Abstract
Background
Predictive factors for efficacy of vascular endothelial growth factor pathway-targeted therapies have not been identified or confirmed. Hypertension has been observed as a side effect to anti-vascular endothelial growth factor therapy. The goal of our study was to retrospectively assess if hypertension induced during treatment with bevacizumab was associated with clinical outcome in metastatic colorectal cancer patients treated with bevacizumab.
Patients and methods
We conducted a retrospective chart review of patients with colorectal cancer treated with bevacizumab at Lombardi Comprehensive Cancer Center from 2004 to 2008.
Results
Eighty-four patients with metastatic colorectal cancer were eligible. Eighteen patients (21%) developed grades 3 hypertension. Twelve patients (14%) developed grade 2 hypertension. Six patients (7%) developed grade 1 hypertension. Median overall survival (OS) was 29 months and progression-free survival (PFS) was 10 months. Patients with any grade hypertension while on bevacizumab had an adjusted hazard ratio for death of 0.32 (p=0.03) and adjusted risk of progression of 51% (p=0.02) compared to those without hypertension (HTN). When stratified by metastatic disease, patients presenting with metastases who developed HTN had better OS and PFS (p=0.03 and 0.01.) Among patients without metastases at diagnosis, those with HTN on bevacizumab had better OS and PFS but results were not statistically significant (p=0.60 and 0.62, respectively).
Conclusions
Our data indicate that bevacizumab-induced hypertension may represent an interesting prognostic factor for clinical outcome in advanced colorectal cancer patients receiving bevacizumab.
Keywords: bevacizumab, colorectal cancer, hypertension
Background
Colorectal cancer is the second leading cause of cancer death in the United States of America. Within the past 10 years, the number of chemotherapeutic agents available to combat this grave disease has significantly increased. Novel biologically targeted therapies that interfere with specific molecular pathways affecting cancer proliferation and metastasis have been developed as treatment options for patients. Bevacizumab is a humanized monoclonal antibody that targets vascular endothelial growth factor-A (VEGF-A), a member of a family of VEGF receptor-activating ligands. Colorectal cancer was the first malignancy for which clear evidence for efficacy of an anti-VEGF strategy was shown in randomized trials. In a pivotal early trial, the addition of bevacizumab to the bolus 5-FU/leucovorin/irinotecan regimen significantly improved response rates (45% versus 35%), time to progression (11 versus 6 months), and overall survival (20 versus 16 months) [1]. Since then, the benefit of adding bevacizumab to a variety of irinotecan- and oxaliplatin-containing regimens used for first-line [2–5] and second-line therapy [6] has been confirmed.
Attempts to define molecular or pathologic predictive factors for bevacizumab efficacy in order to identify subgroups of patients who gain greater or lesser degrees of benefit from the drug have not been successful [7, 8]. An important issue is that it is not tumor tissue that is the target for bevacizumab but instead host endothelial cells. Therefore, the effect of anti-VEGF therapy on the host endothelial cells may serve as a surrogate marker of the efficacy of bevacizumab treatment. VEGF increases micro-vascular permeability, induces cell division and migration, and inhibits apoptosis [9]. When VEGF is infused into rats, there is a dose-dependent decrease in mean arterial pressure. This is thought to be secondary to decreased venous return via nitric oxide and prostacyclin synthesis. In a trial of patients with stable coronary artery disease with the goal of revascularization through infusion of VEGF, hypotension was the dose-limiting side effect [10].
Anti-VEGF therapy leads to the common side effect of hypertension (HTN). Grade 2–3 HTN is seen in approximately 15% of patients who receive bevacizumab. The mechanism of action by which bevacizumab causes HTN is uncertain. The hypertensive effect has not been shown to be a dose-dependent response [11]. It has been proposed that impaired angiogenesis or endothelial dysfunction may be the central mechanism of elevated blood pressure [12–15]. Since both the effect of anti-VEGF on tumor vasculature and normal vasculature is through modification of endothelial function, we propose hypertension serves as a surrogate marker for the anti-tumor activity of bevacizumab.
The aim of our study was to retrospectively assess if hypertension was associated with progression free-survival and overall survival among patients treated with bevacizumab-containing therapy for metastatic colorectal cancer.
Methods
We reviewed the cases of patients treated with bevacizumab at Lombardi Comprehensive Cancer Center between January 2004 and August 2008. We collected data from medical records including office visits and imaging reports. IRB approval and informed consent were waived as no patient contact was required and no identifying information was used. Blood pressures during office visits while on bevacizumab were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0, 2003). Grade 1 toxicity is defined as an asymptomatic, transient increase (<24 h) greater than 20 mm Hg diastolic or to greater than 150/100 mm Hg. Grade 2 is recurrent or persistent (>24 h) or a symptomatic increase greater than 20 mm Hg diastolic or to greater than 150/100 mm Hg. Grade 3 HTN is hypertension requiring therapy or more intensive therapy than previously. Grade 4 is hypertensive crisis.
Statistical Methods
Patient characteristics were compared using chi-square tests for categorical characteristics and the Wilcoxon rank-sum test for continuous characteristics. The primary endpoints were overall survival (OS) and progression-free survival (PFS). OS was defined as the time from first course of treatment containing bevacizumab to death from any cause or last contact. PFS was defined as the time from first course of treatment containing bevacizumab to disease progression, death, or last contact.
Overall and progression-free survival for patients with HTN versus those without were presented using Kaplan-Meier estimates and compared with the log-rank test. Cox regression models were used to estimate unadjusted and adjusted hazard ratios and their 95% confidence intervals for hypertension. Age, metastatic disease at diagnosis, past medical history of hypertension, and time from first therapy to first therapy containing bevacizumab were considered as covariates. Hypertension was considered present for grades 1, 2, 3, or 4. All comparisons were made at a two-sided 5% significance level.
Results
A total of 84 patients had available patient records and were included in the analysis. Patient characteristics, overall and by metastatic disease status, are shown in Table 1. Overall, 50% of patients were female, and 74% were age 50 or older at diagnosis. Fifty-two patients (62%) reviewed had metastatic disease at initial diagnosis and the remaining 32 (38%) developed metastatic disease further along in their disease process. In addition, 43% of patients, overall, had hypertension while on bevacizumab, among which 18% of patients had grade 3 or 4 hypertension. There were no statistically significant differences between patients with and without metastatic disease at diagnosis on the basis of gender, age at diagnosis, or hypertension grade. All patients with metastatic colorectal cancer at diagnosis had bevacizumab as part of first-line therapy. For patients with no metastatic disease at diagnosis, the median time from diagnosis to bevacizumab treatment was 19.4 months. Therefore, patients with metastatic disease at diagnosis had shorter time to starting bevacizumab-containing therapy compared to those without metastatic disease (p<0.001).
Table 1.
Patient characteristics by metastatic disease (N=84)
| Characteristic | Metastatic disease at diagnosis
|
p valuea | ||||
|---|---|---|---|---|---|---|
| Total (N=84) | No (N=32)
|
Yes (N = 52)
|
||||
| N | N | % | N | % | ||
| Gender | ||||||
| Male | 42 | 16 | 50 | 26 | 50 | 0.99 |
| Female | 42 | 16 | 50 | 26 | 50 | |
| Age at diagnosis | ||||||
| <50 | 22 | 10 | 31 | 12 | 23 | 0.45 |
| ≥50 | 62 | 22 | 69 | 40 | 77 | |
| HTN grade | ||||||
| 0 | 48 | 17 | 53 | 31 | 60 | 0.69 |
| 1 | 6 | 3 | 9 | 3 | 6 | |
| 2 | 12 | 6 | 19 | 6 | 12 | |
| 3 | 18 | 6 | 19 | 12 | 23 | |
| 4 | 0 | 0 | 0 | |||
| Months to 1st bevacizumab | ||||||
| Median (range) | 19.4 | 0–128 | 0 | 0–150 | <0.001 | |
p values for proportions computed using Fisher’s exact test and for medians using Wilcoxon rank-sum test
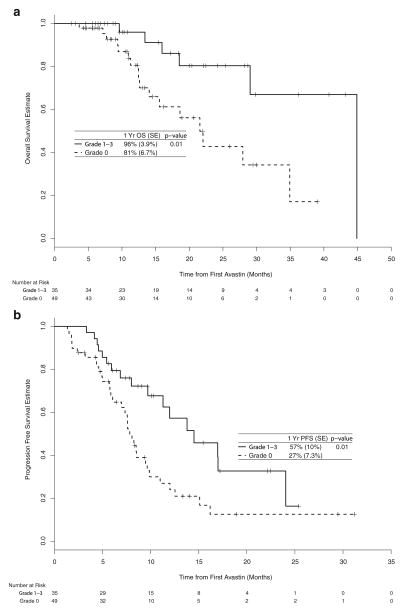
The median OS was 29 months and the 1-year OS rate was 87% (95% CI: 79–96%). The median follow-up time among survivors was 11 months. Patients with hypertension had better OS (Fig. 1a). Specifically, patients with and without hypertension had a 1-year OS rate of 96% (95% CI: 89–100%) compared to 80% (95% CI: 68–95%), respectively (p=0.01).
Fig. 1.
Kaplan-Meier estimates of overall survival estimate (a) and progression-free survival (b)
The median PFS on bevacizumab was 10 months and the 1-year PFS rate for all patients was 40% (95% CI: 29%–54%). Again, patients with versus without hypertension had a one-year PFS rate of 56% (95% CI: 39%–79%) compared to 28% (95% CI: 16%–47%), respectively (Fig. 1b; p=0.02).
After controlling for metastatic disease at diagnosis, patients with hypertension still had a lower risk of death and progression compared to patients who did not have hypertension. (Table 2) The adjusted risk of death for those with hypertension was 32% of the risk for those who did not have hypertension (95% CI: 12%–90%, p=0.03) and the adjusted risk of progression was 51% (95% CI: 28%–91%, p=0.02). A past medical history of HTN was examined as a potential confounder of OS. This was not statistically significant and did not change the estimates for the other covariates, so it was removed from the models.
Table 2.
Unadjusted and adjusted hazard ratios and 95% confidence intervals for hypertension grade (N=84)
| Outcome | HR | 95% CI | p value |
|---|---|---|---|
| Overall survival | |||
| HTN vs. none | 0.27 | 0.10, 0.74 | 0.01 |
| HTN vs. none adjusted for metastases | 0.32 | 0.12, 0.89 | 0.03 |
| HTN vs. none adjusted for metastases and age | 0.32 | 0.12, 0.89 | 0.03 |
| Progression-free survival | |||
| HTN vs. none | 0.48 | 0.26, 0.85 | 0.01 |
| HTN vs. none adjusted for metastases | 0.48 | 0.26, 0.86 | 0.01 |
| HTN vs. none adjusted for metastases and age | 0.46 | 0.26, 0.84 | 0.01 |
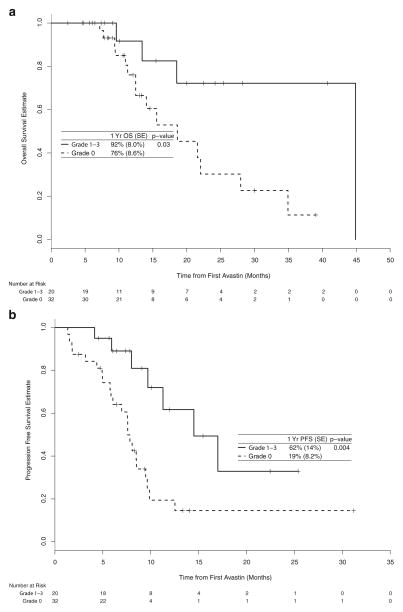
The OS and PFS for those with and without hypertension were also compared within the subgroups of those with and without metastatic disease at presentation (Fig. 2). Among patients with metastatic disease at diagnosis, patients with hypertension had better OS and PFS. Specifically, those with and without hypertension had a 1-year OS rate of 92% (95% CI: 77–100%) compared to a 1-year rate of 76% (95% CI: 61–95%), respectively (p=0.03). Those with versus without hypertension had a 1-year PFS rate of 59% (95% CI: 37–93%) compared to a 1-year rate of 20% (95% CI: 9–46%), respectively (p=0.01).
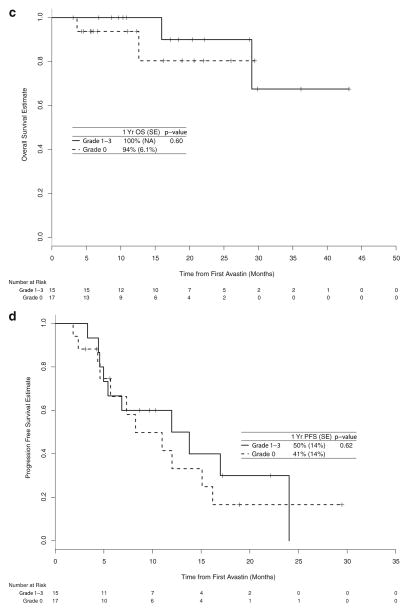
Fig. 2.
Kaplan-Meier estimate of overall survival (a) and progression-free survival (b) among those with metastatic disease and of overall survival (c) and progression-free survival (d) among those without meta-static disease
Among patients without metastatic disease at diagnosis, patients on bevacizumab treatment with hypertension had better OS and PFS compared to patients without hypertension, but these differences were not statistically significant (p=0.60 and 0.62, respectively).
Discussion/Conclusion
We have found in retrospective analysis that the side effect of hypertension while on bevacizumab appears to associate with a statistically significantly improved progression-free survival and overall survival irrespective of age, past medical history of hypertension, or time from diagnoses to receiving bevacizumab. These finding are partially supported by recent data reported in colorectal cancer and renal cell carcinoma patients [16, 17]. Scartozzi et al. reported a retrospective review of 39 metastatic colorectal cancer patients treated with bevacizumab as part of frontline therapy. In their study, eight patients (20%) developed grades 2–3 hypertension. Median PFS was 14.5 months for patients showing bevacizumab-related hypertension, while it was 3.1 months in those without hypertension (p=0.04). Unlike the current study, they did not report a statistically significant improvement in overall survival though. In our study, 18 out of 84 patients (21%) developed grade 3 HTN. The incidence of grade 3–4 hypertension observed in patients treated with anti-VEGF therapy in previous clinical trials ranges from 6–21% [1, 6, 18, 19]. The median OS (29 months) and PFS (10 months) observed in our study are similar to what has been presented in clinical trials [1, 15, 20, 21], the BRiTE Registry [22] and the first BEAT Registry [23].
When stratified by metastatic disease, this result remains significant only among those with metastatic disease at initial presentation. The reason for this dissimilarity is unclear. Those patients without metastatic disease at presentation developed distant spread by the time bevacizumab was administered. Thus, one would think that the groups would act similarly in regards to incidence of HTN and response to therapy. One possible reason for the observed difference is that the sample size for patients without metastatic disease at diagnosis is too small, therefore, no statistical significance is observed. The patient sample size for those without metastatic disease at presentation was much smaller (n=32) than the group with metastatic spread at diagnosis (n=52). Additionally, the effect of hypertension appears to be stronger among patients with metastatic disease at diagnosis. Those starting without metastatic disease do not show the same level of benefit with hypertension. This results in a smaller estimated difference between those with and without hypertension for patients without metastatic disease. A larger study may be able to detect this smaller, but still apparent difference.
With the exponential increase in economic cost contributed by emerging biological therapy, identification of patients with a reliable predictive factor who would benefit from specific treatments would not only relieve some burden of medical expense from society, but also spare patients who do not benefit from the therapy from additional toxicity. Our data support that hypertension induced by anti-VEGF therapy due to the blockade of the effect of VEGF on endothelial function may serve as a useful marker for the effect of bevacizumab on the endothelial function in tumor vasculature, thus affecting overall survival. This is analogous to the significant association found between the occurrence of rash and increased survival in patients treated with anti-epidermal growth factor receptor (EGFR) therapy [24]. In that case, the rash induced by cetuximab, a monoclonal antibody to EGFR, by inhibiting EGFR in hair follicles, serves as a predictive marker of anti-EGFR effect in tumor. In addition, in contrast to gene array, immunohistochemical staining or gene mutation analysis of tumor biopsy, blood pressure monitoring can be utilized in any clinic with little cost.
This study, like all retrospective analyses, is limited since it is not a prospective study and is small in size. Due to lack of randomization and inability to measure all variable, many things may account for why some patients developed a hypertensive response and others did not. In particular, patients’ functional status is not accounted for in our analysis. It may be that those patients able to mount a hypertensive response simply had less aggressive disease and/or better functional status, thus making them more likely to live longer irrespective of hypertensive response. In other words, the elevation in blood pressure may simply be a marker of better prognosis due to functional status and slow-growing nature of the tumor and not a marker of drug efficacy. Secondly, we did not account for time spent on bevacizumab therapy. The side effect of hypertension may be one that simply comes with time or cumulative dose. Those who respond to treatment stay on bevacizumab longer and may develop high blood pressure simply due to a total dose effect. Finally, the accuracy of data is not always reliable or completely clear when evaluated in a retrospective fashion. However, our observations support looking further into this correlation with the creation of a randomized, prospective study that is designed to evaluate HTN as a surrogate marker for the clinical outcome of bevacizumab treatment.
Our observations imply hypertension (including grade 1–4) developed during bevacizumab-containing treatment of colorectal cancer predicts overall survival and progression-free survival, whereas lack of this side effect could indicate an important warning of lack of benefit of bevacizumab and warrant a switch in therapy. Future efforts will be made both to confirm this observation in larger studies and to prospectively investigate the value of tailoring patient dosing to developing grade 1–2 hypertension as a way of maximizing the activity of bevacizumab.
Contributor Information
Rebekah Ryanne Wu, Email: rrf103@gunet.georgetown.edu, Department of Internal Medicine, Georgetown University Hospital, 3800 Reservoir Rd., Washington, DC 20007, USA.
Peter A. Lindenberg, Department of Oncology, Walter Reed Army Hospital, Washington, DC, USA
Rebecca Slack, Department of Biostatistics, Bioinformatics, and Biomathematics and Lombardi Comprehensive Cancer Center, Georgetown University, Georgetown, USA.
Anne-Michelle Noone, Department of Biostatistics, Bioinformatics, and Biomathematics and Lombardi Comprehensive Cancer Center, Georgetown University, Georgetown, USA.
John L. Marshall, Department of Medicine and Oncology Lombardi Comprehensive Cancer Center, Georgetown University, Georgetown, USA
Aiwu R. He, Department of Medicine and Oncology Lombardi Comprehensive Cancer Center, Georgetown University, Georgetown, USA
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs CS, Marshall J, Mitchell E, et al. Randomized controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol. 2007;25:4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 3.Hochester HS, Hart LL, Ramanathan K, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol. 2008;26:3523–9. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 6.Giantonio BJ, Catalano PJ, Meropol N, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOL-FOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 7.Ince WL, Jubb AM, Holden SN, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–9. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–27. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–93. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Annex HTD, McKendall BHGR, et al. The viva trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 11.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–803. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Veronese ML, Flaherty KT, Townsend R, et al. Pharmacodynamic study of the raf kinase inhibitor BAY 43-9006: mechanisms of hypertension. J Clin Oncol. 2004;14(22 Suppl):2035. [Google Scholar]
- 13.Horowitz JR, Rivard A, van der Zee R, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Arterioscler Thromb Vasc Biol. 1997;17:2793–800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 14.Hood JD, Meininger CJ, Ziche M, Grander HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothe-lial cells. Am J Physiol Heart Circ Physiol. 1998;274:H1054–H58. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 15.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab: a crucial role of microcirculation. Ann Oncol. 2008;19:927–34. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]
- 16.Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 17.Bono P, Elfving H, Utriainen T, et al. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009;20:393–4. doi: 10.1093/annonc/mdn729. [DOI] [PubMed] [Google Scholar]
- 18.Kohne C, Bajetta E, Lin E, et al. Final results of CONFIRM 2: A multinational, randomized, double-blinded phase III study in 2nd line patients with metastatic colorectal cancer receiving FOL-FOX4 and PTK787/ZK 222584 or placebo. J Clin Oncol. 2007;18 (25 Suppl):4033. [Google Scholar]
- 19.Miller KD, Chap LI, Holmes F, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 20.Giantonio BJ, Catalano PJ, Meropol NJ, et al. High-dose bevacizumab improved survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2005;16(23 Suppl):2. [Google Scholar]
- 21.Hecht JR, Trarbach T, Jaeger E, et al. A randomized, double-blind, placebo-controlled, phase III study in patients with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) J Clin Oncol. 2005;16(23 Suppl):3. [Google Scholar]
- 22.Kozloff M, Hainsworth J, Badarinath S, et al. Efficacy of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRite) J Clin Oncol. 2006;18(24 Suppl):3537. [Google Scholar]
- 23.Berry S, Cunningham D, Michael M, et al. Preliminary safety of bevacizumab with first-line Folfox, Capox, Folfiri and Capecitabine for metastatic colorectal cancer—first B E A trial. J Clin Oncol. 2006;18(24 Suppl):3534. [Google Scholar]
- 24.Agero AL, Dusza SW, Benvenuto-Adrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Amer Acad Dermatol. 2006;55:657–70. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]