Abstract
Recent studies have indicated an important role of proteinases and proteinase-activated receptors (PARs) in tumorigenesis. Although a role for PARs has been described in various skin tumors including melanoma, the underlying cellular mechanisms have not been understood. Recent studies have suggested PAR1 as a regulator of melanoma cell growth and metastasis by affecting angiogenic and invasive factors. Moreover, changes in the expression patterns of PAR1 and PAR2 correlate with skin cancer progression, and PAR1 is overexpressed in melanoma. Therefore, we sought to elucidate the putative role of PAR1- and PAR2 -mediated signal transduction pathways during melanoma progression. Activation of both PAR1 and PAR2 led to rapid phosphorylation of protein kinase D1 (PKD1) in cultured WM9 melanoma cells. PKD1 is known to be involved in cell migration, integrin regulation, and intracellular vesicle transport. Downregulation of PKD1 by siRNA resulted in diminished proliferation, decreased αvβ3 integrin regulation, and secretion of pro-angiogenic chemokine IL-8 in WM9 cells. In conclusion, our results show that PAR1 and PAR2 are involved in WM9 cell proliferation and secretion of IL-8 by activation of PKD1. Inactivation of the PKD1 pathway may be beneficial for the inhibition of PAR-induced melanoma proliferation and for maintenance of the inflammatory tumor environment.
INTRODUCTION
Proteinase-activated receptors (PARs) are G-protein-coupled receptors with seven transmembrane domains that are stimulated by a unique activation mechanism. The extracellular N-terminus is specifically cleaved by certain serine proteinases (e.g., thrombin, trypsin, tryptase, cathepsin G), exposing a formerly cryptic peptide sequence of six amino acids. Subsequently, this “tethered ligand” can bind to the second extracellular loop of PARs, leading to the induction of specific intracellular cell signaling events, which can also be mimicked by stimulating PARs with synthetic peptides of the tethered ligand sequence. PARs are expressed in numerous tissues that exert several biological effects, including cell proliferation, inflammation, and blood coagulation. In addition, both PAR1 and PAR2 expressions are abundantly upregulated in tumors of various tissues (Tellez and Bar-Eli, 2003; Steinhoff et al., 2005; Melnikova et al., 2008). Semiquantitative RT-PCR revealed that PAR1, but not PAR2, is expressed by primary melanocytes of both newborn and adult origin. Nevertheless, PAR2 on keratinocytes is essential for melanosome transfer between melanocytes and keratinocytes (Seiberg et al., 2000).
The incidence of malignant melanoma is still increasing and causes most deaths related to skin cancer (Bowden, 2004; Larson et al., 2009). PAR1 was reported to be significantly overexpressed in malignant melanomas (Tellez et al., 2003; Melnikova et al., 2008; Tellez and Bar-Eli, 2003; Villares et al., 2009) and PAR2 immunoreactivity was found to be upregulated both in nevi and malignant melanomas (Massi et al., 2005). Thrombin-induced activation of PAR1 on murine melanoma cells led to increased tumor growth and enhanced pulmonary metastasis in vivo (Nierodzik et al., 1992; Nierodzik et al., 1998). In vitro overexpression of PAR1 in a nonmetastatic melanoma cell line (SB2) led to increased adhesion to extracellular matrix molecules and also modulated cytoskeletal reorganization (Nierodzik et al., 1998). Furthermore, activation of PAR1 induced the recruitment of αvβ5 integrin to focal contact sites (Hazarika et al., 2004).
PAR1 expression is controlled by the transcription factor activator protein-2α, and loss of activator protein-2α causes overexpression of PAR1, which correlates with increased metastatic potential (Tellez et al., 2003). Accordingly, thrombin-induced activation of PAR1 on M24met melanoma cells enhanced their migration and metastasis. Interestingly, thrombin coactivated PAR2 in these cells by an indirect cleavage-independent mechanism, indicating that PAR2 may have an additional, possibly collaborative role in melanoma metastasis. In fact, treatment of melanoma cells with a selective PAR2 agonist also enhanced experimentally induced metastasis (Even-Ram et al., 2001). Finally, increased expression of PAR1 in malignant melanomas was recently correlated with increased expression of IL-8 and integrins (Shi et al., 2004).
Activated PARs induce production of diacylglycerol, an important second messenger in cells of which the primary cellular targets are protein kinase C (PKC) kinases, leading to their translocation to the plasma membrane and subsequent phosphorylation of various molecules. Thus, the family of protein kinase D (PKD) kinases is a direct substrate of PKCs. The PKD family of serine/threonine PKs comprises three members: PKD1 (PKCμ), PKD2, and PKD3 (PKCν). PKD1 has important roles in cell migration and invasion. This kinase reportedly arranges cell motility by aiding the transport of αvβ3 integrin to emerging focal adhesions (Tellez et al., 2007). Moreover, PKD1 was found to be directly involved in tumor cell invasion and metastasis, and it promotes proproliferative and anti-apoptotic effects (Tellez et al., 2007). Here, we provide early evidence that both PAR1 and PAR2 can activate PKD1 in malignant melanoma cells, thereby enhancing cell proliferation and motility.
RESULTS
Stimulation of PAR1/PAR2 on WM9 cells leads to phosphorylation of PKD1
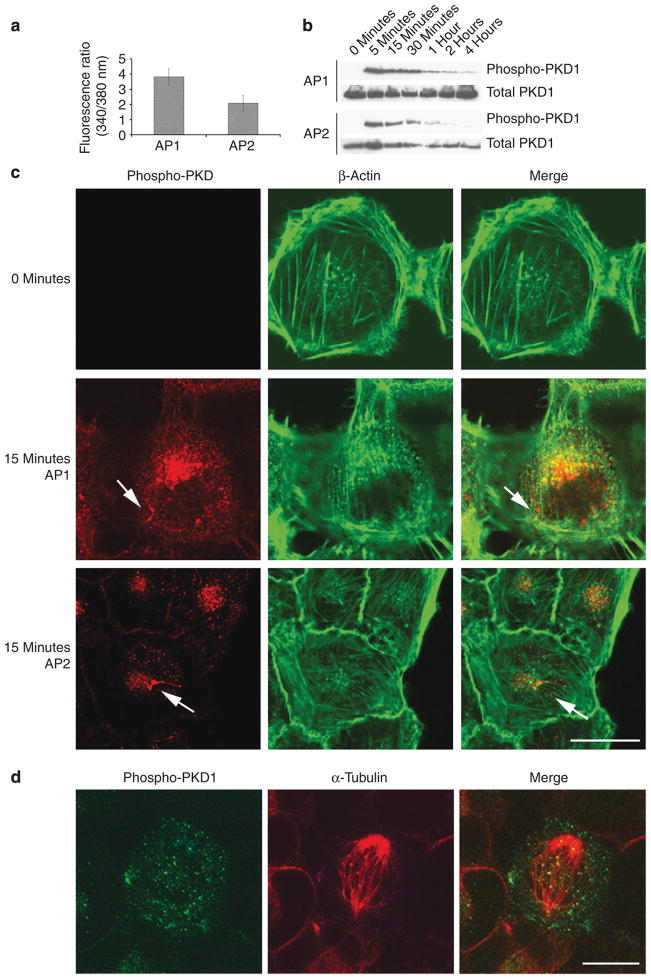
WM9 melanoma cells respond with a fast [Ca2+]i signal to synthetic PAR agonists (Figure 1a). As both PAR1 and PAR2 are known to be involved in melanoma invasion and metastasis, we searched for possible signal transduction molecules after PAR stimulation in melanoma cells. Both PAR1 and PAR2 agonists led to a rapid phosphorylation of PKD1 within 5 minutes after stimulation (Figure 1b). Immunoblot analysis using a specific antibody against phospho-Ser916-PKD1 gave an immunoreactive band of about 110 kDa, which corresponds to the molecular mass of PKD1. Activation started to fade 30 minutes after stimulating peptides were added. However, PKD1 remained partly phosphorylated even after 4 hours. We confirmed this observation by immunofluorescence: in unstimulated WM9 cells no phospho-PKD1 was detectable, whereas in PAR-agonist-stimulated WM9 cells, phospho-PKD1 was detected in intracellular compartments, which appeared to be partly aligned along cytoskeletal fiber structures (Figure 1c). Unstimulated WM9 cells did not show a positive staining for phospho-Ser916-PKD1, unless the cells were in a mitotic state (Figure 1d).
Figure 1. Stimulation of proteinase-activated receptor (PAR)1 and PAR2 induces phosphorylation of protein kinase D (PKD)1.
Incubation with PAR1- and PAR2-activating peptides (activating peptide for PAR1 (AP1), activating peptide for PAR2 (AP2); 0.1mM) induces fast activation of PKD1. (a) Stimulation with AP1 and AP2 induces increase of [Ca2+]i in WM9 cells. (b) AP1- or AP2-induced phosphorylation of PKD1 (110 kDa) peaked after 5 minutes, decreased rapidly within 15 minutes, but lasted up to 4 hours in WM9 cells. (c) Immunoreactivities of phospho-Ser916-PKD1 and β-actin were localized in WM9 cells after 15 minutes of stimulation with AP1 or AP2 (0.1mM). Only agonist-induced phosphorylation of PKD1 was detectable, which was localized in vesicular compartments in the cells. However, there was no costaining with β-actin fibers. (d) Unstimulated cells only showed positive staining for phospho-Ser916-PKD1 during mitosis (bar=10 μm).
Knockdown of PKD1 in WM9 melanoma cells
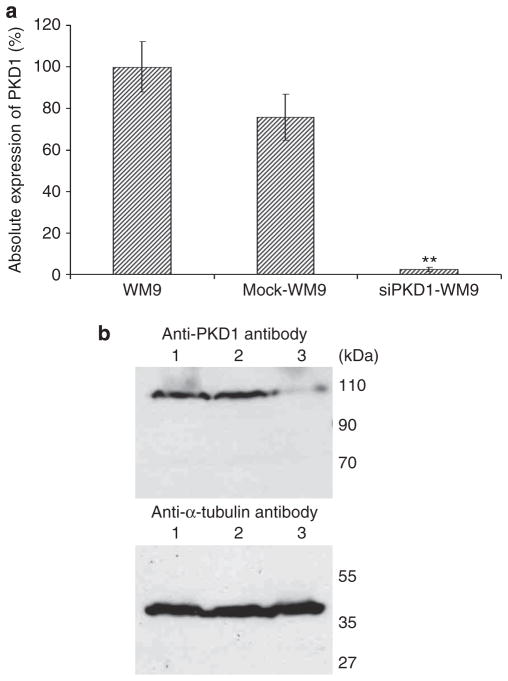
We investigated the role of PKD1 in invasiveness and metastasis of melanoma cells by using RNA interference for PKD1-specific knockdown. For high RNA interference efficiency, WM9 cells were transfected with pSuppressor construct with or without a short interfering RNA specific for PKD1, and cells were selected with the antibiotic G418 for 14 days. Stable cell clones were screened for their ability to silence PKD1 expression by real-time PCR analysis (Figure 2a). In all stable clones, the PKD1 mRNA level was reduced by at least 80% as compared with untransfected WM9 cells, which was confirmed by immunoblot analysis (Figure 2b). The slightly decreased expression of PKD1 in mock-transfected WM9 cells was not significant (Figure 2b). The maintenance of PKD1 suppression was monitored for each experiment (Livak and Schmittgen, 2001).
Figure 2. Protein kinase D (PKD)1 downregulation in WM9 cells by selective knockdown.
PKD1 expression analysis of either normal or PKD1-knockdown WM9 cells. (a) PKD1-knockdown cells present a significant decrease of PKD1 expression in real-time PCR. However, control-transfected cells present a nonsignificant decreased expression of PKD1. Triplicate observation in n=3 experiments; data are presented as means±SEM, and differences between data were tested by Student’s t-test for unpaired data. **P<0.005. (b) Short interfering RNA to PKD1 led to a significant reduction of protein translation in PKD1-knockdown cells (lane 3). Although transcription of PKD1 was decreased in mock cells (lane 2), translation of the PKD1 protein is comparable to that of untransfected WM9 (lane 1) cells.
Stable PKD1 antisense clones exhibit diminished proliferation
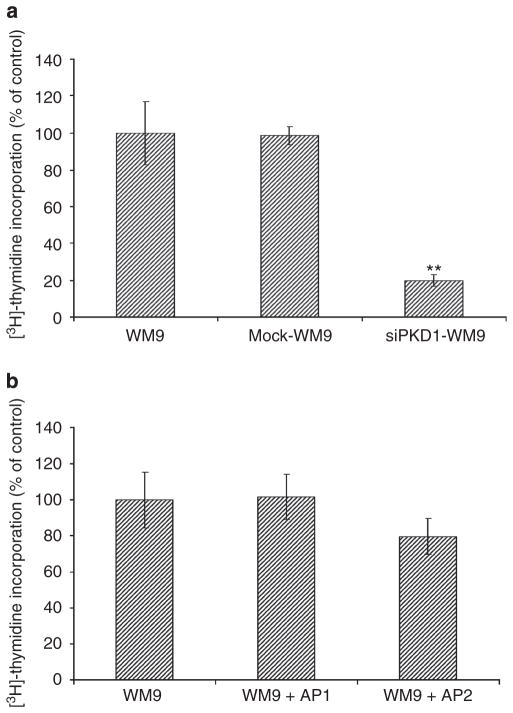
Recently, PKD1 has been reported to be involved in the proliferation of a pancreatic carcinoma cell line (Guha et al., 2002). We elucidated the impact of PKD1 on the proliferation of WM9 cells. First, we compared the capability of cell proliferation of normal (untransfected) WM9 cells and PKD1 antisense-expressing cells by means of a 3[H]-thymidin incorporation assay. Normal WM9 cells, as well as mock-transfected WM9 cells, revealed high 3[H]-thymidin incorporation levels, whereas WM9 cells with low PKD1 expression showed significantly diminished proliferation with a lower 3[H]-thymidin incorporation level (Figure 3a).
Figure 3. Proliferation is compromised in protein kinase D (PKD)1-knockdown melanoma cells.
(a) Proliferation of normal, mock-transfected, and PKD1-knockdown WM9 cells was determined by [3H]-thymidine incorporation. Stably transfected PKD1-knockdown WM9 cells display an 80% inhibition of cell proliferation as compared with nontransfected and mock-transfected WM9 cells. Data are presented as means±SEM. Differences between data were tested by Student’s t-tests for unpaired data. **P<0.005. (b) Stimulation of either proteinase-activated receptor (PAR)1 or PAR2 (0.1mM agonists) had no effect on WM9 cell proliferation. The data shown are n=3 and presented as means±SEM.
Activation of PARs on melanoma cells was recently reported to increase proliferation (Even-Ram et al., 2001). Therefore, we analyzed the proliferation capacities of untransfected WM9 cells after stimulation with PAR1- or PAR2-activating peptides. Interestingly, we found that stimulation of PAR1 or PAR2 on WM9 cells had no obvious effect on cell proliferation (Figure 3b). This could be explained by the possibility that proliferation of WM9 cells was already at a maximum. Kinase protein analyses showed a constitutive activation of ERK1/2 kinases in unstimulated WM9 cells (data not shown). The constitutive activation of ERK1/2 could account for a sustained cell proliferation, which was affected by neither PAR1 nor PAR2 activation in these cells (Satyamoorthy et al., 2003). WM9 cells are highly proliferative but do not display an invasive phenotype, because of which we failed to establish migration assays using Boyden Chamber, wound closure experiments, or time-lapse microscopy.
Knockdown of PKD1 in WM9 cells affects PAR1-mediated secretion of IL-8
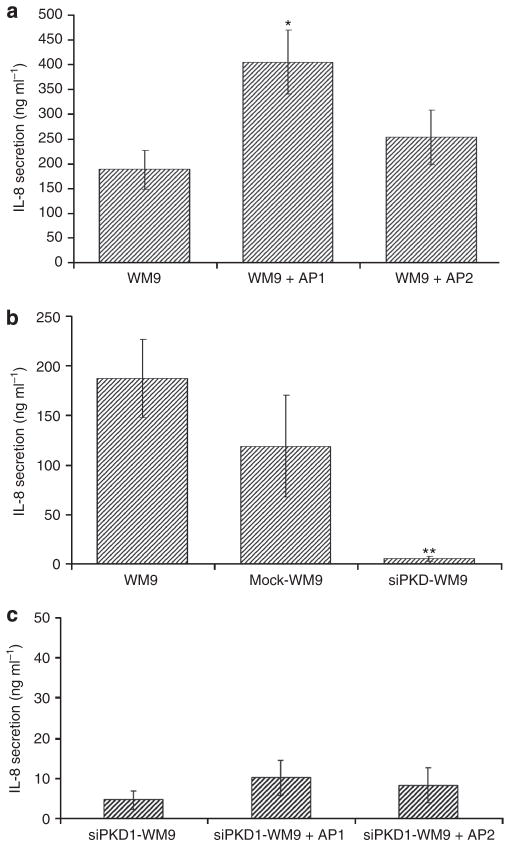
Both PAR1 and PAR2 are known to stimulate the secretion of IL-8 in endothelial and epithelial cells, as well as in fibroblasts (Amadesi et al., 2009). In addition, IL-8 is known to promote progression of prostate cancer and melanoma (Huang et al., 2002; Araki et al., 2007). Thus, we analyzed the role of PAR1 and PAR2, as well as that of PKD1, for IL-8 secretion of WM9 cells. Only PAR1 significantly increased the moderate baseline of IL-8 secretion (216±34% compared with 100±20% of unstimulated cells), whereas PAR2 did not significantly affect IL-8 secretion (135±29%; Figure 4a). Conversely, IL-8 secretion was markedly inhibited in PKD1-knockdown cells down to 3±1.5% (Figure 4b), and stimulation of PAR1 did not increase IL-8 secretion in these cells (Figure 4c). We cannot exclude the possibility that the transfection and selection conditions influenced the secretion of IL-8, because the mock-transfected WM9 cells presented a nonsignificant reduced secretion of IL-8 (63±29%). Together, these results suggest that PAR1-mediated melanoma progression involves the induced secretion of IL-8 by PAR1. This mechanism is partly mediated by PKD1, because the loss of PKD1 expression results in a significant decrease in IL-8 secretion.
Figure 4. IL-8 secretion is enhanced after proteinase-activated receptor (PAR)1 stimulation and decreased after protein kinase D (PKD)1 knockdown in cultured WM9 melanoma cells.
(a) WM9 cells were stimulated with either activating peptide for PAR1 (AP1) or activating peptide for PAR2 (AP2) (0.1mM), and the secretion of IL-8 was determined by ELISA. Activation of PAR1 induced enhanced IL-8 release in WM9 cells, whereas PAR2 did not exert this effect. (b) Secretion of IL-8 is inhibited after PKD1 silencing in WM9 cells (3±1.5%). Triplicate observation in n=4 experiments. (c) Neither stimulation with AP1 nor stimulation with AP2 increased IL-8 secretion of PKD1-knockdown WM9 cells significantly. Data are presented as means±SEM. Differences between data were tested by Student’s t-tests for unpaired data. *P<0.05; **P<0.005.
Knockdown of PKD1 in WM9 cells leads to changes in cell shape and morphology
Immunofluorescence analysis revealed that unstimulated WM9 cells contained a large amount of β-actin at the cell rims. PAR1 and PAR2 activation provoked rapid formations of β-actin-positive stress fibers (Figure 5). These fibers were still present after 2 hours. Unstimulated PKD1-knockdown melanoma cells also showed predominant immunoreactivity for β-actin along the cell rim with some cytoskeletal fibers that contained β-actin. However, these cells revealed much less β-actin-containing filopodia. After stimulation of either PAR1 or PAR2, some stress fibers had formed in the cell cytosol, and filopodia formation was sparse. Nevertheless, no major changes in β-actin redistribution were observed.
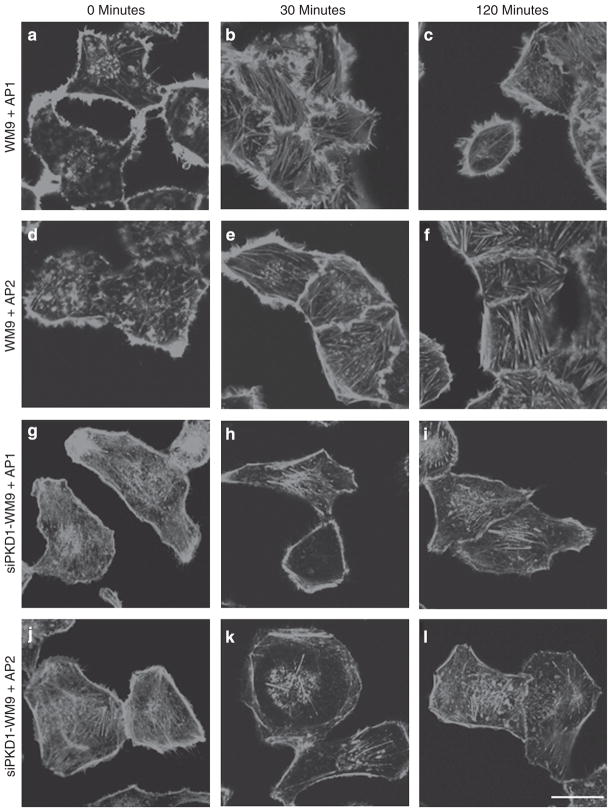
Figure 5. Knockdown of protein kinase D (PKD)1 in WM9 cells leads to changes in cell shape.
Untransfected and PKD1-knockdown WM9 cells were stimulated with activating peptide for proteinase-activated receptor (PAR)1 (AP1) or activating peptide for PAR2 (AP2) (0.1mM) for 120 minutes and fixed, and fiber formation was monitored with β-actin-FITC antibody. In normal, unstimulated WM9 cells, stress fibers did not form (a, d). When PAR1 (b, c) and PAR2 (e, f) were activated, formation of stress fibers was significantly increased in WM9 cells. PKD1 knockdown induced marked changes in the actin cytoskeleton structure (g–l). Unstimulated PKD1-knockdown cells display actin-positive fibers (g, j). Here, the activation of PAR1 and PAR2 in PKD1-knockdown cells did not induce formation of stress fibers, and no changes to unstimulated cells were observed (h, i, k, l) (bar=10 μm).
Knockdown of PKD1 in WM9 cells affects integrin αvβ3 distribution
For normal cell migration, integrins (heterodimeric cell adhesion receptors consisting of alpha and beta subunits) are indispensable. Proper function involves a controlled cycling of the integrin receptors between the cell surface and cytosol. Thus, it is not surprising that several integrins are known to have a direct role in tumor progression. For example, integrin αvβ3 participates in tumor-induced angiogenesis and metastasis (Kumar et al., 2001; Nemeth et al., 2007; Neto et al., 2007). Integrin αvβ3 binds to vitronectin, fibronectin, laminins, matrix-metalloproteinase-2, fibrinogen, prothrombin, thrombospondin, and von-Willebrand factor. Therefore, we analyzed the distribution of αvβ3 after PAR1 and PAR2 activation in wild type and siPKD1-transfected WM9 cells. In unstimulated cells, αvβ3 was mainly found at the cell surface. Fifteen minutes after PAR activation, αvβ3 was widely distributed in the cell; after 60 minutes, αvβ3 was back at the cell membrane (Figure 6a). The PKD1 knockdown in WM9 cells led to a reduced staining of αvβ3 integrin on the cell membrane (Figure 6b). However, PAR activation did not lead to a visible change in integrin distribution in siPKD1-transfected WM9 cells.
Figure 6. Protein kinase D (PKD)1 knockdown affects αvβ3-integrin distribution in WM9 cells.
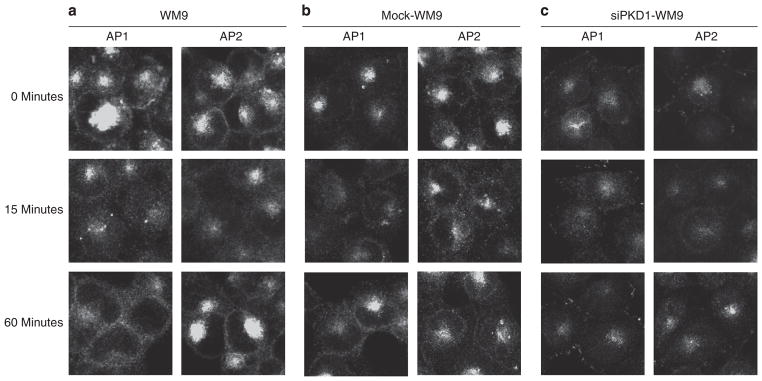
(a, b) Indirect immunofluorescence revealed fast internalization of αvβ3-integrin after activating peptide for proteinase-activated receptor (PAR)1 (AP1) and activating peptide for PAR2 (AP2) stimulation in either WM9 or mock-transfected WM9 cells (0.1mM, 15 minutes) and recycling of the integrin back to the cell membrane after 60 minutes. (c) Staining for αvβ3-integrin on the cell membrane in siPKD1-WM9 cells appears to be reduced, and the integrin is only found in clusters in filopodia-like structures. Stimulation with AP1 and AP2 induced internalization, and recycling of αvβ3-integrin is reduced (bar=10 μm).
DISCUSSION
This study shows that the activation of both PAR1 and PAR2 in the melanoma cell line WM9 leads to fast activation of PKD1, a previously unreported member of the PKC family of kinases involved in tumorigenesis. PKD1 is apparently an important intracellular kinase, as its presence and its PAR1- and PAR2-mediated activation are important for cell proliferation, αvβ3 integrin distribution, and secretion of inflammatory cytokines such as IL-8.
PKD1 is important for cancer cell migration in myeloma and breast cancer (Bowden et al., 1999; Qiang et al., 2004), and activation of PKD1 prevents apoptosis in pancreatic tumor cells (Trauzold et al., 2003). Pancreatic tumor cells present an increased transcription of neurotensin receptor type-1 during tumor progression (Wang et al., 2000). Neurotensin receptor type-1 is a member of the heptahelical G-protein-coupled receptor family, and activation of neurotensin receptor type-1 resulted in rapid intracellular calcium mobilization and PKC phosphorylation (Ryder et al., 2001), properties that are also well known for both PAR1 and PAR2 (reviewed in Amadesi et al., 2009). Neurotensin receptor type-1-mediated PKC activation further led to phosphorylation of PKD1 in the pancreatic tumor cell line PANC-1 (Huang et al., 2002). Recent data showed the direct phosphorylation of PKD isoforms by agonist-activated PAR2 in dorsal root ganglia, indicating PKD1 to depict an important component of a signal transduction pathway for proteinase-induced activation of nociceptive neurons and inflammatory responses (Amadesi et al., 2009). Therefore, we investigated whether PKD1 contributes to PAR1- and/or PAR2-induced modification of melanoma cell behavior.
PAR1 is known to be overexpressed in malignant melanoma, and the expression of PAR1 is known to contribute to melanoma cell invasion (Bromberg et al., 2001; Tellez et al., 2003; Shi et al., 2004; Boire et al., 2005; Goerge et al., 2006). Until now, the underlying mechanisms of PAR1 activation and melanoma progression have barely been understood. PAR1 is normally expressed by thrombocytes, neutrophils, mast cells, granulocytes, endothelial cells, fibroblasts, myocytes, neurons, and astrocytes. PAR1 is upregulated during inflammation and triggers degranulation of mast cells. It can be activated by various serine proteinases such as thrombin, kallikreins, plasmin, and cathepsin G, as well as by metalloproteinase-1, all of which are involved in inflammation and tumorigenesis (Hollenberg et al., 2008; Stefansson et al., 2008; Villares et al., 2009; Wilson et al., 2009). Tumors are often called wounds that do not heal because noticeable similarities exist, similar to neovascularization and extracellular matrix deposition (Dvorak, 1986; Chantrain et al., 2006). Therefore, we investigated the activation and role of both PAR1 and PAR2 in WM9 melanoma cells. In normal skin, PAR2 is expressed by keratinocytes, endothelial cells, fibroblasts, sensory nerve fibers, and cells of the immune system (e.g., mast cells and neutrophils). Our own data revealed upregulation of PAR2 in these cells during inflammation (Shpacovitch et al., 2007). Activation of PAR2 has an important role in diseases that involve acute or chronic inflammation of the skin (e.g., contact dermatitis and atopic dermatitis; Buddenkotte et al., 2005; Grant et al., 2007). The expression of PAR2 in keratinocytes is also important for melanosome transfer and skin tanning. Normal melanocytes do not express PAR2, whereas nevi and malignant melanomas express PAR2 but present no significant differences between benign and malignant melanocytic lesions (Massi et al., 2005).
Others have shown that both PAR1 and PAR2 contribute to melanoma cell metastasis. Interestingly, PAR2 can promote metastasis by means of a thrombin-dependent mechanism. However, the details of this mechanism remain elusive (Even-Ram et al., 2001). We therefore analyzed possible signal transduction pathways triggered in WM9 cells after PAR1 or PAR2 activation. We found that either PAR1- or PAR2-activating peptides temporarily induced the phosphorylation of PKD1 at Ser916. We found phosphorylated PKD1 close to α-tubulin fibers during mitosis of WM9 cells. PKD1 is known to have a role in the cell cycle (Rykx et al., 2003). Indeed, we found that cell proliferation was significantly downregulated in PKD1-silenced melanoma cells.
The immunofluorescence staining of activated PKD1 in WM9 cells revealed that after PAR1 and PAR2 stimulation, PKD1 was mainly found in structures that appeared to be vesicle-like. It is appreciated that PKD1 is important for vesicle transport from the trans-golgi network to the cell surface and for recycling of internalized αvβ3 integrin vesicles back to the cell membrane (Baron and Malhotra, 2002; Jaggi et al., 2007). Therefore, activation of PAR1 and PAR2 could lead to a PKD1-dependent vesicle transport to the cell membrane. However, we could not detect any colocalization of PKD1-positive vesicles with either β-actin or α-tubulin fibers.
Here, we also demonstrate that melanoma cells can secrete the chemoattractive and proangiogenic molecule IL-8 after stimulation of PAR1. IL-8 expression by human melanoma cells is known to correlate with their metastatic capability in vivo (reviewed in Tellez et al., 2007). The secretion of IL-8 is influenced by PKD1, as knockdown of PKD1 led to a decrease in the baseline secretion of IL-8. A reduced release of IL-8 could lead to a diminished invasion and metastasis of melanoma (Bar-Eli, 1999). However, PAR1 stimulation in siPKD1-WM9 cells did not increase IL8-secretion. Therefore, secretion of IL-8 in WM9 cells depends only on PKD1 and could be independent from other signaling pathways. PAR2 expression had no significant impact on IL-8 secretion, which could be explained by the lower PAR2 expression compared with PAR1 expression in these cells. We hypothesize that PKD1 is involved in the secretion of pro-inflammatory cytokines, which will be exocytosed from tumor cells to establish a pro-inflammatory environment and induce neovascularization.
Invasive tumor cells form membrane protrusions to come in contact with extracellular matrix proteins. At these invadopodia, metalloproteinases are secreted, which degrade the extracellular matrix to enable tumor cell invasion (Mauch et al., 1994; Schnaeker et al., 2004; Villares et al., 2008). PKD1 is also enriched at invadopodias (White et al., 2007). Thus, we asked whether filopodia formation might be impaired in PKD1-silenced WM9 cells. In addition, non-transfected cells contained numerous filopodia, whereas WM9 cells expressing PKD1-antisense displayed a rounded appearance with only sparse filopodia formation. The cytoskeletal protein β-actin is abundant in filaments along the cell rims, giving WM9 cells a wispy appearance. When either PAR1 or PAR2 was activated, stress fiber formation was pronounced in WM9 cells. In contrast, WM9 cells expressing PKD1 antisense showed almost no filopodia positive for β-actin. Moreover, PAR activation in these cells induced only minor formation of actin stress fibers. These observations are in accordance with a recent report demonstrating that active PKD1 interacts with F-actin at the leading edge of migrating cells, strongly suggesting a direct role of PKD1 in actin remodeling (Eiseler et al., 2007).
PKD1 was shown to be indispensable for the fast recycling of αvβ3 by antagonizing α5β1 recycling in NIH3T3 fibroblasts, thus enabling persistent migration of cells (Woods et al., 2004; White et al., 2007). Integrin αvβ3 is highly expressed in metastatic melanoma cells, and it contributes to the malignant phenotype (Nemeth et al., 2007). Integrin recycling to the cell surface is an important mechanism for cell migration and invasion. Because we saw that PAR agonists can induce a temporary phosphorylation of PKD1 at Ser916 in WM9 cells, we wondered whether PAR activation or silencing of PKD1 in WM9 cells would induce changes in αvβ3 integrin distribution. Activation of either PAR1 or PAR2 induced a fast internalization of αvβ3 integrin from the cell membrane and recycling of the integrin within 60 minutes. Therefore, activation of PAR1 or PAR2 in melanoma cells could influence cell migration. However, we failed to present this possible phenotype for WM9 cells in the Boyden Chamber assay, wound closure experiments, and time-lapse video microscopy because of a low migration phenotype of the cell line. Knockdown of PKD1 in WM9 cells results in lower cell membrane expression of αvβ3 integrin, and PAR stimulation induced no obvious changes in αvβ3 distribution. Our results maintain the important role of PKD1 for αvβ3 integrin recycling in tumor cells and hint that PAR-induced activity of PKD1 could be important for αvβ3-dependent migration of tumor cells. However, future studies with highly invasive and migrating melanoma cell lines must be conducted to improve this hypothesis.
In conclusion, we present early evidence that activation of both PAR1 and PAR2 induces rapid and transient phosphorylation of PKD1 in WM9 cells, serving as an in vitro model system for studying the mechanisms of melanoma progression, remodeling, and metastasis. Further elucidation of the signal transduction pathways involved in PAR-mediated PKD1 activation may identify new targets for therapeutic intervention in malignant melanoma.
MATERIALS AND METHODS
Materials
Reagents were purchased from the indicated providers: rabbit polyclonal anti-PKD1 and anti-phospho-Ser916-PKD1 from Cell Signalling (Boston, MA), mouse anti-αvβ3-antibody (clone LM609) from Millipore (Billerica, MA), mouse anti-tubulin antibody (clone DM1A) from Calbiochem (Darmstadt, Germany), Fura2AM and FITC-conjugated anti-β-actin and secondary antibodies from Sigma-Aldrich (Taufkirchen, Germany), [3H]-thymidine from GE Healthcare (Braunschweig, Germany), and activating peptides for PAR1 and PAR2 from Bachem (Weil am Rhein, Germany). The PKD1-gene silencing construct and pSuppressor plasmid were described previously (Eiseler et al., 2007).
Cell lines
The human melanoma cell line WM9 was maintained in a standard culture medium containing enriched Earle’s salts, nonessential amino acids, glutamic acid, and 10% heat-inactivated fetal calf serum (Biochrom, Berlin, Germany). Culture medium for stably transfected PKD1-knockdown WM9 cells and mock-transfected WM9 cells was supplemented with 150 μgml−1 G418 (Sigma-Aldrich, Taufkirchen, Germany). Cells were changed to serum-free medium 24 hours before the experiments (except for proliferation assay).
Generation of transfected cells
WM9 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsberg, Canada) with pSuppressor-PKD1 or control vectors. After 2 days the cells were cultured in standard culture medium supplemented with 400 μgml−1 G418 sulfate. The expression of PKD1 was assessed by real-time PCR (forward: 5′-TGCTGTGGGGGCTGGTA CGT-3′ and reverse: 5′-GTGCGGATGGTGCTGACCCC-3′; Livak and Schmittgen 2001).
Calcium mobilization assay
The calcium mobilization assay was performed as described elsewhere (Bocheva et al., 2009) with minor changes. WM9 cells were incubated with 2.5 μM Fura-2AM for 60 minutes in HEPES-buffered Ringer solution and then washed and stimulated with PAR1 and PAR2 agonists (10−4 M).
Immunoblot analysis
WM9 cells were harvested after treating several times with hot lysis buffer (100mM Tris (pH 6.8), 4% SDS, 0.2% bromophenol blue, 20% glycerol, and 5% β-mercaptoethanol). Equal volume samples (20 μl) were separated by denaturing SDS-PAGE and were transferred to nitrocellulose membranes. Membranes were blocked with 5% milk powder/1× phosphate-buffered saline+Tween-20 and incubated with specific antibodies against phosphorylated and unphosphorylated PKD1 and peroxidase-conjugated secondary antibodies. The membranes were developed using the ECL Plus Western blotting detection system (GE Healthcare).
Proliferation assay
For the proliferation assay, 150,000 cells per ml were cultured in 96-well flat-bottom plates in a final volume of 200 μl. Simultaneously, 1 μCi per well [3H]-thymidine was added for 48 hours and thymidine incorporation was measured by liquid scintillation counting.
Immunofluorescence microscopy
Transfected and nontransfected WM9 cells were washed with cold phosphate-buffered saline and then fixed and permeabilized for 30 minutes in 1× phosphate-buffered saline/1% fetal calf serum/ 0.05% saponin at 4 °C. Primary antibodies were detected with FITC-conjugated antimouse IgG (1:250, 1 hour, room temperature). Cells were embedded in Vectashield mounting medium (Vector, Burlingame, CA). Specimens were observed using a Bio-Rad MRC 1000 confocal microscope (Dreieich, Germany). Images were collected at 0.68 μm intervals using a Zeiss 100 Plan Apo 1.4 NA objective and a zoom of × 1.5–2 (Zeiss, Jena, Germany).
Statistical analysis
Microsoft Excel software was used to manage data and create graphs. The mean data of the experiments are given in percentage ± SD unless otherwise indicated. Statistical significance was tested with unpaired Student’s t-test; significant differences of compared values are indicated (P≤0.05).
Acknowledgments
We thank Heike Hinte and Michaela Fastrich for expert technical assistance and Pamela Derish for critically reading the manuscript. This work was supported by the German Research Council (SFB492-B13 to MS, STE 1014/2-2 to MS and AR, and SFB492-A13 and TR23-A9 to SWS).
Abbreviations
- PAR
proteinase-activated receptor
- PKC
protein kinase C
- PKD
protein kinase D
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amadesi S, Grant AD, Cottrell GS, et al. Protein kinase D isoforms are expressed in rat and mouse primary sensory neurons and are activated by agonists of protease-activated receptor 2. J Comp Neurol. 2009;516:141–56. doi: 10.1002/cne.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–8. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–8. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- Bocheva G, Rattenholl A, Kempkes C, et al. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol. 2009;129:1816–23. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, et al. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–9. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Bromberg ME, Bailly MA, Konigsberg WH. Role of protease-activated receptor 1 in tumor metastasis promoted by tissue factor. Thromb Haemost. 2001;86:1210–4. [PubMed] [Google Scholar]
- Buddenkotte J, Stroh C, Engels IH, et al. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol. 2005;124:38–45. doi: 10.1111/j.0022-202X.2004.23539.x. [DOI] [PubMed] [Google Scholar]
- Chantrain CF, Henriet P, Jodele S, et al. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer. 2006;42:310–8. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Eiseler T, Schmid MA, Topbas F, et al. PKD is recruited to sites of actin remodelling at the leading edge and negatively regulates cell migration. FEBS Lett. 2007;581:4279–87. doi: 10.1016/j.febslet.2007.07.079. [DOI] [PubMed] [Google Scholar]
- Even-Ram SC, Maoz M, Pokroy E, et al. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem. 2001;276:10952–62. doi: 10.1074/jbc.M007027200. [DOI] [PubMed] [Google Scholar]
- Goerge T, Barg A, Schnaeker EM, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66:7766–74. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578(Part 3):715–33. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S, Rey O, Rozengurt E. Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res. 2002;62:1632–40. [PubMed] [Google Scholar]
- Hazarika P, McCarty MF, Prieto VG, et al. Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res. 2004;64:7361–9. doi: 10.1158/0008-5472.CAN-04-0823. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Oikonomopoulou K, Hansen KK, et al. Kallikreins and proteinase-mediated signaling: proteinase-activated receptors (PARs) and the pathophysiology of inflammatory diseases and cancer. Biol Chem. 2008;389:643–51. doi: 10.1515/BC.2008.077. [DOI] [PubMed] [Google Scholar]
- Huang S, Mills L, Mian B, et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125–34. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi M, Du C, Zhang W, et al. Protein kinase D1: a protein of emerging translational interest. Front Biosci. 2007;12:3757–67. doi: 10.2741/2349. [DOI] [PubMed] [Google Scholar]
- Kumar CC, Malkowski M, Yin Z, et al. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61:2232–8. [PubMed] [Google Scholar]
- Larson AR, Konat E, Alani RM. Melanoma biomarkers: current status and vision for the future. Nat Clin Pract Oncol. 2009;6:105–17. doi: 10.1038/ncponc1296. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Massi D, Naldini A, Ardinghi C, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–85. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Mauch C, Krieg T, Bauer EA. Role of the extracellular matrix in the degradation of connective tissue. Arch Dermatol Res. 1994;287:107–14. doi: 10.1007/BF00370728. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Villares GJ, Bar-Eli M. Emerging roles of PAR-1 and PAFR in melanoma metastasis. Cancer Microenviron. 2008;1:103–11. doi: 10.1007/s12307-008-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth JA, Nakada MT, Trikha M, et al. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–46. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- Neto DS, Pantaleão L, de Sá BC, et al. Alpha-v-beta3 integrin expression in melanocytic nevi and cutaneous melanoma. J Cutan Pathol. 2007;34:851–6. doi: 10.1111/j.1600-0560.2007.00730.x. [DOI] [PubMed] [Google Scholar]
- Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267–72. [PubMed] [Google Scholar]
- Nierodzik ML, Chen K, Takeshita K, et al. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood. 1998;92:3694–700. [PubMed] [Google Scholar]
- Qiang YW, Yao L, Tosato G, et al. Insulin-like growth factor I induces migration and invasion of human multiple myeloma cells. Blood. 2004;103:301–8. doi: 10.1182/blood-2003-06-2066. [DOI] [PubMed] [Google Scholar]
- Ryder NM, Guha S, Hines OJ, et al. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation. J Cell Physiol. 2001;186:53–64. doi: 10.1002/1097-4652(200101)186:1<53::AID-JCP1004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Rykx A, De Kimpe L, Mikhalap S, et al. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–6. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–9. [PubMed] [Google Scholar]
- Schnaeker EM, Ossig R, Ludwig T, et al. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: pre-requisite in human melanoma cell invasion. Cancer Res. 2004;64:8924–31. doi: 10.1158/0008-5472.CAN-04-0324. [DOI] [PubMed] [Google Scholar]
- Seiberg M, Paine C, Sharlow E, et al. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp Cell Res. 2000;254:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- Shi X, Gangadharan B, Brass LF, et al. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- Shpacovitch VM, Seeliger S, Huber-Lang M, et al. Agonists of proteinase-activated receptor-2 affect transendothelial migration and apoptosis of human neutrophils. Exp Dermatol. 2007;16:799–806. doi: 10.1111/j.1600-0625.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- Stefansson K, Brattsand M, Roosterman D, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18–25. doi: 10.1038/sj.jid.5700965. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–7. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- Tellez C, McCarty M, Ruiz M, et al. Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632–42. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- Tellez CS, Davis DW, Prieto VG, et al. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2007;127:387–93. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Schmiedel S, Sipos B, et al. PKCmu prevents CD95-mediated apoptosis and enhances proliferation in pancreatic tumour cells. Oncogene. 2003;22:8939–47. doi: 10.1038/sj.onc.1207001. [DOI] [PubMed] [Google Scholar]
- Villares GJ, Dobroff AS, Wang H, et al. Overexpression of protease-activated receptor-1 contributes to melanoma metastasis via regulation of connexin 43. Cancer Res. 2009;69:6730–7. doi: 10.1158/0008-5472.CAN-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares GJ, Zigler M, Wang H, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–86. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Friess H, Zhu Z, et al. Neurotensin receptor-1 mRNA analysis in normal pancreas and pancreatic disease. Clin Cancer Res. 2000;6:566–71. [PubMed] [Google Scholar]
- Wilson TJ, Nannuru KC, Singh RK. Cathepsin G recruits osteoclast precursors via proteolytic activation of protease-activated receptor-1. Cancer Res. 2009;69:3188–95. doi: 10.1158/0008-5472.CAN-08-1956. [DOI] [PubMed] [Google Scholar]
- White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–25. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, White DP, Caswell PT, et al. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–43. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]