Abstract
Epidemiological studies show that adverse cardiovascular events peak in the morning (i.e., between 6 AM and noon) and that shift work is associated with cardiovascular disease, obesity, and diabetes. The endogenous circadian timing system modulates certain cardiovascular risk markers to be highest (e.g., cortisol, nonlinear dynamic heart rate control, and platelet activation) or to respond most unfavorably to stressors such as exercise (e.g., epinephrine, norepinephrine, and vagal cardiac modulation) at an internal body time corresponding to the time of day when adverse cardiovascular events most likely occur. This indicates that the circadian timing system and its interaction with external cardiovascular stressors (e.g., physical activity) could contribute to the morning peak in adverse cardiovascular events. Moreover, circadian misalignment and simulated night work have adverse effects on cardiovascular and metabolic function. This suggests that misalignment between the behavioral cycle and the circadian timing system in shift workers contributes to that population’s increased risk for cardiometabolic disease.
Keywords: biological clock, circadian misalignment, glucose metabolism, heart, night work, shift work, suprachiasmatic nucleus
Introduction
Western societies are rife with cardiovascular disease, diabetes, and obesity. In the United States, it is estimated that 83 million adults have cardiovascular disease (Roger et al., 2011), 26 million have diabetes (Centers for Disease Control and Prevention, 2011), and 80 million are obese (Flegal et al., 2010). The influence of behaviors (e.g., poor diet and physical inactivity) as underlying causes for these diseases has been researched for decades. However, more recently, evidence has been accumulating for a contributing role of the endogenous circadian timing system and its disruption in cardiovascular and metabolic disorders. In this review, we discuss—with a focus on mammals and particularly humans—the impact of the circadian timing system, its interaction with behaviors (e.g., exercise), and its disturbance on cardiovascular and metabolic function.
The circadian timing system
Most life on earth—ranging from single cellular organisms, plants, flies, rats, to humans—contains an endogenous timing system that optimally synchronizes physiology and behavior (e.g., rest/ activity and fasting/feedings cycles) with the solar day. The system is known as the circadian (“circa,” around; “dies,” day) timing system and has two core characteristics: (1) endogenous rhythmicity that cycles approximately every 24 h, even in the absence of cyclic changes in external factors such as light and temperature and (2) the capability to adjust its timing in response to external factors such as light and/or food intake. The circadian timing system engineers a “biological day” and “biological night” that transition in a cyclic manner. Here, we define the biological night as the endogenous circadian time window corresponding to the habitual dark episode, that is, the time normally characterized by behavioral inactivity in diurnal (day-active) species and behavioral activity in nocturnal (night-active) species. The opposite holds true for the term biological day. In mammals, the biological night is also the time when melatonin plasma concentrations are high. This is true both for diurnal and nocturnal mammals. The transition between the biological day and night is associated with relatively large changes in many physiological variables, such as circulating levels of melatonin and cortisol, and core body temperature (Dijk et al., 1999; Gooley et al., 2011; Scheer et al., 2009; Van Cauter et al., 1994; Wehr et al., 2001).
In mammals, the circadian timing system is composed of the suprachiasmatic nucleus (SCN) and circadian oscillators in most peripheral tissues. The SCN is situated in the anterior hypothalamus on top of the optic chiasm and next to the third ventricle. The SCN is a bilateral structure that contains approximately 50,000 neurons in humans and 20,000 neurons in rats (Güldner, 1983; Hofman et al., 1988; Swaab et al., 1985; van den Pol, 1980). Various levels of evidence show that the SCN regulates circadian rhythms: (1) physiological and behavioral rhythms are abolished in SCN-lesioned animals (Moore and Eichler, 1972; Stephan and Zucker, 1972; van den Pol and Powley, 1979), (2) SCN-lesioned animals regain circadian rhythms in locomotor activity following receipt of a donor-SCN (Lehman et al., 1987), (3) SCN-lesioned animals that receive an SCN transplantation exhibit the same period length of the donor animal (Ralph et al., 1990), and (4) neural firing rate of the SCN exhibits a circadian rhythm in vivo and in vitro (Green and Gillette, 1982; Groos and Hendriks, 1982; Meijer et al., 1997). Circadian oscillators—cells that generate circadian rhythms autonomously from others—are also located in the periphery (e.g., the heart, liver, and pancreas). In addition to the SCN, they express circadian rhythms in gene expression, which ultimately can produce endogenous cyclic rhythms in biology independent of input from the central pacemaker (Balsalobre et al., 1998; Brown et al., 2005; Ko and Takahashi, 2006; Mühlbauer et al., 2004; Storch et al., 2002; Yamazaki et al., 2000; Yoo et al., 2004). However, the SCN is considered to be the master pacemaker because synchrony between peripheral clocks within organs is typically lost without input from the SCN (Guo et al., 2006).
Circadian rhythms are generated and regulated by the concerted expressions of core clock genes, which compose the primary mammalian oscillatory mechanism (Ko and Takahashi, 2006; Lowrey and Takahashi, 2004). A complex molecular network of positive and negative feedback loops drive circadian rhythms in core clock genes, such as Clock (circadian locomoter output cycles kaput), Bmal1 (brain and muscle arnt-like protein-1), Cryptochrome (Cry1, Cry2), and Period (Per1, Per2, and Per3; Lowrey and Takahashi, 2004; Reppert and Weaver, 2001; Shearman et al., 2000b). Participating in the primary feedback loop are the transcription factors, Clock and Bmal1, which dimerize and activate the transcription of target genes, such as Pers and Crys (Gekakis et al., 1998; King et al., 1997; Reppert and Weaver, 2002). Subsequently, Clock-Bmal1-mediated transcription is negatively regulated by the Per–Cry complex (Jin et al., 1999; Kume et al., 1999; Reppert and Weaver, 2001; Zylka et al., 1998).
The importance of Bmal1 in generating circadian rhythms is demonstrated by homozygous Bmal1-deficient mice (Bmal1−/−), which cannot entrain to light/dark cycles and are arrhythmic (Bunger et al., 2000). Mice possessing the antimorphic (or dominant-negative) mutation (ClockΔ19/Δ19) display a longer period (~27.3 h) and arrhythmicity in constant darkness (Vitaterna et al., 1994). These studies suggest that the Clock:Bmal1 complex is a crucial driving force in circadian clock function. However, this theory is challenged by researchers who recently demonstrated that Clock-deficient mice (Clock−/−) maintain robust circadian rhythmicity in locomotor activity in constant darkness, albeit with a shorter period (~23.2 h; DeBruyne et al., 2006). Disruptions of negative feedback elements (e.g., Per1, Per2, Per3, Cry1, and Cry2) also modify circadian rhythms (Cermakian et al., 2001; Shearman et al., 2000a; Thresher et al., 1998; Van Der Horst et al., 1999; Vitaterna et al., 1999; Zheng et al., 1999, 2001).
Because the cycle length of the circadian timing system is not exactly 24 h (Czeisler et al., 1999; Duffy et al., 2011), external photic input (i.e., the light/dark cycle) and/or nonphotic input (e.g., food intake) is needed to entrain it with the environment. The strongest Zeitgeber (German: “time giver”) to the central pacemaker is light. For brevity, we only discuss entrainment by light in this review. Intrinsically photosensitive retinal ganglion cells containing the photopigment melanopsin, together with rods and cones, initially detect light (Gooley et al., 2001; Hattar et al., 2002). This signal is then passed along the retinohypothalamic tract to the SCN (Moore et al., 1995). The influence of light on the circadian timing system is dependent on the circadian time of exposure. In both diurnal and nocturnal animals, light exposure during the biological evening/early night phase delays circadian time relative to clock time; the opposite occurs (i.e., a phase advance) when light exposure takes place during the biological morning (Khalsa et al., 2003; Rosenberg et al., 1991).
The internal clock and the timing of adverse cardiovascular events
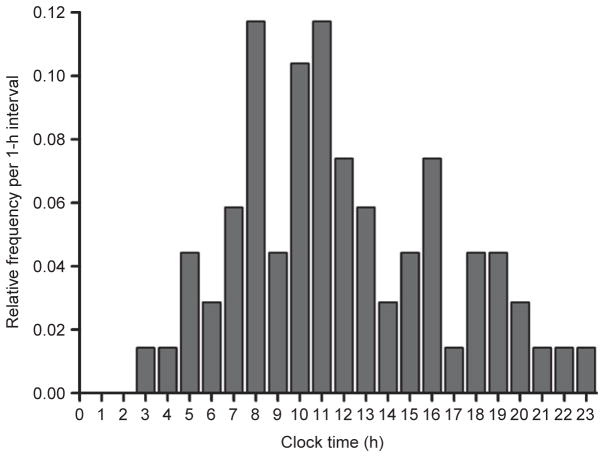
Epidemiological data demonstrate a 24-h rhythm in the frequency of adverse cardiovascular events such as angina, myocardial infarction, stroke, arrhythmias, cardiac arrest, and sudden cardiac death (Fig. 1), with the highest incidence occur ring between approximately 6 AM and noon (Cannon et al., 1997; Cohen et al., 1997; D’Avila et al., 1995; Elliott, 1998; Goldberg et al., 1990; Levine et al., 1992; Marler et al., 1989; Muller et al., 1985, 1987; Twidale et al., 1989; Willich et al., 1987, 1992). The above studies may under-report the occurrence of adverse cardiovascular events during nighttime due to people normally sleeping at this time. However, this possible reporting bias does not apply to the morning peak in arrhythmias because the precise timing of the events was recorded by electrocardiographic recordings (e.g., see Twidale et al., 1989). Furthermore, data obtained from implanted cardioverter-defibrillators—which also record the precise timing of the events—show that the defibrillation threshold (i.e., the amount of energy required) needed for successful defibrillation is greatest in the morning (Venditti et al., 1996). The use of plasma creatine phosphokinase MB activity—which is elevated approximately 4 h after the onset of a myocardial infarction—has confirmed that the incidence of myocardial infarction peaks between 6 AM and noon (Muller et al., 1985; Roberts et al., 1975; Willich et al., 1989). In patients with sleep apnea, however, the peak in adverse cardiovascular events occurs at night, possibly due to the acute hemodynamic, autonomic, and oxidative stress associated with apneas (Gami et al., 2005). This further suggests that, since the peak for adverse cardiovascular events for the population as a whole (including those with sleep apnea) occurs in the morning, the morning peak in those people without sleep apnea may be even more pronounced.
Fig. 1.
Relative frequency histogram for the time of onset of sustained ventricular tachycardia across the day. The figure illustrates a broad peak in the onset of sustained ventricular tachycardia between 8 and 11 AM. Redrawn and reproduced with permission from Twidale et al. (1989).
In order to understand the underlying mechanisms for the morning peak in adverse cardiovascular events, studies have mainly focused on the impact of behavioral changes typical of the morning, such as the change in posture from supine to upright and the start of behavioral activity at awakening. However, the circadian timing system may also play a role in the morning peak in adverse cardiovascular events. That the SCN may modulate cardiovascular functioning is suggested by the presence of multisynaptic projections from the SCN to the heart and organs that regulate cardiovascular function through hormones and blood volume regulation (e.g., adrenal cortex, adrenal medulla, and kidneys; Buijs et al., 1993; Scheer et al., 2001, 2003; Sly et al., 1999). Moreover, the above mentioned organs themselves rhythmically express clock genes, suggesting that the cardiovascular system could be influenced by the circadian timing system at the local level in addition to input from the central pacemaker (Valenzuela et al., 2008; Yamamoto et al., 2004). In light of the above findings and the 24-h rhythm in adverse cardiovascular events, researchers have investigated if cardiovascular variables such as heart rate, blood pressure, autonomic nervous system activity, and platelet function exhibit circadian rhythmicity. From epidemiological studies, it is not possible to deduct the relative role of the circadian timing system versus behavioral and environmental effects because influences of behaviors and the internal circadian timing system occur in synchrony. Thus, this calls for experimental designs that can isolate these factors.
Circadian rhythms in humans can be assessed using either a constant routine protocol or a forced desynchrony protocol. The constant routine protocol, developed by Mills et al. (1978), enables the assessment of circadian rhythms because the influences of behavioral and environmental factors are minimized and equally distributed across the circadian cycle by maintaining constant wakefulness, constant semi-recumbent posture; limiting physical activity, consistent dim light conditions; and evenly distributing isocaloric snacks or continuously infusing nutrients. In the forced desynchrony protocol, developed by Kleitman (1963), subjects are scheduled to live on a fixed sleep/wake cycle that is adequately different from 24 h and in dim light conditions, such that the sleep/wake cycle is outside the range of entrainment of the master oscillator, causing the internal clock to “free run” or drift according to its own internal period. The forced desynchrony protocol allows the separate assessment of circadian and behavioral influence by evenly spreading behavioral (e.g., sleep and wakefulness) factors across the circadian cycle.
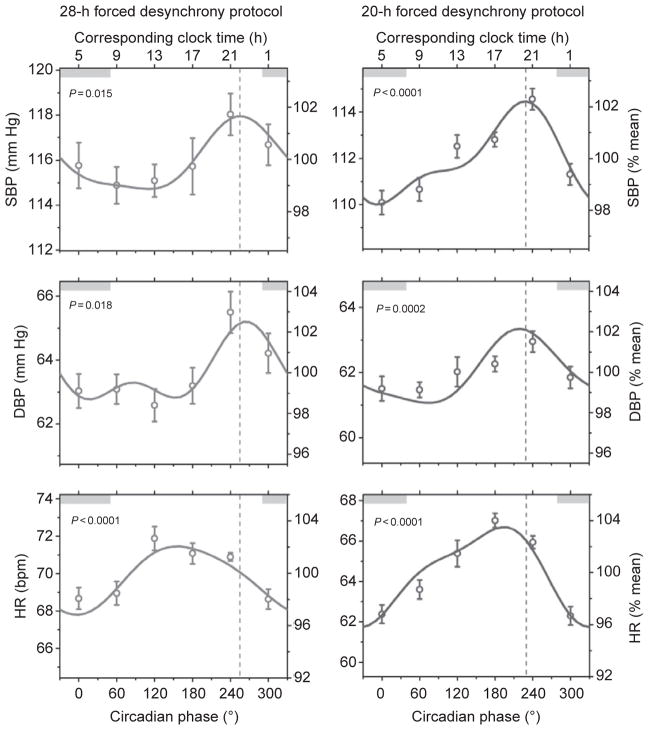
A circadian rhythm in resting heart rate has been reported by different research groups in healthy humans, with a broad peak occurring during the middle of the biological day and a trough during the biological night (Figs. 2 and 3; Burgess et al., 1997; Kräuchi and Wirz-Justice, 1994; Scheer et al., 2010; Shea et al., 2011). On the other hand, the reactivity of heart rate to standardized exercise and postural changes is not influenced by the circadian timing system (Fig. 3; Hu et al., 2011; Scheer et al., 2010). Considering that the circadian peak in heart rate does not occur during the biological morning and that heart rate reactivity to behavioral stressors is not under circadian control, it is unlikely that the circadian timing system’s influence on heart rate contributes to the morning peak in adverse cardiovascular events. In rats, there is also a circadian rhythm in resting heart rate under constant dark conditions, thus even independent of the circadian rhythm in behavioral activity (Scheer et al., 2001). After lesioning the SCN, the circadian rhythm is abolished and the level of resting heart rate is intermediate between that during the biological day and night in intact animals. This suggests that the SCN has an inhibitory and excitatory influence on resting heart rate during the biological day and night, respectively. This is reminiscent of the regulation of melatonin and corticosteroids for which the SCN uses both inhibitory (e.g., GABA) and excitatory neurotransmitters (e.g., glutamate; Kalsbeek et al., 1996; Perreau-Lenz et al., 2003).
Fig. 2.
Circadian rhythm in resting systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) observed in two (28- and 20-h) forced desynchrony protocols. Data are reported as mean±standard error of the mean and are expressed in absolute values (left axes) and as percentages of individual participant’s averages (right axes). Data are plotted according to circadian phase, that is, separated into six 60°-bins which all equate to ~4 h. Gray bars represent the participant’s average normal clock time for sleep under ambulatory conditions in the 2 weeks prior to admission to the laboratory. Solid lines represent the cosinor model fits. Dashed vertical lines indicate the circadian phase at which SBP peaked in both forced desynchrony protocols. Probability data indicate the likelihood of a circadian rhythm in blood pressure and heart rate. Reproduced with permission from Shea et al. (2011).
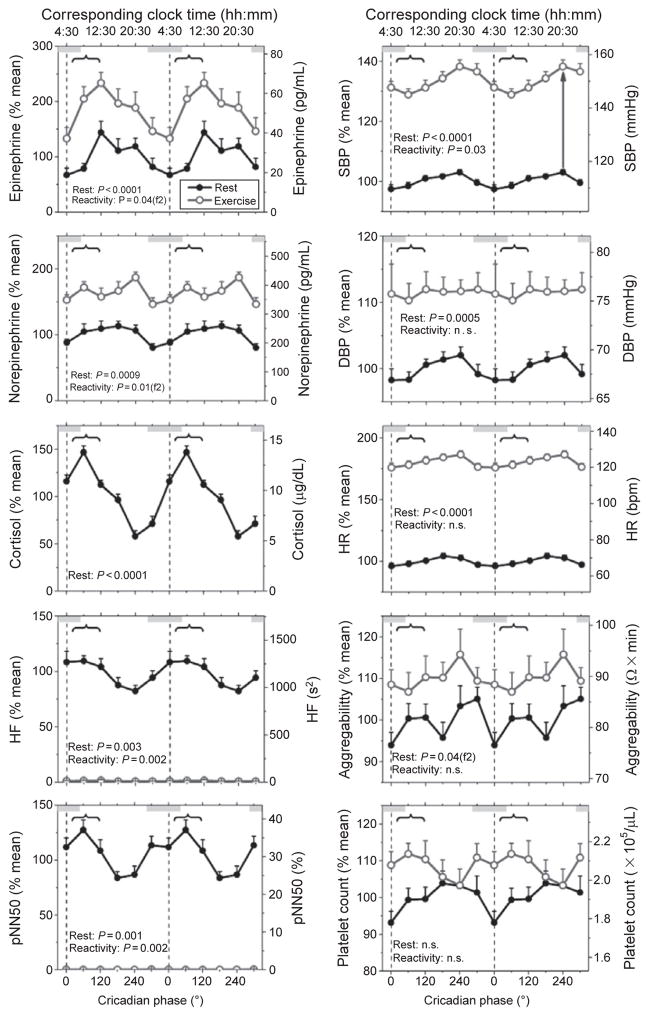
Fig. 3.
Influence of the endogenous circadian timing system on cardiovascular variables at rest and in response to standardized bicycle exercise. Participants undertook an 11-day (including 12 times 20-h “days”) forced desynchrony protocol in which they undertook 15 min of exercise (60% of maximum heart rate) at the same time into each wake period. Data are expressed relative to each participant’s resting value averaged across the whole forced desynchrony protocol (left axes) and in absolute values (right axes) and are plotted according to circadian phase that is separated into six 60°-bins that each equate to ~4 h. Black lines and closed circles indicate resting values, whereas gray lines with open circles represent data obtained during standardized exercise; error bars represent the standard error of the mean. Gray bars indicate the group average habitual clock time for sleep in the 2 weeks prior to admission to the laboratory. The vertical dotted line represents the timing of the group average core body temperature minimum and curly brackets signify the most vulnerable period for adverse cardiovascular events according to epidemiological research (6 AM to noon). Probability data were obtained via cosinor analysis. A statistically significant (P<0.05) second harmonic of a circadian rhythm is indicated by f2 appearing after the probability value. The arrow in the upper right plot is an example of the reactivity of systolic blood pressure to exercise at particular circadian phase. Reproduced with permission from Scheer et al. (2010).
Despite the fact that blood pressure is often mentioned as a key example of a physiological variable under circadian control, two studies using constant routine protocols found no evidence for an endogenous circadian rhythm in blood pressure (Kerkhof et al., 1998; Van Dongen et al., 2001). However, recently, we demonstrated a robust endogenous circadian rhythm in blood pressure in healthy humans, with very similar timing and amplitude across three different protocols (a constant routine and two forced desynchrony protocols) that factor out environmental (e.g., light) and behavioral (e.g., sleep/ wake cycle and physical activity) influences (Fig. 2; Shea et al., 2011). The circadian peak in blood pressure occurred during the biological evening, corresponding to approximately 9 PM. The lack of a circadian rhythm in blood pressure in previous studies was likely related to methodological limitations, including lighting control, posture, and circadian phase assessment (see Shea et al., 2011). The reactivity of blood pressure to exercise, but not postural change, is also controlled by the circadian timing system, again with the greatest response occurring during the biological evening (Fig. 3; Hu et al., 2011; Scheer et al., 2010). Considering that the circadian peak in blood pressure at rest and in response to exercise does not occur during the biological morning, it is unlikely that the circadian timing system’s influence on blood pressure contributes to the morning peak in adverse cardiovascular events. However, future studies are required to determine whether the timing or amplitude of the circadian blood pressure rhythm is changed in individuals at risk for or with underlying cardiovascular disease.
Platelets are a key component in the development of thromboses that cause myocardial infarctions and ischemic strokes. Recent work has demonstrated in healthy humans that platelet size, count, aggregability, and platelet surface expression of activated GPIIb-IIIa, P-selectin, and GP1b—factors involved in the pathway of platelet aggregation and adhesion to subendothelial tissue (Merten et al., 2000; Phillips et al., 1988; Sadler, 1998)—are influenced by the circadian timing system (Scheer et al., 2011). For platelet aggregability and platelet count, a subset of the data regarding the effect of exercise showed no significant circadian rhythms, but a 12-h rhythm in aggregability (see Fig. 3 for platelet aggregability and count data; Scheer et al., 2010). A more comprehensive dataset (more than four times larger) and analysis on the effect of the circadian timing system, mental stress, passive head-up tilt, and exercise demonstrated significant circadian rhythms, not only in platelet aggregability and platelet count, but also in platelet size and platelet surface expression of activated GPIIb-IIIa, P-selectin, and GP1b (Scheer et al., 2011). Platelet count and aggregability peaked at a circadian phase equivalent to 3–8 PM and thus, the circadian timing system’s influence on platelet count and aggregability is unlikely to contribute to the morning peak in myocardial infarctions and ischemic strokes, unless the timing of its influence is disturbed in people at risk for thrombotic events. On the other hand, platelet size and surface expressions of activated GPIIb-IIIa, P-selectin, and GP1b were all greatest during the biological morning, corresponding to 6 AM to noon (Scheer et al., 2011), suggesting that the circadian timing system may have a role in the greater incidence of adverse cardiovascular events in the morning through these thrombotic factors. The reactivity of the above platelet measures to mental, postural, and physical stress was not significantly influenced by the circadian timing system, and thus, the influence of the circadian timing system and that of behavioral stressors appear to be additive (Scheer et al., 2011).
In humans, resting plasma epinephrine and norepinephrine levels—markers of sympathetic nervous activity—exhibit endogenous circadian rhythmicity with a broad peak during the middle of the biological day (Fig. 3; Hu et al., 2011; Scheer et al., 2009, 2010, 2011; Shea et al., 2011). Therefore, if the timing would be similar in vulnerable populations, the circadian peak of catecholamines unlikely contributes to the morning peak in adverse cardiovascular events. Whether the doubling in epinephrine concentrations during the biological morning, possibly coinciding with sensitized adrenergic receptors and the circadian peak in cortisol, is of relevance to the morning peak in adverse cardiovascular events requires further study (Scheer et al., 2010). Another cardiovascular biomarker that is under circadian control is a measure of scale-invariant, or fractal, behavior of heart beat variability, which shows a peak at a circadian phase equivalent to approximately 9 AM (Hu et al., 2004). This fractal pattern breaks down with disease and its change is predictive of mortality (Hu et al., 2009; Mäkikallio et al., 2004; Peng et al., 1995). Furthermore, lesioning of the SCN abolishes such fractal patterns in heart beat variability at time scales between 4 and 24 h, rendering the pattern similar to random noise and demonstrating the SCN as a critical node in the neural feedback network underlying scale-invariant patterns in physiology (Hu et al., 2008).
The reactivity of epinephrine and norepinephrine in healthy humans to exercise is dependent on circadian phase, with two peaks: one in the biological morning and the other in the biological evening (Fig. 3; Scheer et al., 2010). These morning peaks coincide with the circadian peak in cortisol (Scheer et al., 2009, 2010; Wehr et al., 2001), which may further increase cardiac vulnerability since cortisol can potentiate the effects of the sympathetic nervous system (Davies and Lefkowitz, 1984). Also, the epinephrine response to a passive head-up tilt in healthy humans is controlled by the circadian timing system, with a broad peak occurring during biological evening (Hu et al., 2011). Taken all together, the circadian timing system’s influence on the reactivity of epinephrine and norepinephrine to exercise, but not passive head-up tilt, may contribute to the morning peak in adverse cardiovascular events.
Markers of parasympathetic nervous activity display a circadian rhythm at rest in healthy humans, with peaks in the biological night and morning, and thus may be “protective” in the morning, at least in healthy subjects at rest (Fig. 3; Burgess et al., 1997; Hu et al., 2011; Scheer et al., 2010; Shea et al., 2011). The circadian timing system also influences the reactivity of the sympathetic nervous system to behavioral stressors, including exercise and passive head-up tilt, with the largest reduction in parasympathetic markers occurring in the biological morning, corresponding to approximately 9 AM (see Fig. 3 for responses to exercise; Hu et al., 2011; Scheer et al., 2010). If confirmed in vulnerable populations, the impact of the internal circadian timing system on reactivity of parasympathetic modulation of the heart in response to behavioral stressors such as exercise could thus contribute to the morning peak in adverse cardiovascular events (Vanoli et al., 1991).
A recent study in mice demonstrated that 45 min of experimenter-induced ischemia of the coronary artery at the beginning of the biological night (start of the active phase, thus equivalent to the human morning) as compared to the start of the biological day resulted in greater infarct volume, fibrosis, adverse remodeling, and depression of contractile function after 1 month of reperfusion (Durgan et al., 2010). Moreover, deletion of the cardiomyocyte circadian clock gene attenuated the above mentioned outcomes. This indicates that the cardiomyocyte circadian clock gene contributes to the time of day variation in responses to coronary artery ischemia and subsequent reperfusion.
Circadian disruption and cardiovascular function
In the previous section, we provided recent evidence for the impact of the circadian timing system on cardiovascular risk markers that—if confirmed in vulnerable populations—may contribute to the morning peak in adverse cardiovascular events. However, in healthy individuals, the circadian timing system has been proposed to optimally regulate many physiological processes to prepare for the varying demands across the sleep/wake cycle. If this would be the case, it would be expected that circadian disruption et would have adverse health effects. Circadian disruption can be the result of, for example, circadian misalignment (in which circadian function is intact, but not properly aligned with either the external environment or among different components of the circadian timing system), neuroanatomical damage or changes to the SCN, and genetic mutations and variance (for more details, see Rüger and Scheer, 2009). Circadian misalignment occurs when the circadian timing system as a whole is desynchronized from the behavioral and/or environmental cycles, and this can be caused by external factors (e.g., rapidly shifting the light/dark and sleep/wake cycle as seen in shift workers and with jet lag) and internal factors (e.g., blindness preventing entrainment by light of the circadian timing system to the 24-h light/ dark cycle). Circadian misalignment can also occur among the different circadian oscillators in the body, for example, between the SCN and peripheral oscillators in the liver, as a result of “internal desynchrony.” Circadian disruption with underlying neuroanatomical changes in the SCN has been demonstrated in Alzheimer’s disease and depression (Wu et al., 2006; Zhou et al., 2001). Animal experimental SCN lesions and human case studies of tumors in proximity to the SCN have also been shown to lead to circadian disruption (Moore and Eichler, 1972; Schwartz et al., 1986). Examples of circadian clock gene mutations which impact circadian function include familial advanced sleep phase syndrome in humans and the many clock gene mutations in experimental animals (Toh et al., 2001; Turek et al., 2005). These categories are not mutually exclusive, for example, familial advanced sleep phase syndrome can lead to circadian misalignment. In this section, we will provide some examples of the detrimental cardiovascular health consequences of circadian disruption. Adverse metabolic consequences of circadian disruption will be discussed in the next section.
Epidemiological studies indicate that shift work is associated with cardiovascular disease (Kawachi et al., 1995; Knutsson et al., 1986, 1999; Suwazono et al., 2008b). Because differences in socioeconomic status and life style cannot fully explain these observations, researchers have begun investigating whether circadian misalignment itself may be one of the underlying mechanisms of the above associations. Human studies investigating the effect of circadian misalignment or simulated night work on cardiovascular function in controlled laboratory conditions are scarce. Scheer et al. (2009) demonstrated that circadian misalignment (i.e., desynchrony between the circadian timing system and behaviors) increases wake time blood pressure. Recent research has demonstrated that an acute bout of evening exercise significantly lowers blood pressure during subsequent simulated night work, suggesting that the adverse effect of circadian misalignment on blood pressure could be negated by evening exercise (Fullick et al., 2009). Clearly, more experimental human research on the effects of circadian disruption on cardiovascular function is needed.
There is also evidence of adverse cardiovascular effects of desynchrony between the circadian timing system and the imposed light/dark cycle from animal experimental work. Cardiomyopathic hamsters that are subjected to a 12-h shift in the light/dark cycle every week have a shorter life span than cardiomyopathic hamsters that maintain a fixed 24-h light/dark cycle (Penev et al., 1998). Furthermore, mice with surgically induced heart disease (cardiac hypertrophy through transverse aortic constriction) that live on an abnormal light/dark (10:10 h) cycle following surgery show impaired compensatory cardiac and vascular remodeling, reduced ventricular contractile strength, and increased blood pressure as compared to animals that live on a normal light/dark (12:12 h) cycle (Martino et al., 2007). Phenotypic rescue, including reversal/attenuation of abnormal pathology and genes, only occurs when the external rhythm is allowed to correspond with the animals’ innate 24-h internal rhythm.
Further evidence for a critical role of proper synchrony between the circadian timing system and the external environment comes from follow-up studies in Tau mutant hamsters. Tau mutant hamsters have a shorter period (~22 h) than their wild-type (~24 h) counterparts (Ralph and Menaker, 1988). Tau mutant hamsters have a shorter life span and have cardiomyopathy, fibrosis, and impaired heart contractility when they live on a 24-h light/dark cycle which is beyond their circadian timing system’s range of entrainment and thus causes the animal’s behavioral cycle and biological timing system to be out of phase (i.e., desynchronized; Martino et al., 2008). Remarkably, when Tau mutant hamsters are placed in 22-h light/dark cycles, similar to their endogenous circadian period, these pathological changes are largely reversed. Taken together, the above mentioned findings suggest that circadian disruption, such as with shift work, may worsen cardiovascular function in individuals with preexisting heart disease.
It has been hypothesized that dysfunction of the SCN is associated with hypertension. Levels of three key neurotransmitters (vasopressin, vasoactive intestinal peptide, and neurotensin) in the human SCN, required for proper circadian functioning, are lower in postmortem material of patients with essential hypertension than in that of control subjects (Goncharuk et al., 2001). At the same time, more corticotropin-releasing hormone (CRH) neurons and greater CRH mRNA are present in the paraventricular nucleus (PVN) of the hypothalamus of people with essential hypertension than controls (Goncharuk et al., 2002). Because CRH raises blood pressure when administered intraventricularly, it has been proposed that the elevated CRH levels in hypertensive patients may contribute to their hypertension (Kalin et al., 1983). The vasopressinergic neurons from the SCN have an inhibitory effect on CRH neurons in the PVN (Buijs et al., 1993; Hermes et al., 1996). Thus, the reduced levels of vasopressin in the SCN could result in disinhibition of CRH production by the PVN and, in turn, contribute to the abnormal 24-h variation in blood pressure observed in these patients (Goncharuk et al., 2002). Alternatively or additionally, it has been hypothesized that the reverse may be true and that the observed more intense CRH projection to the SCN in hypertensive patients contributes to the decrease in the number of vasopressin, vasoactive intestinal peptide, and neurotensin neurons in the SCN (Goncharuk et al., 2007). Based on the information above, the evidence that the SCN is involved in cardiovascular control (Scheer et al., 2003) and the observation that melatonin administration can amplify or synchronize circadian rhythms (Bothorel et al., 2002; Kostervan Hoffen et al., 1993; Zaidan et al., 1994), Scheer et al. (2004) investigated if 3 weeks of nighttime melatonin administration can improve blood pressure regulation in patients with essential hypertension. Repeated nighttime melatonin administration enhanced the day/night rhythm amplitude of blood pressure and reduced blood pressure during the patient’s scheduled sleep period. Of interest, acute nighttime melatonin intake did not influence blood pressure indicating that the improvement in blood pressure after 3 weeks of melatonin intake was not due to the drugs acute vasodilatory effect but possibly a result of its influence on the circadian timing system. The blood pressure lowering effect of melatonin after repeated administration in hypertensive patients has been replicated by others (Cagnacci et al., 2005; Grossman et al., 2006).
Other examples of an intricate link between the circadian timing system and cardiovascular control come from recent data on the influence of clock genes in hypertension and cardiac functioning. Mice without core clock components Cry1 and Cry2 develop hypertension when their diet contains salt, whereas wild-type mice do not (Doi et al., 2010). This finding may have been due to Cry-null mice overexcreting the hormone aldosterone which increases blood pressure through stimulation of reabsorption of sodium ions and water. Moreover, mice lacking the Clock gene specifically within cardiomyocytes have brachycardia, greater myocardial oxygen consumption, and decreased cardiac efficiency (Bray et al., 2008).
Circadian disruption and metabolic function
Human endogenous circadian rhythms have been observed in many factors related to metabolism. For example, glucose, insulin, cortisol, epinephrine, norepinephrine, and leptin display endogenous circadian variation (Morgan et al., 1998; Scheer et al., 2009, 2010; Shea et al., 2005; Van Cauter et al., 1994; Wehr et al., 2001). Recent research demonstrates that amino acid plasma concentrations are under endogenous circadian control, through Krüppel-like factor 15-control of the rhythmic expression of multiple enzymes involved in mammalian nitrogen homeostasis (Jeyaraj et al., 2012). SCN-intact rats, that have a fixed 6-meals-per-day feeding schedule (to uniformly distribute food intake across the circadian cycle), also have a circadian rhythm in glucose levels, showing that this is not dependent on the feeding/fasting cycle, and this rhythm is abolished in SCN-lesioned rats. Multisynaptic projections from the SCN to the liver, pancreas, adrenal cortex, and adipocytes have been discovered, and these provide neuroanatomical pathways by which the SCN can influence metabolic-related factors (Buijs et al., 1999, 2001; Kreier et al., 2006; la Fleur et al., 2000). The SCN can also influence metabolic-related factors (e.g., cortisol) via hormonal pathways (Buijs et al., 1993, 1999) and peripheral clocks per se can influence metabolism (Marcheva et al., 2010). Taken together, the circadian timing system is an important entity that contributes to the control of many metabolic-related processes, and thus it is conceivable that circadian disruption results in impaired metabolic function. In this section, we will provide some examples of the adverse metabolic-related consequences of circadian disruption.
Numerous epidemiological studies have demonstrated that shift work is a risk factor for metabolic disorders such as diabetes and obesity (Morikawa et al., 2005; Niedhammer et al., 1996; Suwazono et al., 2006, 2008a). As a consequence, researchers have employed experimental models to investigate the possible underlying mechanisms. A 9-h phase advance of the circadian timing system—a simulation of shift work—increased the glucose and insulin response to a test meal given at the same clock time when the test meal was preceded by a high-fat but not low-fat meal (Hampton et al., 1996; Ribeiro et al., 1998). Moreover, a 9-h phase advance only had a detrimental effect on the average triglyceride response to a test meal when the pretest meal is a low- rather than high-fat meal (Hampton et al., 1996; Ribeiro et al., 1998). The influence of macronutrient content of a meal on responses to subsequent meals during night work requires further research. Al-Naimi et al. (2004) extended the work of the above researchers by measuring glucose, insulin, and triglyceride responses to multiple meals across simulated day and night work shifts. The researchers reported that integrated glucose (statistical trend) and triglyceride levels were higher across the night shift than day shift. Integrated insulin levels were not different between the night shift and day shifts. Furthermore, postprandial glucose, insulin, and triglyceride levels are higher during night work than day work under field conditions (Lund et al., 2001). The glucose and insulin, but not triglyceride, postprandial responses normalize 2 days after ceasing night work, indicating that some—but not all—of the adverse metabolic and hormonal effects of night work quickly subside after short-term exposure (Lund et al., 2001; Ribeiro et al., 1998). How long it takes for adverse metabolic changes to normalize after chronic exposure, such as following chronic shift work, requires further study.
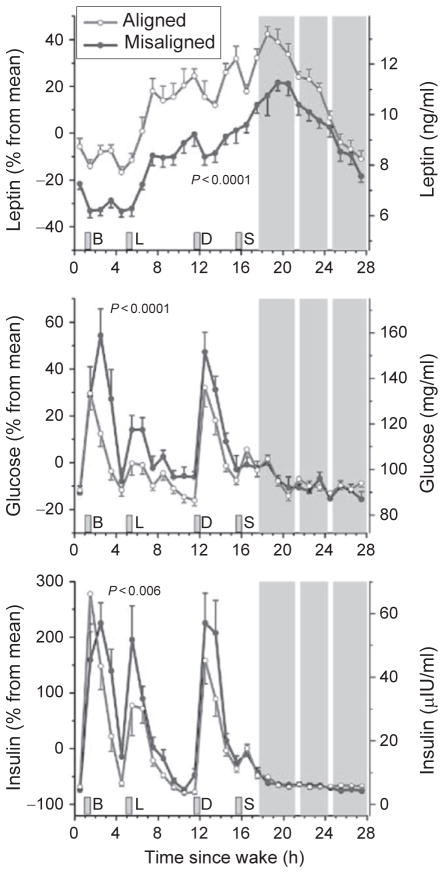
The simulated shift work studies discussed above did not replicate the duration of wakefulness and behavioral activity before tests meals in both experimental conditions. Thus, it is unclear from those studies what the contribution is of circadian misalignment per se on metabolic and hormonal factors. Scheer et al. (2009), utilizing a forced desynchrony protocol, demonstrated that short-term exposure (after 3 days) to circadian misalignment per se increases the postprandial responses of glucose and insulin, demonstrating reduced glucose tolerance and suggesting reduced insulin sensitivity under these conditions (Fig. 4). It has also been demonstrated, in rats prone to the development of type 2 diabetes, that phase advancing the light/dark cycle by 6 h every 3 days for 10 weeks accelerates the onset of type 2 diabetes by causing a hastened loss of beta-cell function and mass (Gale et al., 2011). Two studies have assessed the effect of more prolonged circadian misalignment on human metabolism under controlled laboratory conditions. Morgan et al. (1998) found no significant changes in the concentrations of glucose, insulin, and triglycerides as measured in two constant routine protocols with small hourly liquid meals, before and after a 19-day forced desynchrony protocol. Recently, it was demonstrated in humans that 3 weeks of sleep restriction (5.6 h of sleep opportunity per 24 h) concurrent with circadian disruption increases postprandial glucose levels, possibly due to a reduction in post-meal insulin concentrations (Buxton et al., 2012). These adverse alterations were abolished following 9 days of recovery sleep and circadian re-entrainment to a normal sleep/wake cycle.
Fig. 4.
The response of circulating levels of leptin, glucose, and insulin to circadian misalignment. Data are reported relative to each participant’s average values during circadian alignment on the left axes and in absolute values on the right axes. Error bars represent standard error of the mean. Gray area indicates the participant’s sleep opportunity. The white strips within the scheduled sleep opportunity represent when participants were briefly awoken to perform pulmonary function tests. B, breakfast; L, lunch; D, dinner; S, snack. Reproduced with permission from Scheer et al. (2009).
Short-term circadian misalignment while subjects consume isocaloric meals also decreases circulating levels of leptin (Fig. 4; Scheer et al., 2009). Such an effect, if persisted chronically, could help explain the increased risk for the development of obesity through increased food intake. In addition, rats subjected to simulated night work (forced activity during the normal rest phase) gain significantly more body mass than rats undertaking simulated day work (forced activity during the normal active phase; Salgado-Delgado et al., 2008). Total daily food intake did not differ between the two groups, but the “night workers” were less active and this may explain the above finding. Moreover, feeding mice for 6 weeks only during their biological day (normal rest phase) rather than biological night (normal active phase) increases their body fat percentage (statistical trend) and body mass (Arble et al., 2009). These increases may have been due to lower energy expended via physical activity and greater food intake in the mice fed during the biological day, although neither of these observations reached statistical significance. Exposing mice to light (150 lux of continues light or 16:8 150/5 lux cycle) during the habitual dark phase significantly increases body and fat mass and impairs glucose tolerance (Fonken et al., 2010). These results were not associated with any change in total 24-h food consumption or locomotor activity, but the mice in the 16:8 light/dim light cycle altered their feeding behavior, with more food being consumed during the habitual light phase.
Weekly 6-h phase advances and delays—experimental models applicable to shift work—significantly hasten mortality in mice (Davidson et al., 2006). In an attempt to explore the mechanisms underlying these findings, Castanon-Cervantes et al. (2010) investigated the effect of four consecutive weekly 6-h phase advances of the light/dark schedule on immune responses in mice. Twenty-four hours after a lipopolysaccharide (LPS) challenge, systemic concentrations of the proinflammatory cytokines interleukine-1β (IL-1β), granulocyte macrophage colony-stimulating factor, IL-12, and IL-13 were significantly higher in the shifted than control mice. The circadian phase angle, as assessed by body temperature; locomotor activity; and Per2 expression in four tissues (SCN, liver, thymus, and spleen), at the time of the LPS challenge was similar between the shifted and control animals, and therefore the findings were not due to the stimulus being given at a different circadian time between the two groups. The increased IL-6 release in the shifted mice was also observed during in vitro LPS stimulation of macrophages. Furthermore, the risk of death following the in vivo LPS challenge was significantly greater in the shifted than control mice. The above findings clearly indicate that repeatedly shifting a mouse’s light/dark cycle has an adverse effect on their immune response. Whether similar adverse changes in immune function are also observed in humans requires further study.
It is unclear how simulated night work and circadian misalignment cause adverse effects such as impaired glucose metabolism. Both night work and circadian misalignment decrease total sleep time which also decreases leptin levels and impairs glucose tolerance and insulin sensitivity (Åkerstedt, 1998; Buxton et al., 2010; Ohayon et al., 2002; Spiegel et al., 1999, 2004). Thus, the question rises to what extent the adverse effects of night work and circadian misalignment are mediated via a disturbance of sleep. A covariance analysis performed by Scheer et al. (2009) suggests that the effect of circadian misalignment on leptin is likely to be at least in part independent of concurrent decreases in sleep efficiency. Moreover, slow wave sleep suppression per se has adverse effects on glucose metabolism (Tasali et al., 2008). However, during circadian misalignment, while sleeping during the biological day, there was no decrease in the amount of slow wave sleep (Morris et al., 2012), such that also changes in slow wave sleep could not explain these results. Thus, these data suggest that the adverse effects of night work and circadian misalignment on endocrine factors are not merely mediated by a reduction in sleep quality or quantity. Another possible mechanism underlying some adverse endocrine effects of night work and circadian misalignment relates to internal desynchrony caused by feeding schedules. Feeding rodents only during the rest phase—as similarly occurs during night work when they eat during their night shift—can phase shift peripheral oscillators in the liver, pancreas, kidney, and heart while it has no effect on the phase of the SCN (Damiola et al., 2000; Stokkan et al., 2001). Consequently, such a feeding regime can uncouple peripheral oscillators from the SCN. What the physiological effects of internal desynchrony are on metabolism and whether such internal desynchrony occurs also in humans in a shift work setting is currently unknown.
In addition to their participation in the generation of circadian rhythms, molecular clock genes have also been extensively reported to play a role in metabolic function. Homozygous ClockΔ19 mutant (C57BL/6J) mice have a significantly attenuated diurnal feeding rhythm and are hyperphagic and obese compared to wild-type mice (Turek et al., 2005). In addition, they exhibit adipocyte hypertrophy, lipid enlargement of hepatocytes with pronounced glycogen build-up, hypercholesterolemia, hypertriglyceridemia, hypoinsulinemia, and hyperglycemia. Marcheva et al. (2010) reported that whole-body Clock mutation caused impaired glucose tolerance in mice. The researchers also demonstrated that glucose-stimulated insulin secretion is reduced in isolated pancreatic islets from Clock mutants, which may explain the observed impaired glucose tolerance in these animals. Knockout of Bmal1 alone in C57BL/6J mice showed that isolated mouse embryonic fibroblast (MEF) cells could not successfully differentiate into adipocytes (Shimba et al., 2005). Further, restoration of Bmal1 function by adenovirus gene transfer into the initial Bmal1-deficient mice restored adipocyte differentiation and lipid accumulation in the MEF cells. The presence of high Bmal1 mRNA levels in adipocytes increased lipogenesis activity, further suggesting that Bmal1 is involved in regulating lipid metabolism. The hypoglycemic response following insulin administration is exacerbated in Clock mutant and Bmal1−/− mice as compared in wild-type mice, possibly due to an impaired counterregulatory gluconeogenesis response (Rudic et al., 2004). In parallel to the ClockΔ19 mutant mice (Turek et al., 2005), mPer2−/− mice also had a defective diurnal feeding rhythm compared to their wild-type littermates. These mice were also hyperphagic under a high-fat diet and this may explain why these animals became obese. In mice, genetic elimination of Cry increases the expression of genes responsible for hepatic glucogenesis and also raises fasting blood glucose level (Zhang et al., 2010). The above studies indicate that core clock genes participate in the regulation of metabolic processes and many of the phenotypes induced by dysfunctional/lost clock genes are hallmarks of metabolic syndrome.
Experimenters have also investigated the effect of organ-specific clocks on whole-body metabolism to differentiate the effects from behavioral changes. Some abnormal phenotypes displayed by global Bmal1−/− mice are observed in liver-specific knockouts. The loss of Bmal1 in the liver of mice leads to hypoglycemia and to a loss of rhythmic expression of hepatic genes that regulate glucose homeostasis (Lamia et al., 2008). In recent work, young pancreas-specific Bmal1 mutant mice showed increased ad libitum glucose levels, impaired glucose tolerance, lowered insulin secretion, and decreased insulin responsiveness to glucose (Marcheva et al., 2010). These mice had normal body weight, activity, and feeding rhythms, suggesting that the above mentioned phenotypes are attributable to pancreatic clock disruption per se rather than to secondary changes in behavior. These findings clearly reveal that the abscission/disruption of the pancreatic clock can lead to the onset of detrimental diseases such as diabetes. However, more evidence is still needed to determine which organ-specific clocks contribute to global metabolic functions and what the underlying mechanisms may be.
Countermeasures that prevent or attenuate the adverse endocrine effects associated with night work are needed. Researchers have demonstrated in humans that appropriately timed light exposure and/or melatonin intake can entrain the central pacemaker to an inverted (relative to the solar day) sleep/wake cycle (Boivin and James, 2002; Crowley et al., 2003; Czeisler et al., 1990; Smith et al., 2009). One would think that such a strategy would attenuate the adverse effects of night work on metabolic and hormonal factors; however, little research has been undertaken in this area. Furthermore, it is not known if the above strategy would entrain, in addition to the central pacemaker, pertinent peripheral oscillators, for example, in liver and pancreas. Recent work has demonstrated that restricting food intake in rodents to the biological night (typical active period) undertaking simulated night work prevents the gain in body mass and the accumulation of abdominal fat deposits induced by such a work schedule; perhaps by preventing internal desynchrony (Salgado-Delgado et al., 2010). This appears to be a promising countermeasure, and investigations are needed to determine if such a strategy is helpful in humans. Morning exercise can suppress daytime hunger and levels of acylated ghrelin (Broom et al., 2007). Morris et al. (2010) investigated if evening exercise suppresses hunger, acylated ghrelin levels, and increases leptin concentrations during simulated night work. Evening exercise increased levels of both acylated ghrelin and leptin but had no influence on mean hunger ratings. Participant’s activity—as measured by a wrist accelerometer—was higher during the simulated night shift that was preceded by exercise which could have beneficial implications for their energy balance. Clearly, more research is needed to determine the optimal countermeasures for the adverse endocrine effects of night work.
Summary
The circadian timing system orchestrates cyclic variations in numerous cardiovascular and metabolic functions independent of external influences such as darkness/light, sleep/wakefulness, rest/ activity, and fasting/eating. At rest, the circadian timing system causes some factors to peak during the biological morning (e.g., cortisol, platelet expression of activated GPIIb-IIIa, P-selectin, and GP1b), which could potentiate the onset of adverse cardiovascular events at that time. This suggests that the morning peak in adverse cardiovascular events may not be only due to the acute transition from a resting (sleeping, inactive, supine, and fasting) to an active (awake, active, standing, and eating) state. Moreover, the circadian timing system causes the greatest increase in epinephrine level and the largest decrease in parasympathetic nervous activity in response to exercise to occur in the biological morning. This further suggests that the circadian timing system contributes to the morning peak in adverse cardiovascular events. Previous work in the above area has been conducted using healthy people; thus, further research needs to be undertaken in individuals who are at risk of having an adverse cardiovascular event to determine whether there are changes in timing and amplitude of the endogenous circadian rhythms in cardiovascular risk markers. Research has also demonstrated that circadian disruption has a profound effect of cardiovascular and metabolic function. More research is needed to fully understand the underlying mechanisms. Such research may help in the development of novel therapies in the treatment of circadian related disorders.
Acknowledgments
C. J. M. was supported by the National Space Biomedical Research Institute through NASA NCC 9-58. F. A. J. L. S. was supported by National Institute of Health Grants P30-HL101299 and R01 HL094806.
References
- Åkerstedt T. Shift work and disturbed sleep/wakefulness. Sleep Medicine Reviews. 1998;2:117–128. doi: 10.1016/s1087-0792(98)90004-1. [DOI] [PubMed] [Google Scholar]
- Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiology International. 2004;21:937–947. doi: 10.1081/cbi-200037171. [DOI] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. Journal of Biological Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- Bothorel B, Barassin S, Saboureau M, Perreau S, Vivien-Roels B, Malan A, et al. In the rat, exogenous melatonin increases the amplitude of pineal melatonin secretion by a direct action on the circadian clock. The European Journal of Neuroscience. 2002;16:1090–1098. doi: 10.1046/j.1460-9568.2002.02176.x. [DOI] [PubMed] [Google Scholar]
- Bray MS, Shaw CA, Moore MWS, Garcia RAP, Zanquetta MM, Durgan DJ, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. American Journal of Physiology. Heart and Circulatory Physiology. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of Applied Physiology. 2007;102:2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biology. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. The Journal of Comparative Neurology. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: A light and electron microscopic study. The Journal of Comparative Neurology. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MGP, Ter Horst GJ, Romijn HJ, et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. The European Journal of Neuroscience. 1999;11:1535–154. 4. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Redcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. American Journal of Physiology. Heart and Circulatory Physiology. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse Metabolic Consequences in Humans of Prolonged Sleep Restriction Combined with Circadian Disruption. Science Translational Medicine. 2012;4(129):129ra143. doi: 10.1126/scitranslmed.3003200. http://dx.doi.org/10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. American Journal of Hypertension. 2005;18:1614–1618. doi: 10.1016/j.amjhyper.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cannon MDCP, McCabe BSCH, Stone MDPH, Schactman MSM, Thompson PB, Theroux MDP, et al. Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB) The American Journal of Cardiology. 1997;79:253–258. doi: 10.1016/s0002-9149(97)00743-1. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, et al. Dysregulation of inflammatory responses by chronic circadian disruption. The Journal of Immunology. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. The EMBO Journal. 2001;20:3967–3974. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. The American Journal of Cardiology. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. Journal of Biological Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. The New England Journal of Medicine. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- D’Avila A, Wellens F, Andries E, Brugada P. At what time are implantable defibrillator shocks delivered? European Heart Journal. 1995;16:1231–1233. doi: 10.1093/oxfordjournals.eurheartj.a061080. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & Development. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Current Biology. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AO, Lefkowitz RJ. Regulation of β-adrenergic receptors by steroid hormones. Annual Review of Physiology. 1984;46:119–130. doi: 10.1146/annurev.ph.46.030184.001003. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. Journal of Physiology (London) 1999;516(2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nature Medicine. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MY, Gronfier C, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, et al. Short communication: Ischemia/reperfusion tolerance is time-of-day–dependent. Circulation Research. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott WJ. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, et al. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullick S, Morris C, Jones H, Atkinson G. Prior exercise lowers blood pressure during simulated night-work with different meal schedules. American Journal of Hypertension. 2009;22:835–841. doi: 10.1038/ajh.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. Journal of Biological Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. The New England Journal of Medicine. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Brady P, Muller JE, Chen Z, de Groot M, Zonneveld P, et al. Time of onset of symptoms of acute myocardial infarction. The American Journal of Cardiology. 1990;66:140–144. doi: 10.1016/0002-9149(90)90577-n. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, Buijs RM, Swaab DF. Corticotropin-releasing hormone neurons in hypertensive patients are activated in the hypothalamus but not in the brainstem. The Journal of Comparative Neurology. 2007;503:148–168. doi: 10.1002/cne.21387. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, van Heerikhuize J, Dai JP, Swaab DF, Buijs RM. Neuropeptide changes in the suprachiasmatic nucleus in primary hypertension indicate functional impairment of the biological clock. The Journal of Comparative Neurology. 2001;431:320–330. doi: 10.1002/1096-9861(20010312)431:3<320::aid-cne1073>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: Activation of corticotropin-releasing hormone neurons. The Journal of Comparative Neurology. 2002;443:321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Research. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neuroscience Letters. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, et al. Melatonin reduces night blood pressure in patients with nocturnal hypertension. The American Journal of Medicine. 2006;119:898–902. doi: 10.1016/j.amjmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Güldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Experimental Brain Research. 1983;50:373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: Effects of transplanting the pacemaker. The Journal of Neuroscience. 2006;26:6406–6412. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, et al. Post-prandial hormone and metabolic responses in simulated shift work. The Journal of Endocrinology. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. The Journal of Physiology. 1996;496(Pt. 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: Sex differences and age-dependent changes. Journal of Anatomy. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Buijs RM, Shea SA. The endogenous circadian pacemaker imparts a scale-invariant pattern of heart rate fluctuations across time scales spanning minutes to 24 hours. Journal of Biological Rhythms. 2008;23:265–273. doi: 10.1177/0748730408316166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011;123:961–970. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Van Someren EJW, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraj D, Scheer FA, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, et al. Klf15 orchestrates circadian nitrogen homeostasis. Cell Metabolism. 2012;15:311–323. doi: 10.1016/j.cmet.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Kraemer GW, McKinney WT. Corticotropin-releasing factor administered intraventricularly to rhesus monkeys. Peptides. 1983;4:217–220. doi: 10.1016/0196-9781(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamopituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. The Journal of Neuroscience. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- Kerkhof GA, Van Dongen HPA, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? American Journal of Hypertension. 1998;11:373–377. doi: 10.1016/s0895-7061(97)00461-5. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. Journal of Physiology (London) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N. Sleep and wakefulness. Chicago: University of Chicago Press; 1963. [Google Scholar]
- Knutsson A, Åkerstedt T, Jonsson BG, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. The Lancet. 1986;2:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- Knutsson A, Hallquist J, Reuterwall C, Theorell T, Åkerstedt T. Shiftwork and myocardial infarction: A case-control study. Occupational and Environmental Medicine. 1999;56:46–50. doi: 10.1136/oem.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Human Molecular Genetics. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kostervan Hoffen GC, Mirmiran M, Bos NP, Witting W, Delagrange P, Guardiola-Lemaitre B. Effects of a novel melatonin analog on circadian rhythms of body temperature and activity in young, middle-aged, and old rats. Neurobiology of Aging. 1993;14:565–569. doi: 10.1016/0197-4580(93)90040-i. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. The American Journal of Physiology. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from fat tissue, liver, and pancreas: A neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Research. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. The Journal of Neuroscience. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Pepe PE, Fromm RE, Curka PA, Clark PA, Chernow B, et al. Prospective evidence of a circadian rhythm for out-of-hospital cardiac arrests. Journal of the American Medical Association. 1992;267:2935–2937. [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annual Review of Genomics and Human Genetics. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. The Journal of Endocrinology. 2001;171:557–564. doi: 10.1677/joe.0.1710557. [DOI] [PubMed] [Google Scholar]
- Mäkikallio AM, Mäkikallio TH, Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllylä VV. Heart rate dynamics predict poststroke mortality. Neurology. 2004;62:1822–1826. doi: 10.1212/01.wnl.0000125190.10967.d5. [DOI] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, et al. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476. doi: 10.1161/01.str.20.4.473. [DOI] [PubMed] [Google Scholar]
- Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49:1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Schaap J, Watanabe K, Albus H. Multiunit activity recordings in the suprachiasmatic nuclei: In vivo versus in vitro models. Brain Research. 1997;753:322–327. doi: 10.1016/s0006-8993(97)00150-9. [DOI] [PubMed] [Google Scholar]
- Merten M, Chow T, Hellums JD, Thiagarajan P. A new role for P-selectin in shear-induced platelet aggregation. Circulation. 2000;102:2045–2050. doi: 10.1161/01.cir.102.17.2045. [DOI] [PubMed] [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. The Journal of Physiology. 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. The Journal of Comparative Neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morgan L, Arendt J, Owens D, Folkard S, Hampton S, Deacon S, et al. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. The Journal of Endocrinology. 1998;157:443–451. doi: 10.1677/joe.0.1570443. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scandinavian Journal of Work, Environment & Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Molecular and Cellular Endocrinology. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Fullick S, Gregson W, Clarke N, Doran D, MacLaren D, et al. Paradoxical post-exercise responses of acylated ghrelin and leptin during a simulated night shift. Chronobiology International. 2010;27:590–605. doi: 10.3109/07420521003663819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Letters. 2004;564:91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. The New England Journal of Medicine. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- Niedhammer I, Lert F, Marne MJ. Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. International Journal of Obesity and Related Metabolic Disorders. 1996;20:625–633. [PubMed] [Google Scholar]
- Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. Journal of Psychosomatic Research. 2002;53:577–583. doi: 10.1016/s0022-3999(02)00438-5. [DOI] [PubMed] [Google Scholar]
- Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. The American Journal of Physiology. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics: Long-range correlations and their breakdown with disease. Journal of Electrocardiology. 1995;28(Suppl 1):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, et al. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. The European Journal of Neuroscience. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- Phillips D, Charo I, Parise L, Fitzgerald L. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71:831–843. [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. The Journal of Endocrinology. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- Roberts R, Gowda KS, Ludbrook PA, Sobel BE. Specificity of elevated serum MB creatine phosphokinase activity in the diagnosis of acute myocardial infarction. The American Journal of Cardiology. 1975;36:433–437. doi: 10.1016/0002-9149(75)90890-5. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics—2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RS, Zee PC, Turek FW. Phase response curves to light in young and old hamsters. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 1991;261:R491–R495. doi: 10.1152/ajpregu.1991.261.2.R491. [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biology. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger M, Scheer FA. Effects of circadian disruption on the cardiometabolic system. Reviews in Endocrine & Metabolic Disorders. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annual Review of Biochemistry. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R, Ángeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154:922–931. doi: 10.1016/j.neuroscience.2008.03.066. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biological Chemistry. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Michelson AD, Frelinger AL, III, Evoniuk H, Kelly EE, McCarthy M, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6:e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Ter Horst GJ, van der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Van Montfrans GA, Van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Busis NA, Tessa Hedley-Whyte E. A discrete lesion of ventral hypothalamus and optic chiasm that disturbed the daily temperature rhythm. Journal of Neurology. 1986;233:1–4. doi: 10.1007/BF00313981. [DOI] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circulation Research. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/ wake regulation of adipokines and glucose in humans. The Journal of Clinical Endocrinology and Metabolism. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: Subtle effects on circadian clock function. Molecular and Cellular Biology. 2000;20:6269–6275. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Colvill L, McKinley MJ, Oldfield BJ. Identification of neural projections from the forebrain to the kidney, using the virus pseudorabies. Journal of the Autonomic Nervous System. 1999;77:7–82.3. [PubMed] [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: Study 4. Journal of Biological Rhythms. 2009;24:161–172. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Tasali E, Penev P, Van Cauter E. Sleep curtailment results in decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity. 2008a;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, et al. Shift work is a risk factor for increased blood pressure in Japanese men. Hypertension. 2008b;52:581–586. doi: 10.1161/HYPERTENSIONAHA.108.114553. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Sakata K, Okubo Y, Harada H, Oishi M, Kobayashi E, et al. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. Journal of Occupational and Environmental Medicine. 2006;48:455–461. doi: 10.1097/01.jom.0000214355.69182.fa. http://dx.doi.org/10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twidale N, Taylor S, Heddle WF, Ayres BF, Tonkin AM. Morning increase in the time of onset of sustained ventricular tachycardia. The American Journal of Cardiology. 1989;64:1204–1206. doi: 10.1016/0002-9149(89)90881-3. [DOI] [PubMed] [Google Scholar]
- Valenzuela FJ, Torres-Farfan C, Richter HG, Mendez N, Campino C, Torrealba F, et al. Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: Is the adrenal a peripheral clock responsive to melatonin? Endocrinology. 2008;149:1454–1461. doi: 10.1210/en.2007-1518. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, et al. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. American Journal of Physiology. Endocrinology And Metabolism. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: Intrinsic anatomy. The Journal of Comparative Neurology. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Research. 1979;160:307–326. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- Van Der Horst GTJ, Muijtjens M, Kobayashi K, Takano R, Kanno SI, Takao M, et al. Mammalian cry1 and cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiology International. 2001;18:85–98. doi: 10.1081/cbi-100001178. [DOI] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari G, Stramba-Badiale M, Hull S, Foreman R, Schwartz P. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circulation Research. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- Venditti FJ, John RM, Hull M, Tofler GH, Shahian DM, Martin DT. Circadian variation in defibrillation energy requirements. Circulation. 1996;94:1607–1612. doi: 10.1161/01.cir.94.7.1607. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proceedings of the National Academy of Science of the United States of America. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Aeschbach D, Duncan WCJ. Evidence for a biological dawn and dusk in the human circadian timing system. The Journal of Physiology. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. The American Journal of Cardiology. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the framingham heart study population. The American Journal of Cardiology. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- Willich SN, Linderer T, Wegscheider K, Leizorovicz A, Alamercery I, Schroder R. Increased morning incidence of myocardial infarction in the ISAM Study: Absence with prior beta-adrenergic blockade. ISAM Study Group. Circulation. 1989;80:853–858. doi: 10.1161/01.cir.80.4.853. [DOI] [PubMed] [Google Scholar]
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. The FASEB Journal. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Molecular Biology. 2004;5:18. doi: 10.1186/1471-2199-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidan R, Geoffriau M, Brun J, Taillard J, Bureau C, Chazot G, et al. Melatonin is able to influence its secretion in humans: Description of a phase-response curve. Neuroendocrinology. 1994;60:105–112. doi: 10.1159/000126726. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nature Medicine. 2010;16:1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJG, van Heerikhuize JJ, Hofman MA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Archives of General Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside the brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]