Abstract
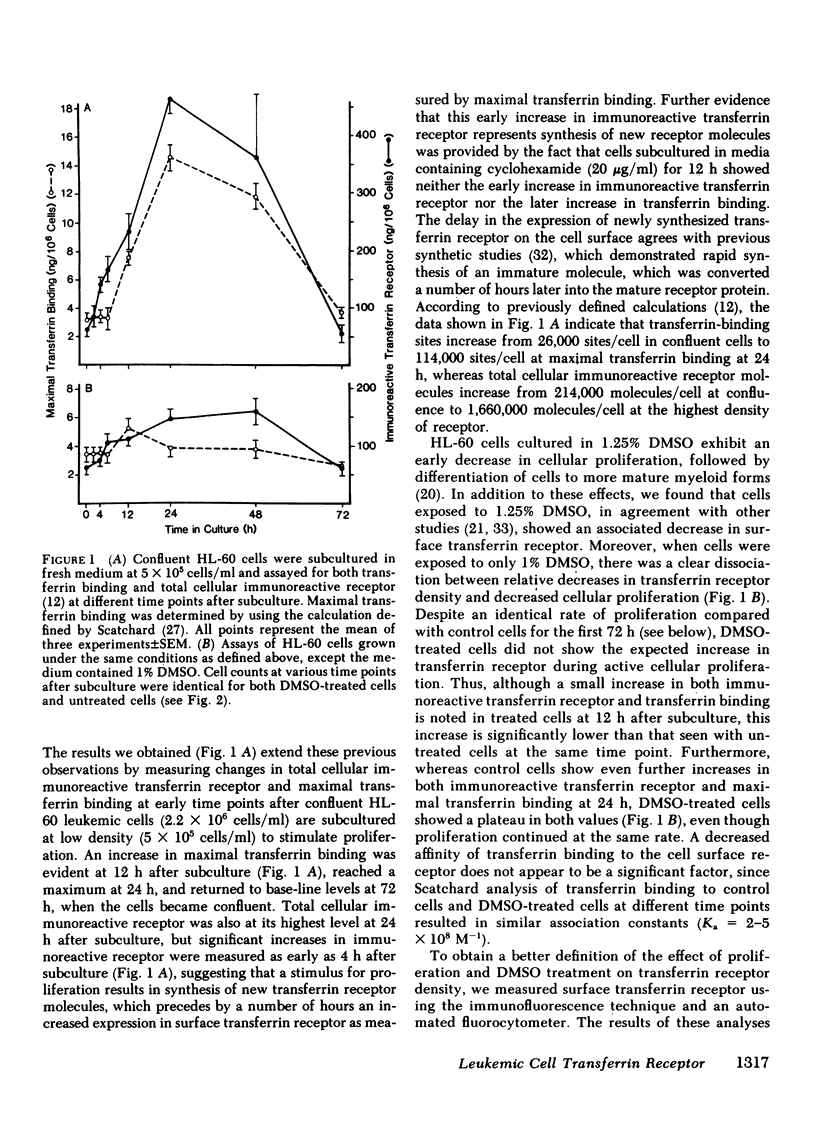
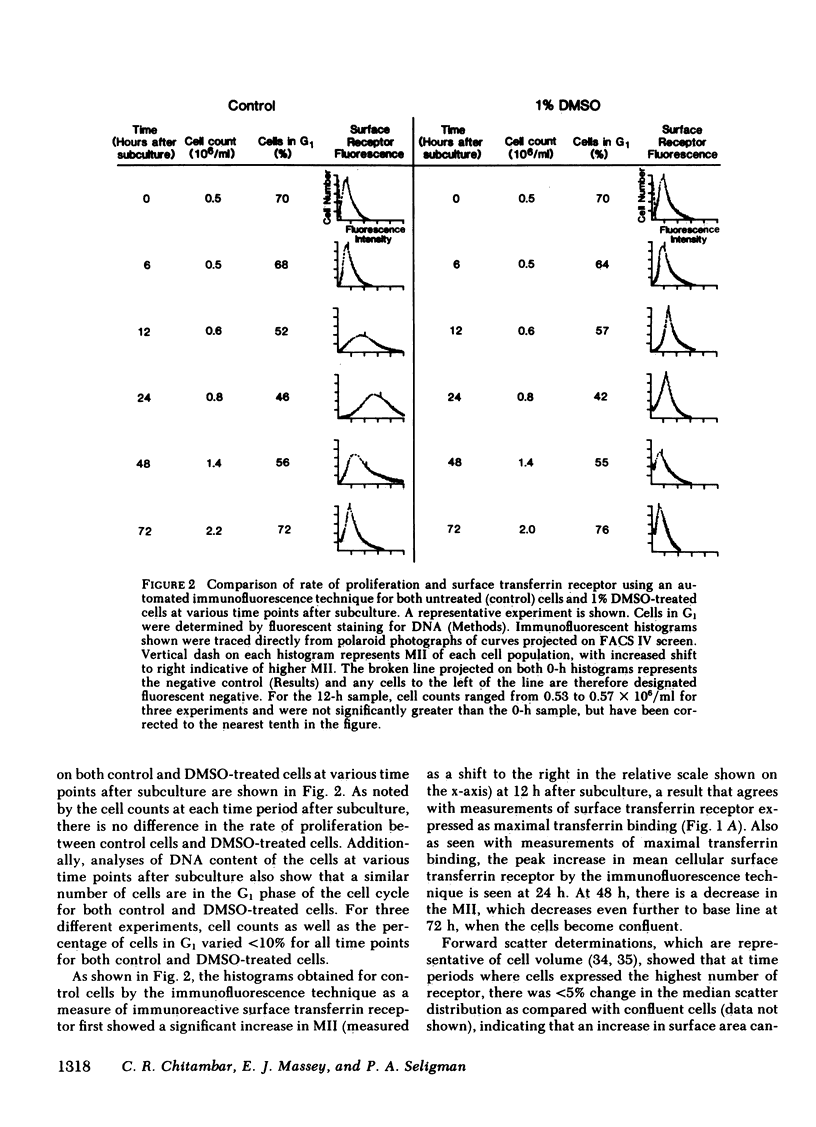
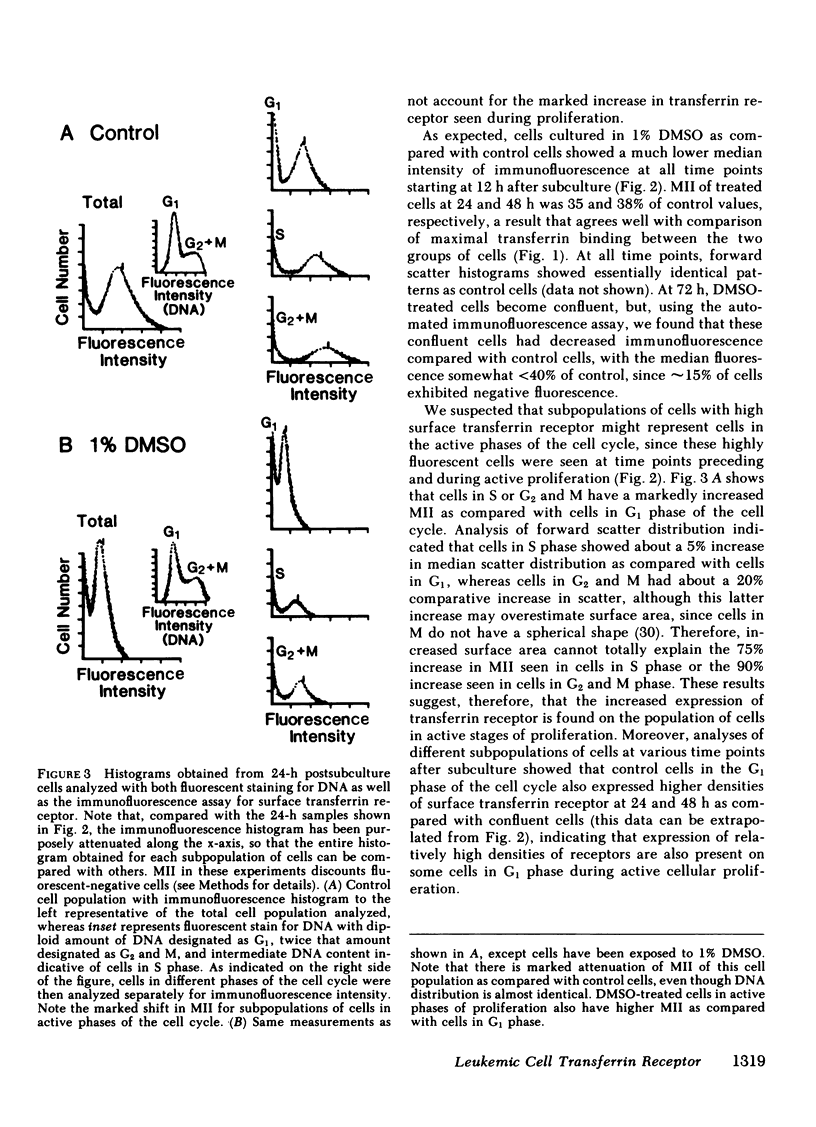
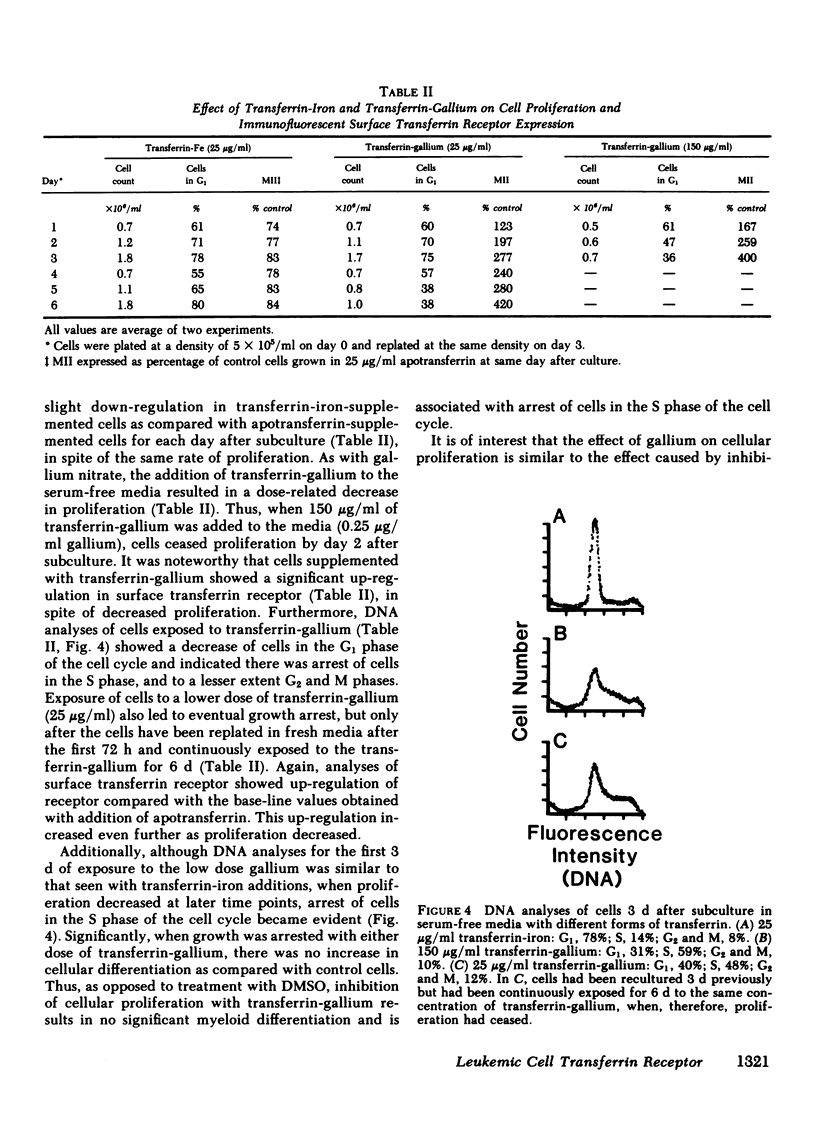
The association of transferrin receptor expression with cellular proliferation has been studied extensively, but a number of events have not been defined. We therefore assayed receptor on promyelocytic leukemia (HL-60) cells at early times after exposure to a stimulus for proliferation (subculture), as well as agents that either induce differentiation (dimethylsulfoxide [DMSO] ) or inhibit iron uptake (transferrin-gallium). Within 4 h after subculture, we found that a significant increase in total cellular immunoreactive receptor occurred that preceded by 8 h the increase in cell-surface transferrin binding. Automated fluorocytometric analysis of cells in an immunofluorescent assay indicated that increased surface receptor density appeared on cells in the S, G2, and M phases of the cell cycle. DMSO-treated cells proliferated at the same rate as untreated (control) cells for the first 72 h, but as early as 12 h after treatment transferrin receptor was significantly decreased (65% of control cells). Further decreases occurred at later time points until transferrin receptor was undetectable after 7 d, when proliferation had ceased, cells were arrested in G1 phase of the cell cycle, and myeloid differentiation occurred. After exposure to transferrin-gallium, proliferation ceased, but cells exhibited increased surface receptor and were arrested at S phase of the cell cycle without associated myeloid differentiation. We conclude that events preceding cell division provide the regulatory stimulus for the synthesis and subsequent appearance of the transferrin receptor on the cell surface. Additionally, decreased receptor expression may be important in causing cessation of proliferation and/or differentiation. Finally, the way in which gallium salts are currently being investigated as chemotherapeutic agents should be reevaluated in light of our findings concerning transferrin-gallium effects on cellular proliferation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. Structure and function of transferrin. Prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Replacement of serum by insulin and transferrin supports growth and differentiation of the human promyelocytic cell line, HL-60. Exp Cell Res. 1980 Apr;126(2):494–498. doi: 10.1016/0014-4827(80)90296-7. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram L. S., Brunsting A. Fluorescence and light-scattering measurements on hog cholera-infected PK-15 cells. Exp Cell Res. 1973 Mar 30;78(1):209–213. doi: 10.1016/0014-4827(73)90056-6. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns C. A., Shindelman J. E., Tonik S. E., Sussman H. H. Radioimmunochemical measurement of the transferrin receptor in human trophoblast and reticulocyte membranes with a specific anti-receptor antibody. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4222–4225. doi: 10.1073/pnas.78.7.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero D., Tarella C., Gallo E., Ruscetti F. W., Breitman T. R. Terminal differentiation of the human promyelocytic leukemia cell line, HL-60, in the absence of cell proliferation. Cancer Res. 1982 Nov;42(11):4421–4426. [PubMed] [Google Scholar]
- Frazier J. L., Caskey J. H., Yoffe M., Seligman P. A. Studies of the transferrin receptor on both human reticulocytes and nucleated human cells in culture: comparison of factors regulating receptor density. J Clin Invest. 1982 Apr;69(4):853–865. doi: 10.1172/JCI110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. M., Galbraith G. M. Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: relation to cellular activation and related metabolic events. Immunology. 1981 Dec;44(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A. Regulation of transferrin receptor expression in concanavalin A stimulated and Gross virus transformed rat lymphoblasts. J Cell Physiol. 1982 Oct;113(1):40–46. doi: 10.1002/jcp.1041130109. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. M., Adamson R. H. Antitumor activity and toxicity of salts of inorganic group 3a metals: aluminum, gallium, indium, and thallium. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1623–1626. doi: 10.1073/pnas.68.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. S. Clinical applications of gallium in oncology. Int J Nucl Med Biol. 1981;8(4):249–255. doi: 10.1016/0047-0740(81)90030-9. [DOI] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Loh T. T., Higuchi D. A., van Bockxmeer F. M., Smith C. H., Brown E. B. Transferrin receptors on the human placental microvillous membrane. J Clin Invest. 1980 May;65(5):1182–1191. doi: 10.1172/JCI109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Rasey J. S., Nelson N. J., Larson S. M. Relationship of iron metabolism to tumor cell toxicity of stable gallium salts. Int J Nucl Med Biol. 1981;8(4):303–313. doi: 10.1016/0047-0740(81)90037-1. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Durbin H., Clingan D., de Asua L. J. Iron salts and transferrin are specifically required for cell division of cultured 3T6 cells. Biochem Biophys Res Commun. 1977 Apr 11;75(3):556–562. doi: 10.1016/0006-291x(77)91508-x. [DOI] [PubMed] [Google Scholar]
- Scott R. E., Florine D. L., Wille J. J., Jr, Yun K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proc Natl Acad Sci U S A. 1982 Feb;79(3):845–849. doi: 10.1073/pnas.79.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W. Nitroblue-tetrazolium tests. Lancet. 1974 Nov 23;2(7891):1248–1252. doi: 10.1016/s0140-6736(74)90758-2. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Allen R. H., Kirchanski S. J., Natale P. J. Automated analysis of reticulocytes using fluorescent staining with both acridine orange and an immunofluorescence technique. Am J Hematol. 1983 Feb;14(1):57–66. doi: 10.1002/ajh.2830140107. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Sephton R. G., Harris A. W. Gallium-67 citrate uptake by cultured tumor cells, stimulated by serum transferrin. J Natl Cancer Inst. 1975 May;54(5):1263–1266. doi: 10.1093/jnci/54.5.1263. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarella C., Ferrero D., Gallo E., Pagliardi G. L., Ruscetti F. W. Induction of differentiation of HL-60 cells by dimethyl sulfoxide: evidence for a stochastic model not linked to the cell division cycle. Cancer Res. 1982 Feb;42(2):445–449. [PubMed] [Google Scholar]
- Thomopoulos P., Roth J., Lovelace E., Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976 Jul;8(3):417–423. doi: 10.1016/0092-8674(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. Biological effects of transferrin on human lymphocytes in vitro. Exp Cell Res. 1972 Sep;74(1):220–226. doi: 10.1016/0014-4827(72)90500-9. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Warrell R. P., Jr, Coonley C. J., Straus D. J., Young C. W. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983 Jun 1;51(11):1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Yeh C. J., Papamichael M., Faulk W. P. Loss of transferrin receptors following induced differentiation of HL-60 promyelocytic leukemia cells. Exp Cell Res. 1982 Apr;138(2):429–433. doi: 10.1016/0014-4827(82)90192-6. [DOI] [PubMed] [Google Scholar]
- van Bockxmeer F. M., Morgan E. H. Transferrin receptors during rabbit reticulocyte maturation. Biochim Biophys Acta. 1979 Apr 18;584(1):76–83. doi: 10.1016/0304-4165(79)90237-x. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]



