google Scholar]
- Webster B., Bain J. Antidiuretic effect and complications of chlorpropamide therapy in diabetes insipidus. J Clin Endocrinol Metab. 1970 Feb;30(2):215–227. doi: 10.1210/jcem-30-2-215. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R., Handler J. S. Inhibition of vasopressin-stimulated prostaglandin E biosynthesis by chlorpropamide in the toad urinary bladder. Mechanism of enhancement of vasopressin-stimulated water flow. J Clin Invest. 1977 Dec;60(6):1348–1353. doi: 10.1172/JCI108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig S. M., Ettinger B., Earley L. E. Mechanism of antidiuretic action of chlorpropamide in the mammalian kidney. Am J Physiol. 1971 Sep;221(3):911–915. doi: 10.1152/ajplegacy.1971.221.3.911. [DOI] [PubMed] [Google Scholar]



